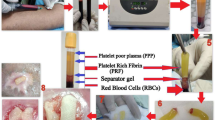
Abstract
Study design:
Prospective clinical case series.
Objectives:
The objective of this study is to evaluate the local application of platelet-rich plasma (PRP) in relation to pressure ulcers (PrUs) healing on one PrU (case) versus saline dressing on another PrU (control) in the same patient.
Setting:
Tertiary Level Care Centre, India.
Methods:
Twenty-five spinal cord injury patients with at least two PrUs were included. All 25 PrUs (case) were grade IV, and PrUs (control) were grade II (n=11), grade IV (n=10) and grade III in 4 patients. Evaluation of PrU healing was done by measuring wound surface area, Pressure Ulcer Scale for Healing (PUSH), biopsy and clinical examination.
Results:
Statistically significant decrease in mean PUSH scores of PrUs (case) (t=6.13, P<0.000) and PrUs (control) (t=3.98, P=0.000) was observed after 5 weeks. The wound surface area of PrU (case) decreased significantly (t=4.98, P=0.000); however, the decrease was not significant (t=0.095, P=0.924) in PrUs (control). Majority of histopathological pictures of PrUs (case) showed necrosis and suppuration (56%) at the time of enrollment and well-formed granulation tissue and epithelialization (60%) at the 5th week. Twenty-four (96%) PrUs (case) improved and only 1 deteriorated with PRP therapy, whereas in control group 17 (68%) PrUs improved, 7 (28%) deteriorated and 1 wound showed no change.
Conclusions:
Advanced wound therapy using local applications of PRP seems to be a promising alternative to standard saline dressings in PrU healing. With the advantages of simple preparation, biocompatible safety, low cost and significant clinical effectiveness, it may be beneficial to study the effects of PRP in large-scale trials to validate it as an ideal therapy for enhanced wound healing in PrUs.
Similar content being viewed by others
Introduction
As life expectancy is steadily improving through modern spinal unit care, the increased survival in spinal cord injury (SCI) patients is associated with secondary complications, which continue to pose management challenges and impair the quality of life of such patients.1, 2 Pressure ulcers (PrUs) are one of the major secondary complications of SCI and are a source of suffering for the patients and their caregivers.1, 2 These wounds are typically non-healing, resulting in a downward spiral of chronic inflammation, which can be a source of morbidity and even mortality in immobile populations.3
Wound healing is a complex and dynamic process. Soft tissue wound healing involves physiologic cascades in which cellular and hormonal factors have pivotal roles.4 Wounds on persons with SCI may be even more difficult to heal because of the physiological deficits that an SCI causes.5 When chronic wounds do not respond, a more aggressive, and sometimes more expensive, treatment is required to stimulate natural healing.6 Platelet-rich plasma (PRP) gel is considered to be an advanced wound therapy for chronic and acute wounds.7 A myriad of growth factors released by platelets regulate the orchestrated and complex events in normal wound healing.5 Promoting accelerated healing of PrUs would provide an improvement of patient’s quality of life and reduce the economic impact that chronic wounds have on the health care system.3
Since the 1980s, efforts to improve the clinical outcomes have explored the use of growth factor-based therapies.8 The clinical use of PRP has been reported for a wide variety of applications, most predominantly for the problematic wounds, maxillofacial applications and spine.9 Many studies in the literature provide support for clinical use of PRP in the treatment of acute and chronic wounds.10, 11, 12, 13 Authors have not come across any pre-clinical evidence of PRP in PrUs; however, recently few studies have shown usefulness of PRP in PrUs, as it leads to reactivation and accelerated healing in PrUs.3, 5, 10, 11, 14
The purpose of the present prospective study was to evaluate the application of PRP in relation to PrUs healing on the basis of clinical, wound size measurement and histopathological features in one PrU (case) versus saline dressing on the another PrU (control) in the same patient.
Materials and methods
Twenty-five patients with SCI with PrUs presenting to the author’s institute, a tertiary-level referral centre, between May 2009 and March 2012 were included in the present prospective study. All patients who met the following eligibility criteria were included in the present study: (1) occurrence of a traumatic event resulting in SCI with at least two PrUs; (2) PrUs that had not shown any progress (decrease in size, formation of granulation tissue and epithelization) after conventional treatments; (3) a minimum regular follow-up of 6 months; (4) signed informed consent; (5) age older than 18 years; and (6) injury below C4. Exclusion criteria were as follows: (1) single PrU; (2) associated malignant disorder; and (3) non-traumatic spinal cord lesion.
