Abstract
Study design:
Retrospective study.
Objectives:
Few studies have reported a relationship between central motor conduction time (CMCT), which evaluates corticospinal function, and degree of spinal cord compression in patients with myelopathy. Thus, there is no consensus on predicting the degree of prolonged CMCT on the basis of the degree of spinal cord compression. If a correlation exists between CMCT and spinal cord compression, then spinal cord compression may be a useful noninvasive clinical indicator of corticospinal function. Therefore, this study evaluated the relationship between CMCT and cervical spinal cord compression measured by magnetic resonance imaging (MRI) in patients with cervical spondylotic myelopathy (CSM).
Setting:
Hiroshima University Hospital in Japan.
Methods:
We studied 33 patients undergoing laminoplasty. Patients exhibited significant cervical spinal cord compression on both MRI and intraoperative electrophysiological examination. We assessed transcranial magnetic stimulation measurement of CMCT; spinal cord compression parameters such as area, lateral diameter, anteroposterior diameter and flattening of the spinal cord at the lesion site and C2/3 levels on MRI; and pre- versus postoperative Japanese Orthopaedic Association (JOA) scores.
Results:
Correlations between CMCT and flattening as well as anteroposterior diameter of the spinal cord at the lesion level were observed. Strong correlations between CMCT and the ratio of the flattening and anteroposterior diameter parameters at the lesion level to that at the C2/3 level were also observed.
Conclusions:
Measurement of spinal cord compression may be useful for the evaluation of corticospinal function as a proxy for CMCT in patients with CSM.
Similar content being viewed by others
Introduction
Cervical spondylotic myelopathy (CSM) is a common degenerative spine disorder. Several methods are used for the diagnosis of CSM,1 including magnetic resonance imaging (MRI). However, although MRI is a popular diagnostic tool for CSM,2 it reveals only morphological and not functional abnormalities of the spinal cord. Thus, the use of MRI carries a risk for overdiagnosis of asymptomatic spinal cord compression.
Electrophysiological diagnosis using central motor conduction time (CMCT) as a quantitative measurement of spinal cord function has been reported in patients with CSM.3, 4, 5 However, there are few reports on the relationship between CMCT and the degree of spinal cord compression in patients with myelopathy. Thus, there is no consensus on predicting the degree of prolonged CMCT on the basis of the degree of spinal cord compression.6 If a correlation exists between CMCT and spinal cord compression, then spinal cord compression may be a noninvasive clinical indicator of corticospinal function. The purpose of this study, therefore, was to evaluate the relationship between CMCT and cervical spinal cord compression as measured by MRI in patients with CSM (Table 1).
Materials and methods
Patients
We studied 33 patients (13 women and 20 men) who were undergoing laminoplasty for CSM at Hiroshima University Hospital in Japan. The mean age of patients was 66.1 years (range 44–84 years). In these patients, the level at which the cervical spinal cord was observed to be compressed on MRI was confirmed by intraoperative electrophysiological findings. Lesions were observed at C3/4 in 11 patients, at C4/5 in 12 patients and at C5/6 in 10 patients. All patients demonstrated clinical recovery post surgery.
Central motor conduction time measurements
Preoperatively, surface recording electrodes were placed bilaterally on the abductor digiti minimi and abductor hallucis muscles using the standard belly-tendon method for motor-evoked potential (MEP) recordings. Transcranial magnetic stimulation was delivered using a round 14-cm outer diameter coil (Model 200; Magstim, Whitland, UK). A clockwise current in the left hemisphere and a counterclockwise current in the right hemisphere were used as stimulation. The magnetic stimulus intensity was set at 20% above the threshold for MEP. The center of the coil was maintained over the vertex of the cranium, whereas MEPs were recorded from the abductor digiti minimi muscle. The coil was then shifted anteriorly to record MEPs from the abductor hallucis muscle. MEPs were recorded at least four times from each location; all responses were superimposed and their latencies were measured. Compound muscle action potentials and F-waves were recorded following continuous current stimulation at supramaximal intensity (0.2-ms square-wave pulses) of the ulnar and tibial nerves at the wrist and ankle, respectively. Thirty-two serial responses were obtained, and the shortest F-wave latency was measured.
