Abstract
Study design:
Cross-sectional analyses.
Objective:
To determine whether cervical spinal cord lesions predict the presence of thoracic cord lesions in multiple sclerosis (MS) patients.
Setting:
Single MS Clinic, AZ, USA.
Methods:
All MS patients, with MRI studies of the brain, cervical and thoracic spine obtained during a single scanning session, were acquired during a 1-year period. Clinical, demographic and imaging covariates were used in a multivariate regression model to refine predictors of thoracic cord involvement.
Results:
A total of 687 patients were evaluated, and patients were excluded because of a diagnosis of other neurological disorders, not meeting the 2010 McDonald criteria for MS (n=222) or incomplete neuraxis imaging (n=339). The study cohort comprised 126 patients. There was an increase in the odds ratio (OR) of thoracic spine involvement when any cervical spine lesion was present (OR=6.08, 95% confidence interval (2.21–16.68), P<0.001). The multivariate logistic regression model demonstrated a substantial and significant increase in the odds of thoracic spine involvement when more than two cervical spine lesions were present, two lesions (OR 4.44, (0.91–21.60), P=0.06), three lesions (OR 19.76, (3.51–111.17), P=0.001), four or more lesions (OR 20.49, (1.97–213.23), P=0.012) and diffuse lesions (OR 71.94, (5.28–979.88), P=0.001), when adjusting for significant covariates including clinical symptoms, brain lesions, disease duration and treatment exposure.
Conclusions:
Thoracic spinal cord lesions appear to be predicated on the degree of cervical spine involvement in patients with MS, a risk that appears to be independent of brain findings or clinical features.
Similar content being viewed by others
Introduction
Multiple sclerosis (MS) is a heterogeneous autoimmune disorder affecting myelin within the brain and spinal cord. Involvement of the spinal cord parenchyma is correlated with disability outcomes in MS,1, 2, 3 as well as higher conversion rates to clinically definite MS and risk for a first clinical event related to central nervous system demyelination in clinically and radiologically isolated syndromes, respectively.4, 5, 6, 7
To improve visualization of the spinal cord, cervical and thoracic segments are acquired separately. Unfortunately, previous scientific efforts either focused on the cervical spine exclusively or lacked differentiation between clinical correlations of the cervical versus thoracic spine.8, 9 Although the cervical spine is more frequently involved,10, 11, 12 approximately half of the lesions would be missed with the exclusion of thoracic spinal cord data.13 Contributing to the limited amount of data are patient costs, increased time within the magnetic resonance imaging (MRI) scanner and the lack of payer coverage in the absence of clinical symptoms that localize to that region.
The principle aim of this investigation was to determine whether the presence of cervical spine lesions predicted thoracic spinal cord involvement on the basis of the tendency of lesions to co-localize.14 An understanding of predictors of thoracic spinal cord involvement in MS and the association between cervical and thoracic lesions may allow for further insights into disease mechanisms, guide utilization of thoracic spinal imaging and influence both clinical surveillance and therapeutic recommendations by health-care providers.
Materials and Methods
Selection of participants
All patients who were seen in a single MS clinic during the 2012 calendar year with MRI studies of the brain, cervical and thoracic spine performed were consecutively included in the study. Scans were acquired at varying MRI facilities; however, the acquisition of the brain, cervical and thoracic spinal cord during the same scanning session was required. Clinical and radiological data were comprehensively reviewed to ensure that patients fulfilled the 2010 McDonald criteria for MS. Included were patients with relapsing remitting, secondary progressive, primary progressive or progressive relapsing who were classified by the treating MS specialist. Patients were excluded if they did not meet the 2010 McDonald criteria for MS, carried a diagnosis of other neuroinflammatory disease including neuromyelitis optica or lacked complete MRI study sets acquired consecutively during a single imaging session.
Standard protocol approvals, registrations and patient consents
The research protocol was approved by the Institutional Review Board.
Structural neuroimaging
All patients included had MRI studies of the brain and spinal cord at magnetic field strengths of 1.5 T or 3.0 T at multiple facilities, yielding images from nonuniform protocols. All examinations included T1- and T2-weighted spin-echo sequences in multiple planes of view (axial, coronal and sagittal) with and without gadolinium. All brain images included a fluid attenuation inversion recovery (FLAIR) sequence, and all spinal cord studies contained short tau inversion recovery (STIR) sequences.
