Abstract
Objectives:
To investigate whether patients with spinal cord injury (SCI) are at an increased risk of developing Parkinson’s disease (PD).
Study design:
A population-based, propensity score-matched, longitudinal follow-up cohort study.
Setting:
The study was conducted using the National Health Insurance (NHI) Research Database.
Methods:
A total of 10 125 patients with at least 2 ambulatory visits with a diagnosis of SCI in 2001 were enrolled in the SCI group. The non-SCI group comprised 10 125 propensity score-matched patients without SCI. The propensity scores were computed using a logistic regression model that included age, sex, comorbidities and socioeconomic status. The PD-free survival rates of the two groups were estimated using the Kaplan–Meier method. Stratified Cox proportional hazard regression was used to estimate the effect of SCI on subsequent occurrence of PD.
Results:
During the 3-year follow-up period, 99 subjects in the SCI group and 59 in the non-SCI group developed PD. The hazard ratio of PD for the SCI group compared with the non-SCI group was 1.65 (95% confidence interval 1.16–2.33, P=0.0049). The PD-free survival rate for the SCI group was lower than that for the non-SCI group (P=0.0017).
Conclusions:
This study shows that SCI is associated with a subsequent increased risk of PD. Further studies are needed to elucidate the mechanism underlying this association.
Similar content being viewed by others
Introduction
Parkinson’s disease (PD), the second most common neurodegenerative disorder, affects millions of people worldwide, causing substantial disability and decreased quality of life.1 The characteristic histopathological feature of PD is the formation of Lewy bodies, which consist mainly of a cytotoxic protein, alpha-synuclein.2 Recent studies have suggested that traumatic brain injury (TBI) is etiologically linked to PD3 and that abnormal alpha-synuclein deposition in the brain after TBI may explain how TBI is linked to PD.4 In addition, neuroinflammation has been shown to have an important role in PD development.5, 6 Neuroinflammation can be elicited after central nervous system (CNS) injuries such as TBI7 and spinal cord injury (SCI)8 and can have both immediate and delayed detrimental effects leading to neurodegeneration.9 However, to our knowledge, no research has been conducted on the relationship between SCI and PD, and the aim of this population-based, propensity score-matched, longitudinal follow-up study was therefore to investigate whether patients with SCI are at a higher risk of developing PD.
Materials and methods
This study used the National Health Insurance (NHI) Research Database between 2000 and 2003. The NHI program is a single-payer health-care system that covers >97% of population (>21.9 million persons) in Taiwan. The study population consisted of an SCI group and a non-SCI group, both selected from the NHI database in 2001. In order to minimize the potential confounding effects from an imbalance in the distribution of the observed variables between the SCI and non-SCI groups, we used the propensity score matching method to balance the observed baseline characteristics between these two groups.10
The SCI group consisted of subjects aged between 20 and 90 years who received a diagnosis of SCI (International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM) codes 806, 952) in ambulatory medical care visits between 1 January 2001 and 31 December 2001. To optimize the diagnostic accuracy, only patients who had at least two ambulatory visits with the diagnosis of SCI in this period were included (n=11 976). The first ambulatory visit in 2001 during which a diagnosis of SCI was recorded was defined as the index visit. The exclusion criteria for the SCI group were (1) a previous diagnosis of PD (ICD-9-CM code 332.0) or secondary parkinsonism (ICD-9-CM code 332.1) before the index visit and (2) a previous diagnosis of TBI (ICD-9-CM codes 801–804, 850–854) before the index visit, leaving 10 173 subjects with SCI. The comorbidities included in the propensity score matching were diabetes (ICD-9-CM code 250), hypertension (ICD-9-CM codes 401–405), dyslipidemia (ICD-9-CM code 272), stroke (ICD-9-CM codes 430–438) and coronary heart disease (ICD-9-CM codes 410–414). The information on these comorbidities was obtained by retrieving all outpatient and inpatient records in the year before the index visit. The ascertainment of the diagnosis of these comorbidities was defined as ⩾1 hospital discharge or ⩾2 ambulatory medical care visits with a relevant diagnosis code. Socioeconomic status indicators, including income, geographical region and levels of urbanization, were also included in propensity score matching, as they have been suggested to affect the risk of PD.11 The information of these factors was obtained from the NHI database and household registry. We used the monthly insured payroll-related amount as a surrogate for individual income, which was stratified into four levels: new Taiwan dollar (NT$) 0, NT$1–15 840, NT$15 841–25 000, and ⩾NT$25 001. NT$15 840 was chosen as the first cutoff point, as it was the government-stipulated minimum monthly wage for full-time employees in Taiwan. The geographic areas in which patients resided were classified as geographic region of Northern, Central, Eastern or Southern Taiwan. The urbanization levels were categorized into seven levels based on the publication of Taiwan National Health Research Institute,12 with level 1 referring to the ‘most urbanized’ and level 7 referring to the ‘least urbanized’ communities. However, as there were relatively small number of subjects in levels 5, 6 and 7, these three levels were merged into a single group labeled as level 5. A total of 48 subjects in the SCI group were excluded because of missing data in the household registry information, resulting in 10 125 subjects in the final SCI group.