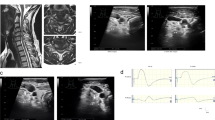
The method used to calculate CMCT from the recordings is outlined in Figure 1. In brief, all muscle responses were recorded using a commercially available system (Viking IV; Nicolet Biomedical, Madison, WI, USA) with a bandpass filter of 0.5–2000 Hz. The 100-ms epoch immediately following stimulation was digitized at a 5-KHz sampling rate. The peripheral conduction time, excluding the turnaround time at the spinal motor neuron (1 ms), was calculated from the latencies of the compound muscle action potential and F-wave as follows:7 (latency of compound muscle action potential+latency of F-wave−1)/2. Conduction time from the motor cortex to the spinal motor neurons (that is, the CMCT) was calculated by subtracting the peripheral conduction time from the onset latency of the MEPs.
Calculations for CMCT. Upper: MEP waveforms detected from the abductor digiti minimi (ADM) and abductor halluces (AH) following transcranial magnetic stimulation (TMS). Lower: Superimposed M-waves and F-waves were detected from the ADM or AH following ulnar or tibial nerve electrical stimulation. The peripheral conduction time (PCT), excluding the turnaround time at the spinal motor neuron (1ms), was calculated from the latencies of the M-wave (compound muscle action potential) and F-wave as follows: (latency of M-wave+latency of F-wave−1ms)/2. CMCT was calculated by subtracting the PCT from the onset latency of the MEP.
Intraoperative electrophysiological recording and analysis
Multimodal spinal cord-evoked potentials (SCEPs) were recorded intraoperatively using the parameters described herein. Ascending SCEPs were recorded after stimulation of the lower thoracic spinal cord with a 0.2-ms long, 10 mA square-wave pulse. Descending SCEPs were recorded after transcranial electric stimulation at 100 mA. Segmental SCEPs were recorded after ulnar or median nerve stimulation at three times the motor threshold. The responses were recorded from platinum needle electrodes (Unique Medical, Tokyo, Japan) inserted into the ligamentum flavum in the surgical field, and averaged and stored using the Nicolet Viking IV system (Nicolet Biomedical, Madison, WI, USA). A needle electrode inserted into the erector spinae muscle served as the reference electrode. SCEP findings of a decrease in amplitude of the negative peak of more than 50% or its disappearance, or monophasic positive potentials, were regarded as signifying electrophysiological abnormalities of the spinal segment.
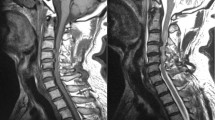
Spinal cord compression parameters on MRI
All patients underwent preoperative MRI examination (1.5-T Signa; General Electric Systems, Milwaukee, WI, USA) using a circular surface coil. T1- and T2-weighted axial and sagittal images were obtained with a spin-echo sequence. T1-weighted axial and sagittal images were obtained with a repetition time of 400–500 ms, echo time of 13–20 ms, four excitations and 5-mm slice thickness. T2-weighted axial and sagittal images were obtained with a repetition time of 3000–4000 ms, echo time of 120–130 ms, four excitations and 5-mm slice thickness.
As defined visually in Figure 2, several spinal cord compression parameters were measured on MRI at the lesion level, including the anteroposterior diameter, lateral diameter, area and flattening. Flattening was defined as the anteroposterior diameter divided by the lateral diameter.
C2/3 ratio
All spinal cord compression parameters were also measured at the C2/3 level (Figure 3). The C2/3 ratio was defined as the values of the parameters at the lesion level divided by their corresponding values at the C2/3 level. This ratio is based on the assumption that original spinal cord shapes may be significantly different across individuals; therefore, using C2/3 as a contrast may reduce individual differences. We considered that the influence of individual differences on original spinal cord shapes could be reduced by the C2/3 ratio.
Parameters of spinal cord compression using the C2/3 ratio. The ratio was defined as the value of parameters at the lesional level divided by the value at the C2/3 level. This ratio is designed to reduce interindividual differences in the natural spinal cord shape. a1: C2/3 level of case 1; b1: lesional level of case 1. a2: C2/3 level of case 2; b2: lesional level of case 2. a3: C2/3 level of case 3; b3: lesional level of case 3.
Japanese orthopaedic association scores
Japanese Orthopaedic Association (JOA) scores were assessed preoperatively and postoperatively, and the recovery rate was calculated. We further analyzed the data for a possible correlation between JOA scores and CMCT as well.
