Abstract
Study design:
Systematic review.
Objective:
To determine the effectiveness of body weight-support treadmill training (BWSTT) for muscle atrophy management in people with spinal cord injury (SCI).
Setting:
Studies from multiple countries were included.
Methods:
The following databases were consulted from January to October 2013: PubMed, Institute for Scientific Information (ISI), Science Direct and Lilacs. The methodological quality of the articles included was classified according to Jovell and Navarro-Rubio.
Results:
A total of five studies were included. These studies reported a significant association between BWSTT and increased trophism of the lower limb muscles of humans with SCI, which was observed as an increase in the cross-sectional area. Moreover, improvements in the ability to generate peak torque, contract the knee extensors and ankle plantarflexors with reduction of body weight support were observed after BWSTT.
Conclusion:
The results were considered inconclusive because of the low methodological quality of the articles, which was because of the absence of sample homogeneity, thereby providing a low level of evidence for clinical practice.
Similar content being viewed by others
Introduction
Spinal cord injury (SCI) can be defined as trauma that occurs in any portion of the spinal cord and results in complete or incomplete impairment in motor, sensory and autonomic functions below the injury level.1 The traumatic SCI primarily affects the population of young men aged 15–34 and has enormous social repercussions.2, 3 Furthermore, in most cases, the neurological deficit contributes to drastic physical activity reduction in individuals with SCI. The inactivity can lead to substantial changes in the body fat content, including the accumulation of intramuscular fat,4 reduction of bone mineralization4, 5, 6 and muscle atrophy.7
The muscle atrophy caused by disuse8, 9 is directly associated with ‘morphofunctional’ changes in the musculoskeletal system, such as a decrease in the muscle cross-sectional area (CSA)8 and peak torque10 and low fatigue resistance from the reduction of the fiber type I;11 these changes are negatively associated with motor function in individuals with SCI. In addition, muscle atrophy can negatively affect the immune response and wound healing because the fragile muscle is less able to synthesize the proteins that are involved in the production of antibodies and enzymes.12 It is also associated with decreased bone mineralization density13, 14, 15, 16 and adverse metabolic frames, including glucose intolerance and insulin resistance,17, 18 which are commonly observed in patients with SCI.
To restore/maintain muscle trophism below the injury level after SCI, some therapeutic approaches have been described in the literature, including functional electric stimulation to activate the neuromuscular system, which is usually associated with resistance training with an overload applied during static actions (isometric contraction),19 cycling20, 21, 22, 23 and lifting ankle weight (isotonic contraction).24, 25, 26 Over the last few decades, activity-based therapy using body weight-support treadmill training (BWSTT) has shown beneficial effects in the motor function recovery of SCI patients and has been widely used.5, 6, 26, 27, 28, 29
BWSTT involves the practice of stepping on a motorized treadmill while the individual remains stabilized by a belt connected to a counterweight system that regulates the unloading percentage of body weight on the lower limbs during the stance phase of gait.30, 31 This repetitive locomotor training is associated with sensory stimuli, particularly those related to loading and stretching muscles, and contributes to the refinement of locomotor output after SCI.32, 33 Furthermore, BWSTT has shown beneficial effects on musculoskeletal system adaptation in rats with SCI.34, 35 Such changes have been positively correlated with locomotor activity improvement,35 particularly for activities related to muscle trophism.
However, it is not clear whether BWSTT used as a therapeutic strategy in SCI rehabilitation is effective for restoring and/or maintaining muscle trophism. Therefore, the purpose of this systematic review was to determine the effectiveness of BWSTT for muscle atrophy management in people with SCI.
Materials and methods
Strategies of search
To select the studies, the following databases were consulted from January to October 2013: PubMed, ISI (Institute for Scientific Information), Science direct and Lilacs, using the following terms: ‘spinal cord injury’ and ‘muscle atrophy’ or ‘muscle mass’ or ‘muscular atrophy’ and ‘locomotor training’ or ‘treadmill training’or ‘step training’ or ‘gait training’, which were always in combination with the following: spinal cord injury, locomotor training and muscle atrophy. In addition, updates in the database researches were performed on August 2014.
Three independent authors performed the search in the databases described above.
Study selection
Two independent authors performed the selection of the titles and abstracts of scientific articles in the literature to determine whether the inclusion and exclusion criteria were met. The selected studies were compared, and differences were resolved by discussion until the investigators reached consensus.