The non-SCI group was sampled from the subjects without a diagnosis of SCI from the NHI database in 2001. The index visit was defined as the first ambulatory medical care visit in 2001. The exclusion criteria for the non-SCI group were (1) a previous diagnosis of SCI (ICD-9-CM codes 806, 952) before the index visit, (2) a previous diagnosis of PD (ICD-9-CM code 332.0) or secondary parkinsonism (ICD-9-CM code 332.1) before the index visit and (3) a previous diagnosis of TBI (ICD-9-CM codes 801–804, 850–854) before the index visit. Information on comorbidities and socioeconomic status was obtained as described above. We adopted a two-stage approach to perform the propensity score matching in the large-scale NHI database.13 For each subject in the SCI group, we first randomly sampled 20 age- and sex-matched non-SCI subjects who met the above criteria, giving a total of 202500 non-SCI subjects. In the second stage, a logistic regression model that included age, sex, comorbidities and socioeconomic status as covariates was used to predict the probability (that is, propensity score) of SCI. An 8-to-1 digit greedy matching algorithm14 was then used to identify the unique matched control from the non-SCI subjects for each SCI patient according to the propensity score, leading to a total of 10 125 subjects in the propensity score-matched non-SCI group.
Outcome
Medical records of each subject were retrieved from the index visit to the end of 2003. The mortality data were obtained from the national mortality registry. The first diagnosis of PD (ICD-9-CM codes 332.0) during the follow-up was defined as the end point event. The ascertainment of the diagnosis of PD required ⩾1 hospital discharge or ⩾2 ambulatory medical care visits with a diagnosis of PD. All subjects were followed from the index visit to the first occurrence of PD, death or end of follow-up (whichever occurred first).
Statistical analysis
The Chi-square test and Student’s t-test were used to examine differences in demographic variables, comorbid medical disorders and propensity scores between the SCI and non-SCI groups. The covariate balance before and after propensity score matching was assessed using the standardized difference method.15 An absolute standardized difference of 0 for a covariate indicates no between-group imbalance for that covariate, and values <0.1 indicate an inconsequential imbalance. The incidence rate of PD was calculated as the number of incident PD cases divided by PD-free person-years. The PD-free survival curves for the propensity score-matched SCI and non-SCI groups were plotted using the Kaplan–Meier method, and the differences between groups were compared using the log-rank test. Stratified Cox proportional hazard regression was used to estimate the effect of SCI on the risk of PD. An alpha level of 0.05 was considered statistically significant for all analyses. The analyses were performed using the SAS 9.4 software (SAS Institute, Cary, NC, USA).
Statement of ethics
All personal identification numbers in the NHI database were converted into scrambled numbers before data processing. The retrieved data were kept confidential and were analyzed anonymously, which complies with the Personal Information Protection Act and the regulations of the Department of Health, Executive Yuan, Republic of China. As the data were used in a de-identified manner for research purposes, this study was exempt from full review by the National Taiwan University Hospital Research Ethics Committee, and the need for informed consent was waived.
Results
Table 1 shows the demographic and clinical characteristics for the SCI and non-SCI groups before propensity score matching. The mean age of the SCI group was 56.3 years, with an s.d. of 17.8 years. The SCI group had a higher prevalence of several medical comorbidities, including diabetes (P<0.0001), hypertension (P=0.0046), stroke (P<0.0001) and coronary heart disease (P=0.0317), than the non-SCI group. There were also significant differences in the distribution of monthly income, urbanization level and geographic region between the SCI and non-SCI groups. The absolute standardized differences were >0.1 for stroke, monthly income, urbanization level, geographic region and propensity score before matching (Table 1). After propensity score matching, the matched cohorts were well balanced in terms of all measured covariates, as the absolute standardized difference between the two matched groups was <0.1 for all baseline characteristics (Table 2).