Statistical analyses
Outcome measures included CMCT, spinal cord compression parameters (area, lateral diameter, anteroposterior diameter and flattening at both the lesion level and in the C2/3 ratio described above) and JOA scores.
All statistical analyses were performed with Easy R (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria). More precisely, EZR is a modified version of the R commander that was designed to add statistical functions frequently used in biostatistics.
Correlation analyses were performed using Pearson's correlation coefficient (r). Correlations were accepted as significant if P<0.01 and |r|>0.40.
Statement of ethics
We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during the course of this research. This study was approved by the local Institutional Review Board, and all patients provided informed consent prior to the initiation of the study.
Results
Correlation between upper-limb CMCT and spinal cord compression parameters
Significant correlations were observed between (Figures 4 and 5) upper-limb CMCT and anteroposterior diameter (r=−0.465, P<0.01) as well as flattening (r=−0.600, P<0.01). In contrast, no significant correlation was observed between upper-limb CMCT and lateral diameter (r=0.234, P=0.19) or area (r=−0.168, P=0.35).
Correlation between upper-limb CMCT (U-CMCT) and C2/3 ratio of spinal cord compression parameters. (a) Anteroposterior diameter (C2/3 ratio) and U-CMCT (r=−0.610, P<0.01); (b) lateral diameter (C2/3 ratio) and U-CMCT (r=0.316, P=0.07); (c) area (C2/3 ratio) and U-CMCT (r=−0.263, P=0.14); (d) flattening (C2/3 ratio) and U-CMCT (r=−0.686, P<0.01).
Similarly, significant correlations were observed between upper-limb CMCT and the C2/3 ratio of the anteroposterior diameter (r=−0.610, P=<0.01) as well as the C2/3 ratio of flattening (r=−0.686, P<0.01). In contrast, no significant correlation was observed between upper-limb CMCT and the C2/3 ratio of the lateral diameter (r=0.316, P=0.07) or area (r=−0.263, P=0.14).
Correlation between lower-limb CMCT and spinal cord compression parameters
Significant correlations were observed between lower-limb CMCT and anteroposterior diameter (r=−0.636, P<0.01; Figures 6 and 7) as well as flattening (r=−0.735, P<0.01). In contrast, no significant correlation was observed between lower-limb CMCT and lateral diameter (r=0.151, P=0.40) or area (r=−0.343, P=0.05).
Correlation between lower-limb CMCT (L-CMCT) and C2/3 ratio of spinal cord compression parameters. (a) Anteroposterior diameter (C2/3 ratio) and L-CMCT (r=−0.771, P<0.01); (b) lateral diameter (C2/3 ratio) and L-CMCT (r=0.381, P=0.03); (c) area (C2/3 ratio) and L-CMCT (r=−0.283, P=0.11); (d) flattening (C2/3 ratio) and L-CMCT (r=−0.855, P<0.01).
Similarly, significant correlations were observed between lower-limb CMCT and the C2/3 ratio of the anteroposterior diameter (r=−0.771, P<0.01) as well as the C2/3 ratio of flattening (r=−0.855, P<0.01). In contrast, no significant correlation was observed between lower-limb CMCT and the C2/3 ratio of the lateral diameter (r=0.381, P=0.03) or area (r=−0.283, P=0.11).
Correlation between JOA score and CMCT
Significant correlation was observed between (Figure 8) preoperative JOA score and upper-limb CMCT (r=−0.678, P<0.01) as well as lower-limb CMCT (r=−0.545, P<0.01). In contrast, no significant correlation was observed between recovery rate of the JOA score and upper-limb CMCT (r=–0.119, P=0.51) or lower-limb CMCT (r=−0.085, P=0.64).
Correlation between JOA score and upper (U-) and lower (L-) CMCT. (a) Preoperative JOA score and U-CMCT (r=−0.678, P<0.01); (b) preoperative JOA score and L-CMCT (r=−0.545, P<0.01); (c) recovery rate (RR) of the JOA score and U-CMCT (r=−0.119, P=0.51); (d) recovery rate (RR) of the JOA score and U-CMCT (r=–0.085, P=0.64).
