Abstract
Study design:
Here we describe a patient who developed myelopathy due to gouty tophi of the ligamentum flavum in the thoracic spine. We also review similar cases previously reported in the literature.
Objective:
Our aim was to present a case of myelopathy due to thoracic spinal gouty tophus.
Methods:
We report the case of a 56-year-old male with history of peripheral gout and renal insufficiency. The patient complained of back pain and paraparesis of the left lower limb. Multiple tophi were noted over several interphalangeal and metatarsophalangeal joints. Neurological examination showed decreased left lower limb strength and a positive Babinski sign. Magnetic resonance imaging of the thoracic spine revealed hypertrophy of the ligamentum flavum at the level of T3/T4, T5/T6, T9/T10, T10/T11 and T11/T12.
Results:
A thoracic laminectomy at T1-T5 was performed. Chalky white granular material was found in the ligamentum flavum during surgery. Histological analysis of the specimen demonstrated a gouty tophus. The patient’s back pain and paraparesis of the lower left limb improved.
Conclusion:
The clinician should include spinal gout in the differential diagnosis when dealing with patients with gout and axial pain with or without neurologic deficits. If this diagnosis is seriously entertained, then a CT scan or magnetic resonance imaging as well as tissue biopsy may be needed to establish the diagnosis.
Similar content being viewed by others
Background
Gout is monosodium urate crystal-induced inflammatory arthritis associated with hyperuricemia.1 The incidence of gout is estimated to be 0.2–0.4% worldwide, with an annual incidence of 0.01–0.015%.2 Gout is more common in men with a male-to-female ratio of 4:1 below the age of 65 years and 3:1 above the age of 65 years.3 Predisposing factors for an acute attack include trauma, surgery, increasing alcohol intake, high levels of intake of meat and fish and medications including diuretics and cyclosporine. Tophaceous gout is characterized by precipitation of urate crystals in the joints and periarticular tissues, and deposits commonly are found in the metatarsophalangeal joints, ankles, knees, wrists, fingers and shoulders. Gouty arthritis of the axial joints, particularly of the spine, is very rare.
Gout could involve all the segments of the spine. King et al. reviewed the records of reported cases of axial gouty tophi and ~44% of the axial gout patients had involvement of the lumbar vertebrae, 39% the cervical vertebrae and 17% the thoracic vertebrae.4 Tophaceous gout could impact anatomic components of the spine, such as facet joint,5 vertebral bodies,6 pedicle,7 lamina8 and ligamentum flavum.9 Patients present with features of spinal stenosis, lumbar radiculopathy, spondylolisthesis, cauda equine syndrome or spinal infection.
We present a case of a newly diagnosed patient with thoracic spine tophaceous gout involving the ligamentum flavum.
Case presentation
A 54-year-old Chinese male with a 12-year history of gout and hyperuricemia reported a 3-day history of lower limb edema and elevated serum creatinine. He suffered episodic gouty attacks, despite intermittently being treated with nonsteroidal anti-inflammatory drugs. Tophaceous deposits were present in the hands and toes for at least 5 years. Furthermore, the patient reported high alcohol intake spanning 20 years. He denied any history of trauma or spinal injuries. After he had been admitted to inpatient ward, he was found to have tenderness in the left anterior leg with anesthesia. Five days later, he complained of progressive back pain radiating to his anterior chest. The level of skin anesthesia increased from the lower extremities to his chest. Several days later, the paraparesis progressed to difficulty walking.
On physical examination the patient was found to have a fever of 38.8 °C. He had significant tenderness in his back and obvious difficulty with ambulation secondary to pain. Multiple tophi were also noted over several interphalangeal joints and metatarsophalangeal joints. Neurological examination showed that his lower limb strength had decreased (Grade 4/5) on the left and he had left ankle clonus. The lower abdominal and cremasteric reflexes were normal. The left lower limb also showed exaggerated reflexes as well as positive Babinski and Rossolimo’ sign. Reflexes were intact and within normal range on the straight-leg raise test. Laboratory values at the time of admission are depicted in Table 1.