Eligible studies
The inclusion/exclusion eligibility criteria are presented in Table 1.
Methodological quality assessment
The initial exploration of the literature showed that randomized clinical trials were scarce. Therefore, we also included nonrandomized clinical trial and case studies. To obtain insight into the methodological quality of the included trials, the studies were classified according to Jovell and Navarro-Rubio36 by the type of study design (see Kloosterman et al.37).
We made sure that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed in all of the studies selected for this systematic review.
Results
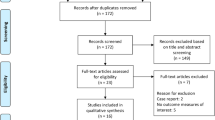
The initial search resulted in 420 titles. Four hundred and fifteen of these were excluded, leaving only five articles5, 6, 10, 27, 28 that fulfilled the selection criteria, which were therefore included in the present review (Figure 1).
An overview of the characteristics of the reviewed articles is presented in Table 2.
Methodological quality judgment
Three5, 10, 27 of the selected studies received a methodological score of IX, indicating a poor strength of evidence for a case report and case series. The remaining two studies by Giangregorio et al.6 and De Abreu et al.28 received scores of VI and III, respectively, indicating fair and good to fair levels of evidence according to Jovell and Navarro-Rubio.36, 37
Patients
We found one case report,27 two case series5, 10 and two nonrandomized clinical trials.6, 28 The samples ranged from 1 to 15 individuals with SCI and an average age of 33 years; most of the individuals were male. The neurologic level ranged from C3 to T12, and there was a predominance of cases of motor-incomplete tetraplegia. Only one case series10 and one nonrandomized clinical trial6 included subjects with paraplegia. Regarding the time of injury, only two studies5, 10 included individuals with acute SCI (injury time of less than a year). The remaining studies had samples of individuals with chronic SCI and a maximum injury time of 24 years.6
Characteristics of the intervention with BWSTT
As shown in Table 2, the period of intervention with BWSTT therapy ranged from 9 to 48 weeks, with a minimum session number of 45 and a maximum of 144, and a mode of 48 sessions was the predominant quantity number. The frequency of therapeutic approach ranged from two to five times a week, and there was a predominance of two sessions per week, lasting a minimum of 5 min (initial sessions) and a maximum of 30 min. In two studies,5, 28 the intervention period and the frequency of the therapeutic approach were exactly equal, at 24 weeks and twice a week, respectively.
In four of the five selected articles,5, 6, 10, 27 the BWS and treadmill speed were mentioned as parameters modulating the intensity of the training, meaning that, during the training period, the BWS decreased while the speed increased. Therefore, the initial BWS ranged from 30 to 60%, according to the severity of the injury; it was higher for individuals with complete SCI and lower at the end of the sessions, reaching a minimum support of 10% in the study by Jayaraman et al.10 It is important to clarify that the aim of the initial BWS was the proper positioning of the participant on the treadmill, prioritizing an upright trunk, knee in extension and heel strike with the ground during the stance phase of gait.
The treadmill speed, another parameter of training intensity, was initially established at an average of 0.3 m s−1. For two studies,5, 6 the reduction of the BWS resulted in increased speed; however in one article,6 the final speed was omitted. The average of the final evaluated speed was 0.6 m s−1.
All protocols of BWSTT included at least two therapists to assist with the positioning of the lower limbs and to facilitate trunk control in individuals with SCI during gait training. Only one study27 guided the participants to swing their arms and/or use parallel support for balance only. In addition, a single study employed overground training immediately following step training on the treadmill.10 Overground training incorporated the use of assistive devices; however, the participants were bearing full weight on their lower extremities.
Outcome measures
Muscle changes
Morphological changes related to muscle trophism. For a morphological analysis of muscle trophism, were measured the CSA of muscle fibers obtained after biopsy27 and of the great muscle groups5, 6, 10, 28 using imaging, such as computed tomography and magnetic resonance imaging.
In acute incomplete SCI, the CSA of the thigh muscles and calf increased in 12 and 14% of the participants, respectively, compared with baseline.5 This increase was more pronounced in the plantarflexors5, 10 after treadmill step training.