The patients were given detailed information about the purpose of study and written consent was obtained from all the participants. The complete history of patients was taken to rule out any other occult medical or neuropsychological problem, and the complete general physical and neurological examination was done. X-rays of the injury site as well as of PrUs sites were done. Routine hematological investigations (viz. hemoglobin, bleeding time, clotting time, blood urea, blood sugar, serum sodium ion, serum potassium ion, total serum protein and serum albumin) were done.
The eschar was adequately removed and PrUs were staged according to the European Pressure Ulcer Advisory Panel.15 The normal protocol for management of PrUs at our institute is as follows: the PrUs are debrided thoroughly to remove necrotic and infected tissue, and then are graded. Dressings are done daily with normal saline and repeat debridements are done if needed. Once wound is healthy, decision regarding skin grafting, flap surgery or spontaneous healing is taken depending on the need of the particular PrU.
PrUs over which PRP was applied was designated as PrUs (case) and PrUs over which saline dressing was done was designated as PrUs (control). As per study protocol, bigger among all the PrUs present on the patient was chosen as PrU (case) irrespective of the site. Patients were made mobile and were actively participating in routine recreational and rehabilitation activities depending on the SCI level and neurological status. General principles of care of SCI patients viz. support surface, posture and complete wound care were followed. Tables 1 and 2 show demographic, injury and PrU characteristics of the study population.
Methods of dressing
PrU dressing with PRP
PRP was prepared in the Department of Blood Transfusion using the standard preparation techniques on the day of application from the patient’s own blood in a sterile environment using a Cryfuge 6000i (Thermofisher Scientific, Schwerte, Germany). A total of 30 ml of blood was drawn from the patient’s antecubital vein. Blood was anticoagulated with citrate phosphate dextrose adenine with a ratio of 1:9 (citrate phosphate dextrose adenine:blood). After a 10-min centrifugation at 2000 r.p.m., the blood was layered in three basic components: red blood cells, platelets and platelet-poor plasma. The red blood cells layer was at lowest level because of different sediment coefficient. The platelet layer was in the middle and the platelet-poor plasma layer was at the top. Red cells layer was drawn from the tube. The remainder was agitated for several seconds and underwent a second centrifugation at 2000 r.p.m. for 10 min, the blood was then centrifuged into two layers, the supernatant was platelet-poor plasma, while the lower layer was concentrated platelets. About three quarters of the supernatant was discarded and the residual PRP (∼6 ml) was introduced into two 5-ml vacutainer tubes. Calcium chloride (10%) was taken in to a 2-ml syringe and injected into both vacutainer tubes in a ratio of 6:1 (PRP: 10% calcium chloride) to activate the PRP. Vacuutainer tubes were agitated for 5–10 s to initiate the gel formation. After cleaning the wound with normal saline and debridement (if needed), activated PRP was applied to the ulcer. When activated PRP was spread over the wound it transformed in to a gel. Non-absorbent Vaseline gauze was applied over the wound after application of PRP, and then dry cotton gauze and cotton pad was applied to absorb the any discharge from the wound. A transparent drape was used to cover the wound. Twice-weekly dressings were done for a minimum of 10 dressings. After this, wound was evaluated whether further dressing was required or not.
PrU dressing with saline
Other PrU was dressed daily with normal saline as a control.
Evaluation of the PrU was done as follows:
Wound-site measurement. The wound surface area was calculated by linear measurement of length and width with a measuring tape.
Pressure Ulcer Scale for Healing. Pressure Ulcer Scale for Healing (PUSH16; as described in Table 3).
Biopsy. A punch biopsy was taken from the margins of the wound initially, at the end of first, third and fifth week to monitor histopathological signs of healing in the entire grade III and IV PrUs.
Clinical examination. Wound was assessed in terms of formation of granulation tissue, moisture balance, infection and measurement. This assessment was used to document the wound as improved, unchanged or deteriorated.
Weekly evaluation of the wound healing was done clinically, by wound measurement and photographs till the end of 5th week of treatment. Later on, monthly follow-up was done for aminimum of 6 months.
Statistical analysis
The χ2-test and two-tailed paired t-test were used for statistical analysis. A P-value ⩽0.05 was considered statistically significant. Normalization of data was checked using Kolmogorov–Smirnov test. In case data were found to be non-normal, it was normalized using log transformation. In the statistical analysis, apart from pre- and post-measurement comparison in cases and controls separately, comparison between cases and controls was done applying mixed-model analysis of variance.