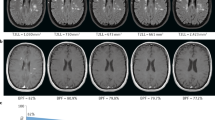
MRI characteristics were classified by two MS specialists (SLD, DTO), blinded to clinical characteristics. From the T2-weighted images the following items were scored for the brain––the number of brain lesions, the presence of gadolinium enhancement and the location of lesions (periventricular, infratentorial, juxtacortical and optic nerve)––and for the spine––the presence of cervical or thoracic spinal cord lesions typical for MS, spine lesion enhancement, the lesion level and lesion location. For the lesion number, diffuse was defined as large confluent signal changes in brain or coalescing appearance of multifocal lesions in the spinal cord on sagittal view. Longitudinally extensive lesions (greater than three spinal segments) were not considered diffuse. Spinal cord lesion locations were defined by the following: anterior (ventral 2/3 of spinal cord), posterior (posterior 1/3 of spinal cord), lateral (lateral 1/3 of cord, right or left), central (surrounding central canal with normal periphery) or patchy (admixed, well-defined, extensive and ‘dirty’ T2-hyperintensities) (Figure 1).
Statistical analysis
Median with interquartile ranges (IQRs) (25–75th percentiles) were used to summarize the demographic, clinical and radiological data.
Disease duration at the time of MRI acquisition was based on diagnosis date and date of initial symptom onset. Symptoms experienced either before or at the time of MRI acquisition were clinically localized on the basis of the following definitions: unilateral (involving one side of the body such as monoplegia or hemiplegia), bilateral (both sides involved such as paraplegia), optic neuritis (vision loss with or without pain lasting >24 h and/or treatment with steroids by their treating physician), cerebellar (gait ataxia, dysmetria, clumsiness, central nystagmus without weakness or other explanation), brainstem (central cranial nerve findings, intranuclear ophthalmoplegia), spinal cord (paraplegia, sensory level, dissociated symptomatology and/or bladder/bowel difficulties), long tract motor (motor symptoms not explained by nerve root impingement or peripheral nerve distribution), long tract sensory (sensory changes not in nerve root or peripheral nerve distribution) or cognitive (acute worsening of cognition or executive functions concerning for relapse).
The performance of the presence of cervical spine lesions in predicting thoracic spinal cord lesions was determined with true positives (subjects with abnormal cervical spine and thoracic spine lesions), false positives (subjects with abnormal cervical spine lesions and normal thoracic spine), true negatives (subjects with normal cervical and thoracic spine) and false negatives (subjects with normal cervical spine and abnormal thoracic spine lesions) used to determine sensitivity, specificity, positive predictive value and negative predictive value.
A multivariate logical regression model was used to further refine predictive characteristics for the presence of thoracic spine involvement. Covariates considered were sex, race/ethnicity (defined and classified by participant), positive family history of MS, MS subtype, the presence of cervical spine lesions, the number of cervical spine lesions, the presence of cervical spine gadolinium enhancement, the number of brain lesions, the presence of brain gadolinium enhancement, location of brain lesions, clinical localization of symptoms, exposure to disease modifying therapies, exposure to glucocorticosteroids, age, disease duration based on diagnosis date and disease duration based on first symptom onset. Covariates were screened in a univariate model; those that appeared to be meaningful were included in the multivariate regression model, generating odds ratios (OR), 95% confidence intervals (CIs) and P-values. Sensitivity analyses were conducted to obtain a final multivariate regression model. A P-value of <0.05 was considered significant. Statistical analyses were performed using Stata/SE 12.0 (Stata Corporation, College Station, TX, USA).
Results
A total of 687 patients were consecutively evaluated over a 1-year period. From this group, 561 patients were excluded because of not meeting the 2010 McDonald criteria, carrying a diagnosis of other neuroinflammatory disease (n=222) or incomplete neuraxis imaging (n=332). The final study cohort comprised 126 patients with MRI scans performed in all three regions (that is, brain, cervical and thoracic) during a single scanning session. Our cohort primarily comprised women (74.6%), ages 14–71 years (median 39 years, IQR 32–49 years), who were white (61.9%) and with a relapsing remitting disease course (90.5%). A total of 35% of the patients underwent MRI scans of all three regions related to the assessment of baseline level of disease at the time of diagnosis. The most common clinical symptom experienced before or at the time of the first spinal MRI involved impaired sensation (77.0%) that was unilateral (71.4%). Only 28.5% of our cohort demonstrated symptoms referable to the spinal cord on the basis of our definitions. Table 1 summarizes the demographic data and clinical characteristics of the study cohort.