Furthermore, in chronic incomplete SCI, BWSTT was also effective for increasing the muscle trophism of the lower limbs (thigh and calf), and the values increased from 2.3 to 16.8%, compared with the reference group that did not receive locomotor training.6 The analysis of the muscle biopsy showed that atrophic fibers of the vastus lateralis experienced an overall increase of 27.1% in CSA, which was primarily because of hypertrophy of fiber types IIa and IIx. In a more detailed observation of each muscle fiber, BWSTT reduced the CSA of type I fibers and increased the CSA of type IIa and IIx fibers. However, training significantly increased the distribution of type I fibers with a reduction in the percentage of both type IIa and IIx fibers. The expansion of the type I fiber distribution promoted an increase in the total area occupied by type I fibers, which is characterized by the sum of each muscle fiber.27
Contrary to what has already been stated in this paper, De Abreu et al.28 reported that 6 months of BWSTT alone were not sufficient to cause any significant change in the CSA of the quadriceps muscle (41.81±8.45 cm2 before versus 41.75±7.87 cm2 after BWSTT). However, the authors noted that further studies with larger samples are needed to achieve more reliable conclusions. In their study, muscle trophism was only observed when BWSTT was combined with NMES, where the quadriceps CSA increased from 49.81±9.36 to 57.33±10.32 cm2.28
Changes in the peak isometric force, voluntary activation and contraction related to muscle function. Jayaramam et al.10 in addition to investigating the muscle morphological changes, examined the implication of BWSTT on muscle function. The findings of this study showed that all subjects demonstrated improvement in their ability to generate peak torque and contract the knee extensors and ankle plantarflexors after BWSTT, whereas in plantarflexors the torque production was more robust and accompanied by increases in the CSA of these muscles.10
Discussion
To the best of our knowledge, this is the first systematic review to examine the efficacy of BWSTT in morphological changes, particularly those related to muscle trophism in individuals with acute and chronic SCI. Overall, the evidence concerning the effects of BWSTT of increasing the muscle trophism in individuals with traumatic SCI was characterized as low quality, according to the scientific rigor in the design of selected studies: only one study28 received a ‘good’ score according to the classification scheme developed by Jovell and Navarro-Rubio.36 The failure of most studies is primarily associated with small sample sizes, absence of follow-up and problems related to internal validity (bias), such as a lack of study subjects who are blind to the intervention, researchers who are blind to the main outcomes of the intervention and randomization into intervention groups.
Although the extracted data suggested that BWSTT was able to provoke musculoskeletal system changes and improve the treadmill performance and walking ability of subjects with SCI in both the acute and chronic stages, these findings are not conclusive because of the low quality of the studies that were eligible for this review. In the muscular system, there were increases in the CSA of the thigh and calf muscles, particularly demonstrated by increasing the trophism of type IIa and IIx fibers and increasing the representation of type I fibers. Morphological changes were accompanied by improvement in muscle function, which was characterized by increases in the peak torque and voluntary muscle activation. In addition, there was improvement in treadmill performance, including increased gait speed, distance walking/session and reduced BWS levels provided during training; however, the improvement in the overground ambulatory capacity was limited to a few patients with incomplete SCI. For the skeletal system, BWSTT was inefficient because it did not prevent the reduction in bone mass in the chronic and acute stages of SCI.
The relative contributions of BWSTT in the musculoskeletal dysfunction of humans with SCI emerged from animal models that had a close relationship between treadmill step training and increased muscle trophism.34, 35, 38 BWSTT in rats with incomplete, acute SCI provoked significant increases in the CSA hind limb muscles immediately after the onset of training.35 However, Liu et al.38 showed that this training effectively recovers the soleus fiber size in the locomotor-trained group compared with the control group: reversion of muscle atrophy was observed following moderate contusion. In humans, the chronic state of SCI does not prevent the promotion of morphological changes in muscles with training, indicating that BWSTT can be a therapeutic tool for individuals who have a higher injury time.6, 10, 27, 28
However, the level of neurological involvement interfered with the ability of the muscle to undergo plastic adaptations related to trophism. De Abreu et al.28 observed that tetraplegic subjects with ASIA impairment scale (AIS) category A had an increase in the CSA of quadriceps of ~15% (49.81±9.36 to 57.33±10.32 cm2) only when BWSTT was associated with neuromuscular electrical stimulation (NMES). Dudley et al.19 also assessed the trophic effects of NMES in individuals with complete motor SCI and observed that 8 weeks of encouraging the isometric or dynamic contraction of the quadriceps muscle was sufficient to increase the average CSA. Although it is already known that NMES is capable of increasing the muscle trophism, we cannot affirm that BWSTT depends on NMES to provoke hypertrophy in the muscles of patients with motor complete SCI. This finding is reinforced by the study by Forrest et al.,39 who showed that BWSTT can modify the corporal composition, thereby increasing the lean mass in 4% in both legs, and that of Adams et al.,27 who reported an increase of 27.1% in the CSA of the vastus lateralis in individual tetraplegic subjects with AIS B.