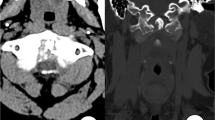
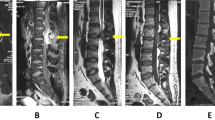
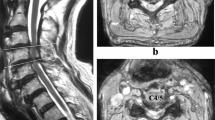
Computed tomography of the thoracic spine showed spinal stenosis at the T3/T4, T9-T12 levels. He also underwent magnetic resonance imaging, which showed hypertrophic ligamentum flavum at the level of T3/T4, T5/T6, T9/T10, T10/T11 and T11/T12 (Figures 1 and 2). The discs of T7/T8, L3/L4, and L5/S1 showed posterior bulge. Degenerative disc disease was found at the levels of T1/T2, T12/L1, L1/L2 and L5/S1.
Sagittal image of hypointensity magnetic resonance imaging shows hypertrophy ligamentum flavum. (a) T1-weighted image shows the lesion at the T3-T4 level. (b) T1-weighted image shows the lesions at the T9-T10, T10-T11 and T11-T12 levels. (c) T2-weighted image shows the lesion at the T3-T4 level. (d) T2-weighted image shows the lesions at the T9-T10, T10-T11 and T11-T12 levels.
A thoracic laminectomy was performed at T1-T5. During the operation, an abnormal mass with a white, chalky, cheese-like and granular appearance was observed. A culture of the chalky material revealed no bacterial growth. A histological examination of the material removed during the laminectomy showed amorphous eosinophilic material with thin needle-shaped crystals that were negatively birefringent on polarizing microscopy. Bacteriologic examinations were negative.
After his operation, his back pain and skin anesthesia of the lower extremities was markedly improved. He was prescribed allopurinol and transferred to a rehabilitation facility.
Discussion
The first radiologic and pathologic description of gouty involvement of the spine was published by Kersley et al. in 1950.10 However, the first case of thoracic gouty spine patient was not discussed until the report by Koskoff et al. in 1953.11 To the best of our knowledge, 21 thoracic spine cases have been reported. The reported cases of spinal gout involvement of thoracic vertebrae are listed in Table 2.7, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30 There was significant gender difference with a male-to-female ratio of 17:4 in the reported cases. Fourteen (66.7%) patients reported a history of gout symptom ranging from 2 to 35 years.11, 12, 13, 15, 16, 17, 18, 19, 20, 22, 23, 24, 25, 30 Peripheral tophi were found in ten (47.6%) patients.7, 11, 12, 13, 15, 16, 17, 18, 19, 25 Although tophi were reported in all thoracic regions, the most frequent involvement was seen in the thoracic region at T7-T10 (Figure 3). The most common location of gouty tophi involvement was extradural space.11, 13, 14, 15, 16, 18, 21, 22, 25, 26 Other locations of axial elements, such as facet joints,23, 29, 30 discs,12 vertebral bodies,7, 12, 17, 19, 20 pedicles7, 13, 27, 30 and costovertebral joint28 have been reported. However, the formation of gouty tophi within the thoracic spine involving the ligamentum flavum causing spinal cord compression, as occurs in our case, have only been reported previously by Wang et al.23 and Hus et al.24
The prevalence of spinal gout is unclear since most of the available information comes from anecdotal case-reports. Konatalapalli et al.31 reviewed 630 patients who were diagnosed with gouty arthritis, tophaceous gout or unspecified gout. Sixty-four patients had computed tomography images of cervical, thoracic or pelvic region. Spinal gout was identified in 9 of these 64 patients (14%). More recently, Konatalapalli et al accomplished a cross-sectional study regarding axial gout. Seventeen of the 48 subjects (35%) had computed tomography evidence of spinal gout and 7 (15%) had spinal tophi.32 On the basis of these observational studies, we speculated that the prevalence of axial gouty tophi was grossly underestimated.