Results
Mean follow-up was 7.36±3.79 (range: 6–18.5) months. Mean total stay in hospital was 83.44±33.81 days (range: 45–212 days). Mean body mass index at enrollment was 20.97±2.96 and at final follow-up increased to 21.78±2.97. Figure 1 shows trend and average PUSH score of PrUs (case) and PrUs (control) during treatment and at final follow-up. The decrease in PUSH scoring was statistically significant in both PrUs (case) (paired t-test, t=6.13, P=0.000) and PrUs (control) (t=3.98, P=0.000) after 5 weeks of treatment (Figures 2 and 3). However, there was no significant difference in decrease in PUSH scores between the groups. A mixed between-within subjects analysis of variance was conducted to compare PUSH scores on the PrUs between cases and control across seven time periods (from enrollment to final follow-up). The main effect comparing the two treatment groups was not significant (P=0.099), suggesting no difference in PUSH scores between cases and control PrUs. There was a significant main effect for time (P<0.001) with both PrUs showing a significant decrease in PUSH scores across the seven time points. The interaction of treatment PrUs (cases and controls) by time was not significant (P=0.087), demonstrating that the decrease in PUSH scores from enrollment to end of study was similar for the PrUs (case) than it was for the PrUs (control) (Table 4).
Clinical and histopathology photographs of the PrU (case) during treatment with PRP. Final outcome is improvement in the ulcer. (a) Clinical photograph at the time of enrollment. European Pressure Ulcer Advisory Panel (EPUAP) Grade IV PrU, wound size 51 cm2 and PUSH score 14. (b) Histopathology slide at enrollment shows ulceration covered with necrotic slough and suppurative exudates (red arrows). Hematoxylin and eosin (H/E) staining, × 100. (c) Clinical photograph after first week of PRP application. Wound size 45 cm2 and PUSH score 13. (d) Histopathology slide after first week of PRP application shows surface is still infiltrated with inflammatory cell (black arrows). Early granulation tissue with capillary formation can be appreciated (red arrows). (H/E staining, × 100). (e) Clinical photograph after third week of PRP application. Wound size 37.5 cm2 and PUSH score 13. (f) Histopathology slide after third week of PRP application shows well-formed granulation tissue. The stroma shows neovascularization (black arrows), early fibrosis and collagenization (red arrows). (H/E staining, × 100). (g) Clinical photograph after fifth week of PRP application. Wound size 26 cm2 and PUSH score 11. (h) Histopathology slide after fifth week of PRP application shows regenerating epidermis (epithelization) at the edge (black arrows). Maturation of the granulation tissue is seen in the form of fibrosis and collagenization (red arrows). A full color version of this figure is available at the Spinal Cord journal online.
Clinical and histopathology photographs of the PrU (control) treated with daily saline dressing. Final outcome is deterioration in the ulcer. (a) Clinical photograph at the time of enrollment. European Pressure Ulcer Advisory Panel (EPUAP) Grade III PrU, wound size 12 cm2 and PUSH score 13. (b) Histopathology slide at enrollment shows sloughed out epidermis (ulceration) (black arrow) and foci of suppuration (red arrows). (Hematoxylin and eosin (H/E) staining, × 40). (c) Clinical photograph after first week. Wound size 12 cm2 and PUSH score 13. (d) Histopathology slide after first week shows granulation tissue is still not well formed and surface is super layered with necrotic suppurative slough (red arrows). (H/E staining, × 40). (e) Clinical photograph after third week. Wound size 25 cm2 and PUSH score 15. (f) Histopathology slide after third week shows capillary buds that have not still vascularized (red arrows). The loose stroma is infiltrated with inflammatory cells (H/E staining, × 100). (g) Clinical photograph after fifth week. Wound size 27.5 cm2 and PUSH score 16. (h) Histopathology slide after fifth week shows poorly developed granulation tissue with some myofibroblastic proliferation. A full color version of this figure is available at the Spinal Cord journal online.
Figure 4 shows average surface area of PrUs (case) and PrUs (control) during treatment and final follow-up. The decrease was statistically significant in PrUs (case) (t=4.98, P=0.000), while it was statistically insignificant (t=0.095, P=0.924) in PrUs (control). A mixed between-within subjects analysis of variance was conducted to compare wound surface area on the PrUs between cases and control across seven time periods (from enrollment to final follow-up). The main effect was significant (P=0.002), suggesting difference in wound surface area between cases and control PrUs. There was a significant main effect for time (P<0.001) with both PrUs showing a decrease in wound surface area across the seven time points. The interaction of treatment groups (cases and controls) by time was also significant (P<0.001), demonstrating that the decrease in wound surface area from enrollment to the end of study was greater for the PrUs (case) than it was for the PrUs (control) (Table 5).