After analyzing the results, it also became clear that the number of BWSTT sessions necessary to cause changes in the CSA of the lower limb muscles is directly proportional to the severity of neurological injuries (see Table 2). While patients with tetraplegia AIS category C required 144 sessions to increase the CSA of the quadriceps by 4.9% and plantarflexors by 8.2%,6 patients with tetraplegia AIS category D received only one-third of this number to increase these CSA values by 6.8% and 21.8%, respectively.10 This suggests that the degree of preservation of motor and sensory functions significantly contributes to plastic changes related to the muscle trophism induced by BWSTT.
The muscle growth provoked by BWSTT in atrophic muscle may be mediated by the expanded intramuscular protein content40 and is possibly because of the activation/proliferation of the satellite cells required to increase muscle trophism in response to functional overload.41 This functional progressive applied overload in both lower limbs during BWSTT is proportional to decreases in the BWS levels, as observed in three of the five studies included in this systematic review.5, 6, 10 Because they had a greater ability to support their own body weight at the end of their sessions, patients with tetraplegic AIS category D averaged 90%,10 followed by individuals with AIS C at 70%6 and those with AIS B at 58%.5
Increasing muscle trophism and muscle strength can improve a patient’s ability to support their weight and training time on the treadmill42 and they are necessary to restore the locomotor function of individuals with SCI.43 In this context, Wirtz et al.44 stated that individuals with tetraplegia need to reach a functional level of muscle contraction in their lower limbs to allow for locomotor function. Although it is not the main objective of this review, it is noteworthy to observe that none of the selected studies were able to establish a positive, significant correlation between the functional improvement of overground walking and muscle changes related to muscle trophism.
Conclusion
Although all of the studies selected for inclusion in this systematic review reported increased muscular trophism, measured by the CSA of the lower limb muscles of humans with SCI in acute and chronic stages after BWSTT, we suggest that these results are inconclusive. This is because of the lower methodological quality of the articles, which is associated with the absence of sample homogeneity, thereby providing a low level of evidence for clinical practice. Furthermore, additional studies, particularly randomized clinical trials, need to be performed to clarify the effectiveness of BWSTT in morphological changes related to muscle trophism in individuals with SCI.
Data archiving
There were no data to deposit.
References
Dumont RJ, Okonkwo DO, Verma RS, Hurlbert RJ, Boulos PT, Ellegala DB et al. Acute spinal cord injury, part I: pathophysiologic mechanisms. Clinl Neuropharmacol 2001; 24: 254–264.
Da Paz AC, Beraldo PS, Almeida MC, Neves EG, Alves CM, Khan P . Is body weight support treadmill training effevtive for increase the muscle trofism after traumatic spinal Cord injury? A systematic review. Prevalence in Brazilian hospitals. Paraplegia 1992; 30: 636–640.
Sekhon LHS, Fehlings MG . Epidemiology, demographics, and pathophysiology of acute spinal cord injury. Spine 2001; 26: S2–12.
Coupaud S, Jack LP, Hunt KJ, Allan DB . Muscle and bone adaptations afeter treadmill training in incomplete spinal cord injury: a case study using peripheral quantitative computed tomography. J Musculoskelet Neuronal Interact 2009; 9: 288–297.
Giangregorio L, Hicks A, Webber C, Phillips S, Craven B, Bugaresti J et al. Body weight supported treadmill training in acute spinal cord injury: impact on muscle and bone. Spinal Cord 2005; 43: 649–657.
Giangregorio LM, Webber CE, Phillips SM, Hicks AL, Craven BC, Bugaresti JM et al. Can body weight supported treadmill training increase bone mass and reverse muscle atrophy in individuals with chronic incomplete spinal cord injury? Appl Physiol Nutr Metab 2006; 31: 283–291.
Kocina P . Body composition of spinal cord injured adults. Sports Med 1997; 23: 48–60.
Shah PK, Stevens JE, Gregory CM, Pathare NC, Jayaraman A, Bickel SC et al. Lower-extremity muscle cross-sectional area after incomplete spinal cord injury. Arch Phys Med Rehabil 2006; 87: 772–778.