Imaging characteristics of the brain and spinal cord are presented in Table 2. The majority of patients with brain lesions that were typical for MS on the basis of morphological characteristics, size (exceeding 3 mm2) and location were found in the lowest ordinal category of 1–5 lesions (23.0%), compared with higher lesion categories of 6–10 lesions (16.7%) and 11–15 lesions (11.9%). Periventricular lesions were present in 124 (98.4%) patients. Juxtacortical lesions were observed in 99 (78.6%), and gadolinium enhancement was present in 39 (31.0%) patients. At least one or more lesions were present in 83.3% (105/126) of cervical spinal cord studies with a slightly lower observation rate within the thoracic region (68.3% (86/126)). The most frequently affected levels were C2, C3, C4 and C5 in the cervical spine, whereas thoracic spine segments were more evenly distributed (Figure 2). For the cervical cord, lesions were more frequently lateral (51.4%) and posterior (19.3%), whereas thoracic cord lesions were more frequently patchy (32.5%) or lateral (39.8%).
The diagnostic predictive value of cervical spine lesions on thoracic spine involvement was determined with a sensitivity of 91.7% (95% CI 83.95–96.66), specificity of 35.0% (20.63–51.68) and positive predictive value of 75.2%. There was a 6.08 (95% CI 2.21–16.68, P<0.001, χ2 test) increase in odds of thoracic spine involvement when a cervical spine lesion was present.
To further characterize predictors of thoracic spine involvement, a multivariate regression model was used to evaluate the relative influences of several important baseline covariates (that is, age, sex, disease duration, ethnicity, positive family history of MS, the number and location of brain lesions, the number of cervical spine lesions, clinical localization of symptoms and exposure to disease modifying therapies or glucocorticosteroids). Covariates that did not survive initial univariate testing were subsequently incorporated in the sensitivity analyses to verify the final regression model. There was no difference between the group of patients who underwent scanning as part of baseline assessment of disease activity compared with those who had scans performed at other times during their disease course. Results of the final multivariate regression model are presented in Table 3. A substantial and significant increase in the odds of thoracic spine involvement was observed when more than 2 cervical spine lesions were present: two lesions (OR 4.44, 95% CI 0.91–21.60, P=0.06), three lesions (OR 19.76, (3.51–111.17), P=0.001), four or more lesions (OR 20.49, (1.97–213.23), P=0.012) and diffuse involvement of the cervical spine (OR 71.94, (5.28–979.88), P=0.001). Enhancement within the cervical spinal cord (OR 0.13, (0.18–1.00), P=0.05), the presence of brainstem symptoms (OR 0.42, (0.15–1.68), P=0.262) and disease duration from first symptom onset (OR=2.36, (0.97–5.74), P=0.057) were not significantly associated with an increase in the odds of thoracic spine lesions.
Discussion
This study identified a significant relationship between the presence of cervical spine lesions in predicting thoracic spine involvement in MS. Our results suggest an increase in risk for the presence of thoracic spine lesions when one or more lesions are present within the cervical spinal cord. Importantly, this observation was found to be independent of the number of brain lesions, treatment exposure, clinical symptoms and disease duration from first symptom onset.
Despite shortcomings of T2 techniques in identifying spinal involvement, cervical spinal cord involvement has been demonstrated to be related to disability outcomes in MS,2, 15, 16 specifically driven by diffuse rather than focal involvement.17 Little is known about the impact of thoracic spinal cord involvement; however, one study showed modest correlations with thoracic spine T2 lesions and disability measures of EDSS and timed 25 foot walk in a small cohort of patients.18 In our cohort, diffuse lesions were the most frequent occurrence in the thoracic spine (19%, 24/126), and the greatest risk for thoracic spine involvement was associated with diffuse cervical spine lesions (OR 71.94, P=0.001). These findings highlight the importance of including thoracic spine imaging to MS studies. By characterizing diffuse thoracic spine involvement, future studies can aide in our understanding of disability in MS. In addition, it would be useful to exclude compressive processes of the thoracic region that may be contributing to disability, especially in cases of progressive MS.