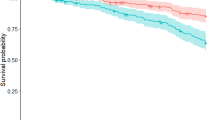
The median follow-up time was 30.4 months (interquartile range 8.7 months). The number of PD cases and the hazard ratios (HRs) of PD for the two propensity score-matched groups are presented in Table 3. For the SCI group, a total of 99 (0.98%) PD cases occurred during 24 113.8 person-years of follow-up, resulting in an incidence rate of 4.10 (95% confidence interval (CI), 3.34–5.00) per 1000 person-years. For the non-SCI group, 59 (0.58%) PD cases occurred during 23 984.7 person-years of follow-up, giving an incidence rate of 2.46 (95% CI, 1.87–3.17) per 1000 person-years. The HR of PD for the SCI group was 1.65 (95% CI, 1.16–2.33, P=0.0049). The PD-free survival rate of the SCI group was significantly lower than that of the non-SCI group (Figure 1, P=0.0017).
As the present study is a retrospective study based on health-care insurance data, the association between SCI and PD may be possibly driven by reverse causation (that is, undetected PD may cause frequent falls and a higher risk of SCI, which leads to an association between SCI and PD) or surveillance bias (that is, earlier detection of PD among people under close surveillance for SCI). To assess the influence of potential reverse causation or surveillance bias, we performed analyses evaluating the risk of PD within and after the first year of follow-up. The HR of PD for the SCI group was 1.39 (95% CI, 0.87–2.20) within the first year of follow-up and was 2.05 (95% CI, 1.19–3.55) after the first year of follow-up. The results show that the association between SCI and PD was more prominent after the first year of follow-up. These findings suggest that the association between SCI and PD found in our study was unlikely to be explained by reverse causation or surveillance bias.
Discussion
In the present population-based, propensity score-matched, longitudinal follow-up study, we found that SCI patients had a 1.65-fold higher risk of PD compared with non-SCI subjects. This is the first longitudinal study showing an increased risk of PD in patients with SCI. Although the exact mechanism underlying the association between SCI and PD is not clear, we propose the following explanations.
First, studies have shown significantly higher levels of proinflammatory cytokines such as tumor necrosis factor-α (TNF-α), interleukin-1β and interleukin-6 in both the acute16 and chronic stages of SCI.17, 18 TNF-α is a mediator in inflammatory responses,6, 17, 19 and its persistent elevation suggests an ongoing inflammatory process in patients with SCI.16, 17, 18 TNF-α can cross the blood–brain barrier and blood–cord barrier by receptor-mediated transport, which is upregulated after SCI.17, 20, 21 After entering the brain, TNF-α can activate microglia, which, in turn, produce more TNF-α and reactive oxygen species5, 22, 23, 24 and cause neuroinflammation in the brain.5, 22, 23, 24 It has been suggested that CNS inflammation and microglial activation can induce high levels of dopaminergic neuron damage,5, 17, 22 as these neurons are particularly vulnerable to inflammatory insults22, 23 and oxidative stress.5, 23, 24 We therefore hypothesize that the increased risk of PD in patients with SCI may be mediated through TNF-α-elicited neuroinflammation, resulting in progressive dopaminergic neuron loss5, 6, 22 and predisposing SCI patients to PD.
Second, animal studies have shown that, after SCI, synuclein expression is induced in the spinal cord25 and synuclein accumulates in brain neurons.26 Such accumulation of synuclein has been associated with injury-related neuron death.26 Alpha-synuclein has been shown to have a pivotal role in the pathogenesis of PD,27, 28 leading to dopaminergic neuron death, both directly27 and indirectly by reactive microgliosis.23, 28 Aggregation of alpha-synuclein is triggered by specific insults and can begin in regions outside the brain and then spread to the midbrain and neocortex in a prion-like manner.27, 29 Through exocytosis and endocytosis, alpha-synuclein aggregates can seed from a cell to neighboring neurons and glia cells, initiating a chain of events that has been suggested as an underlying mechanism for the progressive spreading of neurodegeneration and neuroinflammation.28 We therefore postulate that excessive alpha-synuclein production after SCI may serve as a trigger for PD development in patients with SCI.