Correlation between JOA score and spinal cord compression parameters
Significant correlation was observed between (Figure 9) preoperative JOA score and anteroposterior diameter (r=−0.523, P<0.01) as well as the C2/3 ratio of flattening (r=–0.540, P<0.01). In contrast, no significant correlation was observed between the recovery rate of the JOA score and anteroposterior diameter (r=–0.195, P=0.28) or the C2/3 ratio of flattening (r=–0.116, P=0.53).
Correlation between JOA score and spinal cord compression parameters. (a) Anteroposterior diameter and preoperative JOA score (r=−0.523, P<0.01); (b) flattening (C2/3 ratio) and preoperative JOA score (r=−0.540, P<0.01); (c) anteroposterior diameter and recovery rate (RR) of the JOA score (r=–0.195, P=0.28); (d) flattening (C2/3 ratio) and recovery rate (RR) of the JOA score (r=–0.116, P=0.53).
Discussion
This study revealed a strong correlation between CMCT and flattening of the spinal cord. Moreover, measurement of spinal cord compression may be useful for the evaluation of corticospinal function in patients with CSM. To our knowledge, this study is the first to demonstrate a relationship between CMCT and cervical spinal cord compression as measured by MRI in patients with CSM.
Since the introduction of transcranial magnetic stimulation of the human motor cortex, the use of MEPs in the evaluation of many types of myelopathy has been well described in the literature.4, 5, 8, 9 CMCT, which measures conduction time from the motor cortex to the anterior horn cell, reflects the integrity of corticospinal pathways.7 In particular, CMCT has been found to be more prolonged in patients with more severe cervical spinal cord compression as determined by MRI analysis.10 Delayed CMCT suggests that CMCT prolongation is primarily caused by corticospinal conduction block rather than conduction delay.11, 12, 13
Conventional diagnostic methods for myelopathy include neurologic examination as well as imaging modalities such as MRI and myelograms. However, conclusive diagnosis is sometimes difficult because of the presence of atypical symptoms. In addition, MR images demonstrate morphological abnormalities but not functional impairment of the spinal cord, and not all cord compression shown by MRI is associated with cord dysfunction.14 Transcranial magnetic stimulation may be a more effective diagnostic tool for myelopathy. MEP studies using transcranial magnetic stimulation provide a quantitative evaluation of myelopathy. Previous studies have indicated that MEPs may be a valuable tool in the assessment of the functional relevance of subclinical spondylotic cervical cord compression.15 Furthermore, this test method has advantages in the evaluation of patients for whom neurological detection is difficult, including patients who have developed severe joint deterioration due to rheumatoid arthritis, patients who have difficulty communicating and patients with peripheral nervous disorders. Moreover, MEP facilitates localization of the site responsible for the main functional change in patients with multilevel (for example, cervical and thoracic) compression of the spinal cord. Despite these advantages, there is very little collected information, especially that gathered with more modern techniques, on the relationship between CMCT and the degree of spinal cord compression in patients with myelopathy.
Given that clinical diagnosis relies on images, especially from MRI, there exists the possibility of overdiagnosis of cervical myelopathy when using current diagnostic tools. In contrast, functional evaluation of the spinal cord using CMCT provides a quantitative measure of corticospinal function. However, CMCT requires complicated measurements, which may not be practical in routine clinical practice. In the current study, a correlation between CMCT and spinal cord compression on MRI was observed. This finding suggests that in the absence of an electrophysiological evaluation of spinal cord function, such as by CMCT, physicians can assess spinal cord function via the degree of compression observed on MRI. Thus, significant compression observed on MRI in a patient with subclinical myelopathy symptoms may be a useful indicator for preventive surgery of the cervical spine. It is expected that severe compression observed on MRI would be associated with irreversible spinal cord involvement, even if only mild symptoms are present. Therefore, the findings in this study may lead to significant clinical benefit, which improves the quality of evaluation of patients with myelopathy symptoms.
It is thought that there are interindividual differences in spinal cord anatomy. We felt that the correlation between CMCT and spinal cord compression measured by the ratio of the spinal cord parameters at the lesion level with the C2/3 level would better account for these differences in most cases of CSM.
There are several limitations to the present study. The sample size was small, because only patients with typical single-level myelopathy were selected for the study. In addition, only patients undergoing posterior laminoplasty were selected for the study. There is significant variability in myelopathy pathology across patients. Therefore, clinical results following decompression surgery may differ depending on the surgical procedure.