Ohnishi Y-I, Iwatsuki K, Shinzawa K, Nakai Y, Ishihara M, Yoshimine T . Disuse muscle atrophy exacerbates motor neuronal degeneration caudal to the site of spinal cord injury. Neuroreport 2012; 23: 157–161.
Jayaraman A, Shah P, Gregory C, Bowden M, Stevens J, Bishop M et al. Locomotor training and muscle function after incomplete spinal cord iniury: Case series. J Spinal Cord Med 2008; 31: 185–193.
Scelsi R . Skeletal muscle pathology after spinal cord injury: our 20 year experience and results on skeletal muscle changes in paraplegics. Basic Appl Myol 2001; 11: 75–85.
Forbes GB . Human Body Composition: Growth, Aging, Nutrition and Activity. Springer: New York, USA. 1987.
Terracciano C, Celi M, Lecce D, Baldi J, Rastelli E, Lena E et al. Differential features of muscle fiber atrophy in osteoporosis and osteoarthritis. Osteoporos Int 2013; 24: 1095–1100.
Griffiths HJ, Zimmerman RE . The use of photon densitometry to evaluate bone mineral in a group of patients with spinal cord injury. Paraplegia 1973; 10: 279–284.
Sabo D, Blaich S, Wenz W, Hohmann M, Loew M, Gerner HJ . Osteoporosis in patients with paralysis after spinal cord injury. A cross sectional study in 46 male patients with dual-energy X-ray absorptiometry. Arch Orthop Trauma Surg 2001; 121: 75–78.
Pluskiewicz W, Roberts D, Lee W, Cuneo RC, Wittmann J, Ward G et al. Quantitative ultrasound assessment of acute bone loss following spinal cord injury: a longitudinal pilot study-comment. Arch Orthop Trauma Surg 2003; 14: 785.
Bauman WA, Spungen AM . Disorders of carbohydrate and lipid metabolism in veterans with paraplegia or quadriplegia: a model of premature aging. Metabolism 1994; 43: 749–756.
Myllynen P, Koivisto VA, Nikkila EA . Glucose-intolerance and insulin resistance accompany immobilization. Acta Med Scand 1987; 222: 75–81.
Dudley GA, Castro MJ, Rogers S, Apple DF Jr . A simple means of ncreasing muscle size after spinal cord injury: a pilot study. Eur J Appl Physiol Occup Physiol 1999; 80: 394–396.
Mohr T, Andersen JL, Biering-Sorensen F, Galbo H, Bangsbo J, Wagner A et al. Long-term adaptation to electrically induced cycle training in severe spinal cord injured individuals. Spinal Cord 1997; 35: 1–16.
Crameri RM, Weston A, Climstein M, Davis GM, Sutton JR . Effects of electrical stimulation-induced leg training on skeletal muscle adaptability in spinal cord injury. Scand J Med Sci Sports 2002; 12: 316–322.
Baldi JC, Jackson RD, Moraille R, Mysiw WJ . Muscle atrophy is prevented in patients with acute spinal cord injury using functional electrical stimulation. Spinal Cord 1998; 36: 463–469.
Scremin AM, Kurta L, Gentili A, Wiseman B, Perell K, Kunkel C et al. Increasing muscle mass in spinal cord injured persons with a functional electrical stimulation exercise program. Arch Physl Med Rehabil 1999; 80: 1531–1536.
Rodgers MM, Glaser RM, Figoni SF, Hooker SP, Ezenwa BN, Collins SR et al. Musculoskeletal responses of spinal cord injured individuals to functional neuromuscular stimulation-induced knee extension exercise training. J Rehabil Res Dev 1991; 28: 19–26.
Mahoney ET, Bickel CS, Elder C, Black C, Slade JM, Apple D Jr et al. Changes in skeletal muscle size and glucose tolerance with electrically stimulated resistance training in subjects with chronic spinal cord injury. Arch Phys Med Rehabil 2005; 86: 1502–1504.
Gorgey AS, Shepherd C . Skeletal muscle hypertrophy and decreased intramuscular fat after unilateral resistance training in spinal cord injury: case report. J Spinal Cord Med 2010; 33: 90–95.