Although the etiopathogenesis of the crystal accumulation in the axial skeleton is not completely known, it has been reported that factors such as degenerative disease of the spine, necrosis of the tissues or previous injuries can trigger the process.4, 33, 34 Meanwhile, some predisposing factors such as old age, low temperature, low serum pH level, renal insufficiency, diuretic and cyclospine A agent, IgA nephropathy and high alcohol intake are thought to promote tophi formation and development. The reason for the involvement of peripheral joints in gout is considered to be related to the decrease of the solubility of the crystals in the places with lower temperature and formation of tophi in avascular tissues.4, 35 In addition, lower blood pH causes a decrease in the binding plasma proteins and trauma causes an increase in the precipitation of urate crystals, both of which cause an increase in tophus formation.4, 35, 36 In our review, most of the patients with gouty tophi involved T7 through T10, which was consistent with the possibility that inflammation associated with motion-related damage may create an environment favorable for urate deposition. Renal dysfunction plays a significant role in raising the uric acid levels of the serum. Primary or secondary renal function promotes uric acid levels, causing tophi deposition in the spine and mild spinal stenosis with abnormal nerve compression. In turn, the elevated serum acid level further impairs renal function and contributes to the worsening of tophi deposition, which is supported by Chonchol et al.37 Our patient had a long history of hyperurecimia, with only intermittent pharmaceutical control. Subcutaneous deposition of gouty tophi in the right elbow area was also noted at this time. Thus, a relatively low environmental temperature and decreased renal urate clearance may be prerequisites for urate deposition.
Clinical manifestations of thoracic spinal gout range from back pain, unilateral or bilateral extremities paralysis, limbs weakness, sensory impaired to urinary retention. Neurologic symptoms were dependent on the level of the spine that was affected.
On MR, spinal tophi appear as homogeneous areas of intermediate-to-low signal intensity on T1-weighted images. On T2-weighted images, the signal intensity of the tophi varies from homogeneous hyperintensity to homogeneous hypointensity. This hyperintensity may result from a relative increase in the water content of the tophus and the relative homogeneity of local magnetic field within the tophi. In comparison, the T2-weighted hypointensity may be caused by immobile protons in the tophi. This appearance can be due to regions of calcifications, mature fibrous tissue, or hemosiderin deposition in the tophi. After gadolinium enhancement, the tophi show homogeneous or heterogeneous marginal enhancement. The enhancement of the tophi is thought to be the result of well-vascularized chronic, inflammatory fibrous tissue engendered by urate crystal deposition.4, 24
Gouty tophi are nodular, chalky white in the center, made of monosodium urate crystals, proteins, and mucopolysaccharides. Under microscopy, the urate depositions are found to be surrounded by multinucleated histiocytes, which are giant cells with foreign bodies associated with lymphoplasmocytic cells and fibroblasts. Moreover, monosodium urate crystals can be dissolved by formalin. This may be a reason why in our case there were no birefringent crystals under polarized light. It is important that the specimen should be properly fixed after biopsy or operation.
Surgical decompression such as laminectomy followed by optimization of pharmacological treatment can improve the patient’s clinical symptoms and provide a good prognosis. Modification of risk factors such as alcohol consumption, improvement in renal function, or alteration of the diuretic regimen may be beneficial and should be pursued whenever possible. Frequent follow-ups and imaging studies may permit early diagnosis and minimized complications of this disease.
Conclusion
In conclusion, although spinal gout maybe rare, it is important to be aware of this possibility. The clinician should include spinal gout as a differential diagnosis when dealing with patients with gout and axial pain with or without neurologic deficits. Even a short, uncontrolled period of time in the course of the disease could lead to devastating neurologic deficits necessitating emergent surgery for decompression. If this diagnosis is seriously entertained, then a computed tomography scan or magnetic resonance imaging as well as tissue biopsy may be needed to establish the diagnosis. If gout is suspected at the time of the biopsy, this needs to be communicated to the pathologist because monosodium urate crystals will dissolve during routine histologic processing.
References
Doherty M . New insights into the epidemiology of gout. Rheumatology (Oxford) 2009; 48: ii2–ii8.