In conclusion, in this study, a strong correlation between CMCT and spinal cord compression was observed. Therefore, measurement of spinal cord compression may be useful for evaluation of corticospinal function in patients with CSM.
References
Kim HJ, Tetreault LA, Massicotte EM, Arnold PM, Skelly AC, Brodt ED et al. Differential diagnosis for cervical spondylotic myelopathy: literature review. Spine 2013; 38: S78–S88.
Tetreault LA, Dettori JR, Wilson JR, Singh A, Nouri A, Fehlings MG et al. Systematic review of magnetic resonance imaging characteristics that affect treatment decision making and predict clinical outcome in patients with cervical spondylotic myelopathy. Spine 2013; 38: S89–S110.
Jaskolski DJ, Jarratt JA, Jakubowski J . Clinical evaluation of magnetic stimulation in cervical spondylosis. Br J Neurosurg 1989; 3: 541–548.
Kameyama O, Shibano K, Kawakita H, Ogawa R . Transcranial magnetic stimulation of the motor cortex in cervical spondylosis and spinal canal stenosis. Spine 1995; 20: 1004–1010.
Takahashi J, Hirabayashi H, Hashidate H, Ogihara N, Yamazaki I, kmimura M et al. Assessment of cervical myelopathy using transcranial magnetic stimulation and prediction of prognosis after laminoplasty. Spine 2008; 33: E15–E20.
Lo YL, Chan LL, Lim W, Tan SB, Tan CT, Chen JL et al. Systematic correlation of transcranial magnetic stimulation and magnetic resonance imaging in cervical spondylotic myelopathy. Spine 2004; 29: 1137–1145.
Tavy DL, Wagner GL, Keunen RW, Wattendorff AR, Hekster RE, Franssen H . Transcranial magnetic stimulation in patients with cervical spondylotic myelopathy: clinical and radiological correlations. Muscle Nerve 1994; 17: 235–241.
Taniguchi S, Tani T, Ushida T, Yamamoto H . Motor evoked potentials elicited from erector spinae muscle in patients with thoracic myelopathy. Spinal Cord 2002; 40: 567–573.
Fujimoto Y, Oka S, Tanaka N, Nishikawa K, Kawagoe H, Baba I . Pathophysiology and treatment for cervical flexion myelopathy. Eur Spine J 2002; 11: 276–285.
Lo YL, Chan LL, Lim W, Tan SB, Tan CT, Chen JLT et al. Transcranial magnetic stimulation screening for cord compression in cervical spondylosis. J Neurol Sci 2006; 244: 17–21.
Kaneko K, Taguchi T, Morita H, Yonemura H, Fujimoto H, Kawai S . Mechanism of prolonged central motor conduction time in compressive cervical myelopathy. Clin Neurophysiol 2001; 112: 1035–1040.
Nakanishi K, Tanaka N, Kamei N, Hamasaki T, Nishida K, Touten Y et al. Significant correlation between corticospinal tract conduction block and prolongation of central motor conduction time in compressive cervical myelopathy. Clin Neurophysiol 2007; 256: 71–74.
Nakanishi K, Nobuhiro T, Hujiwara Y, Kamei N, Ochi M . Corticospinal tract conduction block results in the prolongation of central motor conduction time in compressive cervical myelopathy. Clin Neurophysiol 2006; 117: 623–627.
Tanaka N, Fujimoto Y, Yasunaga U, Ochi M . Functionl diagnosis using multimodal spinal cord evoked potentials in cervical myelopathy. J Orthop Sci 2005; 10: 3–7.
Bednarik J, Kadanka Z, Vohanka S, Novotny O, Surelova D, Filipovicova D et al. The value of somatosensory and motor evoked potentials in pre-clinical spondylotic cervical cord compression. Eur Spine J 1998; 7: 493–500.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Rikita, T., Tanaka, N., Nakanishi, K. et al. The relationship between central motor conduction time and spinal cord compression in patients with cervical spondylotic myelopathy. Spinal Cord 55, 419–426 (2017). https://doi.org/10.1038/sc.2016.130
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sc.2016.130
This article is cited by
-
Resting-state functional magnetic resonance imaging indices are related to electrophysiological dysfunction in degenerative cervical myelopathy
Scientific Reports (2024)
-
Multidimensional assessment of cervical spondylotic myelopathy patients. Usefulness of a comprehensive score system
Neurological Sciences (2021)