However, several limitations of this study should be noted. First, as the diagnoses of SCI, PD and medical comorbidities in this study were entirely dependent on the ICD codes from the NHI database, it may raise a concern about the accuracy of the diagnosis. However, the Bureau of the NHI has set up various audit committees that randomly sample the claim data and review the medical records routinely so as to monitor the quality of care and diagnosis. The NHI database is therefore an established database and is widely used for research in a variety of medical fields.30 Second, although we matched the SCI and non-SCI groups for the demographic, clinical, socioeconomic and geographic characteristics to generate comparable cohorts, information pertaining to family history of PD and lifestyle factors, such as smoking and alcohol intake, was not available in the NHI database and was not included in the analysis, which may introduce residual confounding in the association between SCI and PD. Third, although we excluded subjects with a prior diagnosis of PD in both the SCI and non-SCI groups, it is still possible that subclinical or undiagnosed PD in general population may cause easy falls and a higher risk of SCI, which may contribute to the association between SCI and PD. Given the possibility of reverse causality between SCI and PD, we performed sensitivity analysis evaluating the risk of PD within and after the first year of follow-up. The results show that the association between SCI and PD was more pronounced after the first year of follow-up, suggesting that this association is not likely to be driven by reverse causality.
Conclusion
The present population-based, propensity score-matched, longitudinal follow-up study shows that patients with SCI have an increased risk of developing PD. Further studies are needed to validate our findings and to investigate the underlying mechanism.
Data archiving
There were no data to deposit.
References
Huse DM, Schulman K, Orsini L, Castelli-Haley J, Kennedy S, Lenhart G . Burden of illness in Parkinson's disease. Mov Disord 2005; 20: 1449–1454.
Gallegos S, Pacheco C, Peters C, Opazo CM, Aguayo LG . Features of alpha-synuclein that could explain the progression and irreversibility of Parkinson's disease. Front Neurosci 2015; 9: 59.
Gardner RC, Burke JF, Nettiksimmons J, Goldman S, Tanner CM, Yaffe K . Traumatic brain injury in later life increases risk for Parkinson's disease. Ann Neurol 2015; 77: 987–995.
Gardner RC, Yaffe K . Epidemiology of mild traumatic brain injury and neurodegenerative disease. Mol Cell Neurosci 2015; 66: 75–80.
Stojkovska I, Wagner BM, Morrison BE . Parkinson's disease and enhanced inflammatory response. Exp Biol Med (Maywood) 2015; 240: 1387–1395.
Fischer R, Maier O . Interrelation of oxidative stress and inflammation in neurodegenerative disease: role of TNF. Oxid Med Cell Longev 2015; 2015: 610813.
Corrigan F, Vink R, Turner RJ . Inflammation in acute CNS injury: a focus on the role of substance P. Br J Pharmacol 2015; 173: 703–715.
David S, Lopez-Vales R, Wee Yong V . Harmful and beneficial effects of inflammation after spinal cord injury: potential therapeutic implications. Handb Clin Neurol 2012; 109: 485–502.
Park E, Velumian AA, Fehlings MG . The role of excitotoxicity in secondary mechanisms of spinal cord injury: a review with an emphasis on the implications for white matter degeneration. J Neurotrauma 2004; 21: 754–774.
D'Agostino RB . Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med 1998; 17: 2265–2281.
Chen CC, Chen TF, Hwang YC, Wen YR, Chiu YH, Wu CY et al. Different prevalence rates of Parkinson’s disease in urban and rural areas: a population-based study in Taiwan. Neuroepidemiology 2009; 33: 350–357.
Liu C, Hung Y, Chuang Y, Chen Y, Weng W, Liu J et al. Incorporating development stratification of Taiwan townships into sampling design of large scale health interview survey (in Chinese). J Health Manage 2006; 4: 1–22.
Huang YP, Chen LS, Yen MF, Fann CY, Chiu YH, Chen HH et al. Parkinson's disease is related to an increased risk of ischemic stroke-a population-based propensity score-matched follow-up study. PLoS ONE 2013; 8: e68314.
Parsons L. Performing a 1:N Case-Control Match on Propensity Score. Proceedings of the 29th Annual SAS Users Group International Conference; 9–12 May 2004; Montreal, Canada. SAS: North Carolina, USA, 2004.
Austin PC . A critical appraisal of propensity-score matching in the medical literature between 1996 and 2003. Stat Med 2008; 27: 2037–2049.