Hall AP, Barry PE, Dawber TR, McNamara PM . Epidemiology of gout and hyperuricemia. A long-term population study. Am J Med 1967; 42: 27–37.
Wallace KL, Riedel AA, Joseph-Ridge N, Wortmann R . Increasing prevalence of gout and hyperuricemia over 10 years among older adults in a managed care population. J Rheumatol 2004; 31: 1582–1587.
King JC, Nicholas C . Gouty arthropathy of the lumbar spine: a case report and review of the literature. Spine (Phila Pa 1976) 1997; 22: 2309–2312.
Hasturk AE, Basmaci M, Canbay S, Vural C, Erten F . Spinal gout tophus: a very rare cause of radiculopathy. Eur Spine J 2012; 21: S400–S403.
Dharmadhikari R, Dildey P, Hide IG . A rare cause of spinal cord compression: imaging appearances of gout of the cervical spine. Skeletal Radiol 2006; 35: 942–945.
Chan AT, Leung JL, Sy AN, Wong WW, Lau KY, Ngai WT et al. Thoracic spinal gout mimicking metastasis. Hong Kong Med J 2009; 15: 143–145.
Cabot J, Mosel L, Kong A, Hayward M . Tophaceous gout in the cervical spine. Skeletal Radiol 2005; 34: 803–806.
Sanmillan Blasco JL, Vidal Sarro N, Marnov A, Acebes Martin JJ . Cervical cord compression due to intradiscal gouty tophus. Spine (Phila Pa 1976) 2012; 37: E1534–E1536.
Kersley GD, Mandel L, Jeffrey MR . Gout, an unusual case with softening and subluxation of the first cervical vertebra and splenomegaly: result of ACTH administration and eventual post-mortem findings. Ann Rheumat Dis 1950; 9: 282–303.
Koskoff YD, Morris LE, Lubic LG . Paraplegia as a complication of gout. JAMA 1953; 152: 37–38.
Levin MH, Linchtenstein L, Scott HW . Pathologic changes in gout: survey of eleven necropsied cases. Am J Pathol 1956; 32: 871–895.
Leaney BJ, Calvert JM . Tophaceous gout producing spinal cord compression. J Neurosurg 1983; 58: 580–582.
Downey PR, Brophy BP, Sage MR . Four unusual cases of spinal cord compression. Australas Radiol 1987; 31: 136–141.
Yasuhara K, Tomita Y, Takayama A, Fujikawa H, Otake Y, Takahashi K . Thoracic myelopathy due to compression by the epidural tophus: A case report. J Spinal Disord 1994; 7: 82–85.
Dhôte R, Roux FX, Bachmeyer C, Tudoret L, Daumas-Duport C, Christoforov B . Extradural spinal tophaceous gout: evolution with medical treatment. Clin Exp Rheumatol 1997; 15: 421–423.
Pfister AK, Schlarb CA, O'Neal JF . Vertebral erosion, paraplegia and spinal gout. AJR Am J Roentgenol 1998; 171: 1430–1431.
Bret P, Ricci AC, Saint-Pierre G, Mottolese C, Guyotat J . Thoracic spinal cord compression by a gouty tophus. Case report. Review of the literature. Neurochirurgie 1999; 45: 402–406.
Hausch R, Wilkerson M, Singh E, Reyes C, Harrington T . Tophaceous gout of the thoracic apine presenting as back pain and fever. J Clin Rheumatol 1999; 5: 335–341.
Kaye PV, Dreyer MD . spinal gout: an unusual clinical and cytological presentation. Cytopathology 1999; 10: 411–414.
Kao MC, Huang SC, Chiu CT, Yao YT . Thoracic cord compression due to gout: a case report and literature review. J Formos Med Assoc 2000; 99: 572–575.
St George E, Hillier CE, Hatfield R . Spinal cord compression: an unusual neurological complication of gout. Rheumatology (Oxford) 2001; 40: 711–712.