Mean percentage of surface area healed of PrUs (case) was 57.94% and of PrUs (control) was 2.36% at final follow-up (Figure 5). Majority of histopathological pictures of PrU (case) showed necrosis and suppuration (56%) at the time of enrollment and well-formed granulation and epithelialization (60%) at the 5th week (Table 6 and Figures 2 and 3). Similarly, majority of histopathological pictures of PrU (control) showed necrosis and suppuration (78.5%) at enrollment and late granulation (38.48%) at the 5th week (Table 6). In the present study, 36% of the patients were having hemoglobin <10 g% with an average of 10.27±1.11 gm% at the time of enrollment (range: 8–13.5 g%). After treatment of PrUs with PRP, average hemoglobin (for all patients) rose by 0.804±1.15 g% (t=3.48, P=0.001). However, four patients showed decline in hemoglobin levels. Thirteen patients (52%) had low protein values at presentation and there was a rise in serum protein level with an average of 0.236±0.618 g% at final PRR therapy. Rise in serum protein level was statistically insignificant (t=1.90, P=0.068). As a result of PRP therapy, majority of PrUs (case) 24 (96%) improved and only one PrU deteriorated. In control group, 17 (68%) PrUs improved, 7 (28%) PrUs deteriorated and 1 wound showed no change.
Discussion
In the current scientific literature, numerous studies have evaluated PRP in wounds of various etiologies.3, 5, 10, 11, 12, 13, 17, 18, 19 Only very few studies have evaluated its role in PrUs in the SCI population.3, 5, 10, 11, 14 The purpose of the present prospective study was to evaluate the application of PRP in relation to PrUs healing on the basis of clinical, wound size measurement and histopathological features versus saline dressing on the other PrU in the same patient.
There was statistically significant decrease in mean PUSH scores of PrUs (case) (t=6.13, P<0.000) and PrUs (control) (t=3.98, P=0.000) after 5 weeks. The decrease in wound surface area of PrU (case) was statistically significant (t=4.98, P=0.000), whereas in PrUs (control) it was statistically insignificant (t=0.095, P=0.924). Mean percentage of surface area healed of PrUs (case) was 57.9% and in control group was 2.36% at final follow-up. All these changes were signs of progressive healing in PrUs. Mazzuco et al.12 showed 100% healing in nearly half the time with the use of autologous platelet gel in chronic wounds compared with control group of similar category. Anitua et al.10 showed that after 8 weeks of PRP therapy in chronic wounds, the mean percentage of surface area healed in PRP group was 72.94±22.25, whereas it was 21.48±33.5 in control group (P<0.05). Gurgen,13 after 4 weeks of PRP treatment in chronic wounds of various etiologies, reported complete wound healing in 1, decreased in size to an average of 55.2% of their original size in 12 and wound unchanged in 1 patient. Knox et al.17 showed reduction in wound dimension after 14 weeks of PRP treatment combined with a powdered skin substitute in a patient with chronic wounds. Frykberg et al.11 showed reduction in wound area (mean 39.5%, s.d.=41.2) after a mean of 2.8 weeks with 3.2 (s.d.=2.2) application of PRP in 51 chronic wounds of PrUs, venous and diabetic ulcers. A noticeable decrease in exudates amount from wounds was observed. Sell et al.3 showed complete healing of PrUs in 3 SCI patients after PRP therapy. Rappl5 showed average reduction of surface area of PrUs in 20 SCI patients was 53.81% after 3–4 weeks of PRP therapy.
Gardner et al.20 reported that total PUSH score was highly correlated with surface area measurements and this correlation increased over time as wound progressed toward closure. Hon et al.21 also observed a strong relationship (r=0.66) between total PUSH score and surface area. We did not observe such relationship in the present study. Reasons may be the difference in wound surface area measurement methods. Both Gardner et al.20 and Hon et al.21 measured wound surface area using acetate surface area tracings, whereas we measured linear dimensions (length–times–width). Acetate tracings of surface area are different than length–times–width determination of wound size used in PUSH tool, although they both measured wound size.