Adams MM, Ditor DS, Tarnopolsky MA, Phillips SM, McCartney N, Hicks AL . The effect of body weight-supported treadmill training on muscle morphology in an individual with chronic, motor-complete spinal cord injury: A case study. J Spinal Cord Med 2006; 29: 167–171.
de Abreu DC, Cliquet A Jr, Rondina JM, Cendes F . Electrical stimulation during gait promotes increase of muscle cross-sectional area in quadriplegics: a preliminary study. Clin Orthop Relat Res 2009; 467: 553–557.
Behmran AL, Harkema SJ . Locomotor training after human spinal cord injury: A series of case studies. Phys Ther 2000; 80: 688–700.
Field-Fote EC . Combined use of body weight support, functional electric stimulation, and treadmill training to improve walking ability in individuals with chronic incomplete spinal cord injury. Arch Phys Med Rehabil 2001; 82: 818–824.
Hornby TG, Zemon DH, Campbell D . Robotic-assisted, body-weight supported treadmill training in individuals following motor incomplete spinal cord injury. Phys Ther 2005; 85: 52–66.
Pearson KG . Common principles of motor control in vertebrates and invertebrates. Annu Rev Neurosci 1993; 16: 265–297.
Van de Crommert H, Mulder T, Duysens J . Neural control of locomotion: sensory control of the central pattern generator and its relation to treadmill training. Gait Posture 1998; 7: 251–263.
Ilha J, Cunha NB, Jaeger M, de Souza DF, do Nascimento PS, Marcuzzo S et al. Treadmill step training-induced adaptive muscular plasticity in a chronic paraplegia model. Neurosci Lett 2011; 492: 170–174.
Liu M, Bose P, Walter GA, Thompson FJ, Vandenborne K, Kern H et al. A longitudinal study of skeletal muscle following spinal cord injury and locomotor training. Spinal Cord 2008; 46: 488–493.
Jovell AJ, Navarro-Rubio MD . Evaluation of scientific evidence. Med Clin (Barc) 1995; 105: 4.
Kloosterman MGM, Snoeck GJ, Jannink MJA . Systematic review of the effects of exercise theraphy on the upper extremity of patientswith spinal-cord injury. Spinal Cord 2009; 47: 196–203.
Liu M, Stevens-Lapsley JE, Jayaraman A, Ye F, Conover C, Walter GA et al. Impact of treadmill locomotor training on skeletal muscle IGF1 and myogenic regulatory factors in spinal cord injured rats. Eur J Appl Physiol 2010; 109: 709–720.
Forrest G, Sisto S, Barbeau H, Kirshblum S, Wilen J, Bond Q et al. Neuromotor and musculoskeletal responses to locomotor training for an individual with chronic motor complete AIS-B spinal cord injury. J Spinal Cord Med 2008; 31: 509–521.
Hutchinson K, Gomez-Pinilla F, Crowe M, Ying Z, Basso D . Three exercise paradigms differentially improve sensory recovery after spinal cord contusion in rats. Brain 2004; 127: 1403–1414.
Rosenblatt JD, Yong D, Parry DJ . Satellite cell-activity is required for hypertrophy of overloaded adult-rat muscle. Muscle Nerve 1994; 17: 608–613.
Stewart BG, Tarnopolsky MA, Hicks AL, McCartney N, Mahoney DJ, Staron R et al. Treadmill training-induced adaptations in muscle phenotype in persons with incomplete spinal cord injury. Muscle Nerve 2004; 30: 61–68.
Hicks A, Ginis K . Treadmill training after spinal cord injury: It's not just about the walking. J Rehabil Res Dev 2008; 45: 241–248.
Wirtz M, van Hedel HJ, Rupp R, Curt A, Dietz V . Muscle force and gait performance: relationships after sinal cord injury. Arch Phys Med Rehabil 2006; 87: 1218–1222.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
do Espírito Santo, C., Swarowsky, A., Recchia, T. et al. Is body weight-support treadmill training effective in increasing muscle trophism after traumatic spinal cord injury? A systematic review. Spinal Cord 53, 176–181 (2015). https://doi.org/10.1038/sc.2014.198
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sc.2014.198
This article is cited by
-
Strategies used by providers to support individuals with spinal cord injury in weight management: a qualitative study of provider perspectives
Spinal Cord Series and Cases (2021)
-
Locomotor Treadmill Training Promotes Soleus Trophism by Mammalian Target of Rapamycin Pathway in Paraplegic Rats
Neurochemical Research (2018)