Our study reiterates that relying on brain lesions and clinical symptoms may not be sufficient in determining thoracic spine involvement and is consistent with previous studies demonstrating the lack of correlating symptoms to spinal cord lesions.12 Many studies have supported that spinal cord lesions can be asymptomatic and there is value in adding cervical spine imaging.4, 19 The use of routine thoracic spine MR imaging varies widely in clinical practice and is not used as an imaging outcome in clinical trials. Traditionally, MRI studies of the spinal cord have been technically challenging given its small mobile structure, surrounding tissues and susceptibility to artifacts from the heart and great vessels. With technical advances, spinal cord imaging has improved significantly and has become essential in differentiating multiple sclerosis from other processes that present with similar brain MRI findings.1 Historically, thoracic spine involvement was thought to be infrequent compared with cervical spine involvement.9, 10, 11, 12 From an anatomical perspective, the cervical spine has a greater cross-sectional area and contains more myelin per spinal segment when compared with the thoracic spinal cord, and thus the implication is that cervical spine is more frequently involved. Limitations in imaging of this area with decreased spatial resolution, increased susceptibility to pulsation artifact and motion associated with thoracic imaging may be other potential contributors. As a result, and because of the high acquisition cost to patients and to third party payers, routine imaging of the thoracic spinal cord is not performed. Our study challenges this notion, as 68% of our cohort demonstrated thoracic spine involvement. In addition, the observation of the equal spatial distribution of lesions within the thoracic spinal cord suggests that imaging of the neuraxis may be of benefit in the management of patients with MS.
Our study demonstrates that there is an increased risk of thoracic spine involvement with an increasing number of cervical spinal cord lesions. Three or more cervical spinal cord lesions were associated with the presence of one or more thoracic spinal cord lesions. This biologically plausible observation suggests that lesions within the thoracic spinal cord may be forecasted on the basis of the degree of cervical spinal cord involvement, as disease signatures in the rostral cord would result in a higher theoretical risk for thoracic spinal cord segments that are more numerous. Given the lack of an observed independent nature of thoracic spinal cord lesion development, it is unlikely that immune mechanisms with a heightened propensity for the thoracic spinal cord parenchyma were responsible for our findings.
Limitations with this effort include the lack of systematically acquired data with uniform MRI protocols, the cross-sectional nature of the study design, the modest number of patients studied and susceptibility of the study cohort to ascertainment bias, as only patients with imaging studies of the brain, cervical and thoracic spinal cord obtained during a single imaging session were included. Lack of uniform MRI protocols reflects real world clinical practice, but all MRIs contained typical MS sequences. If there were a tendency for triplicate MRI studies to be performed only in those patients with worse disease, one would expect higher lesion loads within the brain and spinal cord regions. However, we did not see this in our cohort as diffuse disease was only observed in 12% of brain studies, 14% of cervical and in 19% of thoracic MRI studies. Other potential limitations include the existing technological limitations with spinal cord imaging and the potential of being unable to visualize lesions that are truly present.20
In vivo imaging of the central nervous system has been invaluable in the diagnosis and clinical surveillance of MS. The accurate assessment of lesions within the spinal cord at the time of diagnosis, during disease evolution and in response to treatment is important for patient counseling, assessing future disability, treatment recommendations and clinical management. Future efforts should be directed toward evaluating the utility of not only cervical imaging but also thoracic spinal cord imaging and the correlates to disability progression.
Data archiving
There were no data to deposit.
References
Bot JCJ, Barkhof F, Lycklama à Nijeholt G, van Schaardenburg D, Voskuyl AE, Ader HJ et al. Differentiation of multiple sclerosis from other inflammatory disorders and cerebrovascular disease: value of spinal MR imaging. Radiology 2002; 223: 46–56.
Nijeholt GJ, van Walderveen MA, Castelijns JA, van Waesberghe JH, Polman C, Scheltens P et al. Brain and spinal cord abnormalities in multiple sclerosis. Correlation between MRI parameters, clinical subtypes and symptoms. Brain J Neurol 1998; 121: 687–697.
Thorpe JW, Kidd D, Moseley IF, Thompson AJ, MacManus DG, Compston DA et al. Spinal MRI in patients with suspected multiple sclerosis and negative brain MRI. Brain 1996; 119: 709–714.
Okuda DT, Mowry EM, Cree BA, Crabtree EC, Goodin DS, Waubant E et al. Asymptomatic spinal cord lesions predict disease progression in radiologically isolated syndrome. Neurology 2011; 76: 686–692.
Okuda DT, Siva A, Kantarci O, Inglese M, Katz I, Tutuncu M et al. Radiologically isolated syndrome: 5-year risk for an initial clinical event. PloS One 2014; 9: e90509.
Patrucco L, Rojas JI, Cristiano E . Assessing the value of spinal cord lesions in predicting development of multiple sclerosis in patients with clinically isolated syndromes. J Neurol 2011; 259: 1317–1320.