Wang LC, Hung YC, Lee EJ, Chen HH . Acute paraplegia in a patient with spinal tophi: a case report. J Formos Med Assoc 2001; 100: 205–208.
Hsu CY, Shih TT, Huang KM, Chen PQ, Sheu JJ, Li YW . Tophaceous gout of the spine: MR imaging features. Clin Radiol 2002; 57: 919–925.
Souza AW, Fontenele S, Carrete H Jr, Fernandes AR, Ferrari AJ . Involvement of the thoracic spine in tophaceous gout. A case report. Clin Exp Rheumatol 2002; 20: 228–230.
EI Sandid M, Ta H . Another presentation of gout. Ann Intern Med 2004; 140: W32.
Popovich T, Carpenter JS, Rai AT, Carson LV, Williams HJ, Marano GD . Spinal cord compression by tophaceous gout with fluorodeoxyglucose-positron-emission tomographic/MR fusion imaging. AJNR Am J Neuroradiol 2006; 27: 1201–1203.
Kwan BY, Osman S, Barra L . Spinal gout in a young patient with involvement of thoracic, lumbar and sacroiliac regions. Joint Bone Spine 2013; 80: 667–668.
Nasseri F, Myers A, Shah K, Moron FE . Severe back pain and lower extremities weakness in a young male. Br J Radiol 2013; 86: 20110685.
Yoon JW, Park KB, Park H, Kang DH, Lee CH, Hwang SH et al. Tophaceous gout of the spine causing neural compression. Korean J Spine 2013; 10: 185–188.
Konatalapalli RM, Demarco PJ, Jelinek JS, Murphey M, Gibson M, Jennings B et al. Gout in the axial skeleton. J Rheumatol 2009; 36: 609–613.
Konatalapalli RM, Lumezanu E, Jelinek JS, Murphey MD, Wang H, Weinstein A . Correlates of axial gout: a cross-sectional study. J Rheumatol 2012; 39: 1445–1449.
Beier CP, Hartmann A, Woertgen C, Brawanski A, Rothoerl RD . A large, erosive intraspinal and paravertebral gout tophus. Case report. J Neurosurg Spine 2005; 3: 485–487.
Draganescu M, Leventhal LJ . Spine gout: case report and review of the literature. J Clin Rheumatol 2004; 10: 74–79.
Mahmud T, Basu D, Dyson PH . Crystal arthropathy of the lumbar spine. J Bone Joint Surg Br 2005; 87: 513–517.
Suk KS, Kim KT, Lee SH, Park SW, Park YK . Tophaceous gout of the lumbar spine mimicking pyogenic discitis. Spine J 2007; 7: 94–99.
See LC, Kuo CF, Chuang FH, Shen YM, Ko YS, Chen YM et al. Hyperuricemia and metabolic syndrome: associations with chronic kidney disease. Clin Rheumatol 2011; 30: 323–330.
Acknowledgements
We thank Dr Fangtan Li for providing magnetic resonance imaging figures.
Author Contributions
Z-F Zheng and Y Xing are responsible for patient clinical diagnosis and therapy. They also collected patient follow-up data and drafted the manuscript. H-L Shi collected the radiological data and provided interpretation. D Li and J-Y Jia reviewed the literature and summarized the data. S Lin prepared the final version of the manuscript. All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Zheng, ZF., Shi, HL., Xing, Y. et al. Thoracic cord compression due to ligamentum flavum gouty tophus: a case report and literature review. Spinal Cord 53, 881–886 (2015). https://doi.org/10.1038/sc.2015.93
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sc.2015.93
This article is cited by
-
Clinical observation of posterior decompression, fusion and fixation in the treatment of spinal gout: a case series
Journal of Orthopaedic Surgery and Research (2023)
-
Tophaceous gout of the atlantoaxial joint: a case report
Journal of Medical Case Reports (2021)
-
Spinal disorders mimicking infection
Insights into Imaging (2021)
-
Gout in the Spine: Imaging, Diagnosis, and Outcomes
Current Rheumatology Reports (2015)