Biglari B, Swing T, Child C, Buchler A, Westhauser F, Bruckner T et al. A pilot study on temporal changes in IL-1beta and TNF-alpha serum levels after spinal cord injury: the serum level of TNF-alpha in acute SCI patients as a possible marker for neurological remission. Spinal Cord 2015; 53: 510–514.
Davies AL, Hayes KC, Dekaban GA . Clinical correlates of elevated serum concentrations of cytokines and autoantibodies in patients with spinal cord injury. Arch Phys Med Rehabil 2007; 88: 1384–1393.
Hayes KC, Hull TC, Delaney GA, Potter PJ, Sequeira KA, Campbell K et al. Elevated serum titers of proinflammatory cytokines and CNS autoantibodies in patients with chronic spinal cord injury. J Neurotrauma 2002; 19: 753–761.
Aggarwal BB . Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol 2003; 3: 745–756.
Pan W, Zhang L, Liao J, Csernus B, Kastin AJ . Selective increase in TNF alpha permeation across the blood-spinal cord barrier after SCI. J Neuroimmunol 2003; 134: 111–117.
Pan W, Kastin AJ . Cytokine transport across the injured blood-spinal cord barrier. Curr Pharm Des 2008; 14: 1620–1624.
Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS et al. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia 2007; 55: 453–462.
Block ML, Hong JS . Microglia and inflammation-mediated neurodegeneration: multiple triggers with a common mechanism. Prog Neurobiol 2005; 76: 77–98.
Block ML, Hong JS . Chronic microglial activation and progressive dopaminergic neurotoxicity. Biochem Soc Trans 2007; 35 (Pt 5): 1127–1132.
Sakurai M, Kawamura T, Nishimura H, Suzuki H, Tezuka F, Abe K . Induction of Parkinson disease-related proteins in motor neurons after transient spinal cord ischemia in rabbits. J Cereb Blood Flow Metab 2009; 29: 752–758.
Busch DJ, Morgan JR . Synuclein accumulation is associated with cell-specific neuronal death after spinal cord injury. J Comp Neurol 2012; 520: 1751–1771.
Sanchez-Guajardo V, Tentillier N, Romero-Ramos M . The relation between alpha-synuclein and microglia in Parkinson's disease: recent developments. Neuroscience 2015; 302: 47–58.
Lee HJ, Bae EJ, Lee SJ . Extracellular alpha—synuclein-a novel and crucial factor in Lewy body diseases. Nat Rev Neurol 2014; 10: 92–98.
Brettschneider J, Del Tredici K, Lee VM, Trojanowski JQ . Spreading of pathology in neurodegenerative diseases: a focus on human studies. Nat Rev Neurosci 2015; 16: 109–120.
Wen CP, Tsai SP, Chung WS . A 10-year experience with universal health insurance in Taiwan: measuring changes in health and health disparity. Ann Intern Med 2008; 148: 258–267.
Acknowledgements
This study was supported by grants DOH93-TD-M-113-030, DOH94-TD-M-113-004 and DOH95-TD-M-113-002 from the Department of Health (DOH), Executive Yuan, Republic of China. This study used the National Health Insurance Research Database provided by the Bureau of National Health Insurance, Department of Health. The conclusions in our study do not necessarily represent those of the Department of Health, Executive Yuan, Republic of China.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Yeh, TS., Huang, YP., Wang, HI. et al. Spinal cord injury and Parkinson’s disease: a population-based, propensity score-matched, longitudinal follow-up study. Spinal Cord 54, 1215–1219 (2016). https://doi.org/10.1038/sc.2016.74
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sc.2016.74
This article is cited by
-
Acute Transcriptomic and Epigenetic Alterations at T12 After Rat T10 Spinal Cord Contusive Injury
Molecular Neurobiology (2023)
-
Chronic MPTP in Mice Damage-specific Neuronal Phenotypes within Dorsal Laminae of the Spinal Cord
Neurotoxicity Research (2021)
-
Motor Neurons Pathology After Chronic Exposure to MPTP in Mice
Neurotoxicity Research (2020)
-
Lentivirus-mediated downregulation of α-synuclein reduces neuroinflammation and promotes functional recovery in rats with spinal cord injury
Journal of Neuroinflammation (2019)
-
Spinal cord injury and Alzheimer’s disease risk: a population-based, retrospective cohort study
Spinal Cord (2018)