Sombekke MH, Wattjes MP, Balk LJ, Nielsen JM, Vrenken H, Uitdehaag BM et al. Spinal cord lesions in patients with clinically isolated syndrome: a powerful tool in diagnosis and prognosis. Neurology 2013; 80: 69–75.
Bag AK, Patel BN, Osman S, Roberson GH . Clinico-radiologic profile of spinal cord multiple sclerosis in adults. Neuroradiol J 2011; 24: 511–518.
Tartaglino LM, Friedman DP, Flanders AE, Lublin FD, Knobler RL, Liem M . Multiple sclerosis in the spinal cord: MR appearance and correlation with clinical parameters. Radiology 1995; 195: 725–732.
Honig LS, Sheremata WA . Magnetic resonance imaging of spinal cord lesions in multiple sclerosis. J Neurol Neurosurg Psychiatry 1989; 52: 459–466.
Kidd D, Thorpe JW, Thompson AJ, Kendall BE, Moseley IF, MacManus DG et al. Spinal cord MRI using multi-array coils and fast spin echo. II. Findings in multiple sclerosis. Neurology 1993; 43: 2632–2637.
Wiebe S, Lee DH, Karlik SJ, Hopkins M, Vandervoort MK, Wong CJ et al. Serial cranial and spinal cord magnetic resonance imaging in multiple sclerosis. Ann Neurol 1992; 32: 643–650.
Bot JCJ, Barkhof F, Polman CH, Lycklama à Nijeholt GJ, de Groot V, Bergers E et al. Spinal cord abnormalities in recently diagnosed MS patients: Added value of spinal MRI examination. Neurology 2004; 62: 226–233.
Mowry EM, Deen S, Malikova I, Pelletier J, Bacchetti P, Waubant E . The onset location of multiple sclerosis predicts the location of subsequent relapses. J Neurol Neurosurg Psychiatry 2008; 80: 400–403.
Filippi M, Colombo B, Rovaris M, Pereira C, Martinelli V, Comi G . A longitudinal magnetic resonance imaging study of the cervical cord in multiple sclerosis. J. Neuroimaging 1997; 7: 78–80.
Oh J, Saidha S, Chen M, Smith SA, Prince J, Jones C et al. Spinal cord quantitative MRI discriminates between disability levels in multiple sclerosis. Neurology 2013; 80: 540–547.
Evangelou N . Pathological study of spinal cord atrophy in multiple sclerosis suggests limited role of local lesions. Brain 2004; 128: 29–34.
Stankiewicz JM, Neema M, Alsop DC, Healy BC, Arora A, Buckle GJ et al. Spinal cord lesions and clinical status in multiple sclerosis: A 1.5 T and 3 T MRI study. J Neurol Sci 2009; 279: 99–105.
O’Riordan JI, Losseff NA, Phatouros C, Thompson AJ, Moseley IF, MacManus DG et al. Asymptomatic spinal cord lesions in clinically isolated optic nerve, brain stem, and spinal cord syndromes suggestive of demyelination. J Neurol Neurosurg Psychiatry 1998; 64: 353–357.
Nair G, Absinta M, Reich DS . Optimized T1-MPRAGE Sequence for Better Visualization of Spinal Cord Multiple Sclerosis Lesions at 3T. Am J Neuroradiol 2013; 34: 2215–2222.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
LHH received personal compensation for consulting and speaking activities from Genzyme and TEVA Neuroscience. SLD received personal compensation for consulting, advisory board and speaking activities from Biogen IDEC, Genzyme, Novartis and TEVA Neuroscience. DTO received personal compensation for consulting, advisory board, and speaking activities from Acorda Therapeutics, Genzyme and TEVA Neuroscience. The remaining authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Hua, L., Donlon, S., Sobhanian, M. et al. Thoracic spinal cord lesions are influenced by the degree of cervical spine involvement in multiple sclerosis. Spinal Cord 53, 520–525 (2015). https://doi.org/10.1038/sc.2014.238
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sc.2014.238
This article is cited by
-
Brainstem lesions are associated with diffuse spinal cord involvement in early multiple sclerosis
BMC Neurology (2022)
-
Imaging in Pediatric Multiple Sclerosis
Clinical Neuroradiology (2021)
-
The Role of Advanced Magnetic Resonance Imaging Techniques in Multiple Sclerosis Clinical Trials
Neurotherapeutics (2017)