The histopathological results of the present study demonstrated that with PRP therapy PrUs can progress from non-healed to wound-healing stage. Various studies on humans in the literature had shown clinically granulation tissue formation after PRP therapy, but none had taken biopsies from the wounds.3, 5, 10, 12, 18, 22 Mazzuco et al.12 reported angiogenesis in chronic wounds with PRP therapy. Anitua et al.10 reported that platelet-derived growth factor in PRP had a role in stimulating fibroblasts proliferation and inducing myofibroblasts. Another growth factor—vascular endothelial growth factor—induced angiogenesis in chronic wounds. PRP promotes capillary growth and accelerates epithelialization in chronic wounds.22 Sell et al.3 also reported granulation tissue development, vascularization and epithelialization in three PrUs in three patients after PRP therapy. Other studies also reported granulation tissue formation after PRP therapy in PrUs, venous and diabetic ulcers.11, 13, 19 Pietramaggiori et al.23 in their study on homozygous genetically diabetic 8- to –12-week-old, Lep/r–db/db male mice (strain C57BL/KsJ-Leprdb) reported that treatment with freeze-dried (FD) platelet, FD and fresh-frozen, sonicated PRPs induced a significant 2.3-, 2.3- and 1.9-fold increase in granulation tissue area, respectively, compared with the non-treatment group (P<0.01). FD treatments resulted in significant (P<0.01) increases in mean vessel count per high-power field when compared with non-treatment. Platelet endothelial cell adhesion molecule-1 analysis showed a significant (P<0.01) fivefold increase in blood vessel density in both the fresh-frozen, sonicated and FD groups, and a sevenfold increase in the FD platelet group when compared with the non-treatment group. Proliferating cell nuclear antigen analysis showed that FD and FD platelet groups resulted in a significant (P<0.01) increase in cell proliferation compared with non-treatment group. Biopsy in the present study substantiated clinical observations of granulation tissue formation, neovascularization and maturation of granulation tissue histopathologically in human beings.
The present study corroborates the findings of the Rappl5 who reported that most healing occurred in first 3–4 weeks of treatment, and also of Scevola et al.14 who reported that in subjects with SCI and deep PrUs , there was a statistically significant increase in the onset of the granulation phase of wound healing, the healing process was triggered faster and there was more healing in the first 2 weeks of treatment with allogenic PRP gel compared with ‘current best-practice approach to chronic wound-dressing protocol’. It is generally accepted that platelet growth factors have a central role in the healing process and tissue formation.20 On the basis of the actions of the various platelet growth factors during the different stages in the wound-healing cascade, the use of autologous platelet leukocyte gel to stimulate wound repair is an interesting proposition. Platelet gels have the supreme advantage in that they synergistically induce various growth factors and promote mitogenesis of mesenchymal stem cells at the wound site.23, 24
In the present study, 96% of PrUs (case) improved with PRP therapy and 68% PrUs (control) treated with conventional therapy showed improvement. Various studies showing improvement in wound healing with PRP applications are shown in Table 7.
No major complication was seen after the treatment with PRP, except that four patients showed decline in hemoglobin levels. Thirty-six percent of the patients in this study had lower hemoglobin and 52% had low serum protein values at the time of enrollment. Similar to the present study, Rappl7 reported that subjects in his study had lower-than-normal levels for hemoglobin and hematocrit, yet healing progressed in all wound parameters. Frykberg et al.11 reported in their study that majority of patients with chronic wounds (PrUs, venous ulcers and diabetic foot ulcers) had low albumin, hematocrit and hemoglobin levels. At 5 weeks of PRP treatment in the present study, there was improvement in the mean hemoglobin level (statistically significant) and serum protein levels (statistically insignificant). In our previous study, we were able to demonstrate that healing in PrUs is associated with improvement in anemia and hypoproteinemia.25 Fuoco et al.26 also reported that both anemia and hypoproteinemia disappeared after PrU healing. Healing of PrUs prevents further loss of proteins through the wound.26 We are of the opinion that PRP application hastens the healing of PU and thus decreases the continuous discharge of proteins and leads to improvement in anemia and hypoproteinemia, although the overall improvement may be due to a summation effect of our comprehensive approach of dietary therapy, blood transfusion and PRP application.
There are some limitations inherent with this study. First, there is diversity in the sites of PrUs, and PrUs (case) and PrUs (control) are not of the same grade. Healing pattern and response to specific therapeutic approach may be varying, depending on the site and grade of the PrU. The control population comprised mainly of trochanteric sores, which are known to be refractory to treatment. We have avoided any selection bias by adhering to strict study protocol of selection of bigger PrU as case irrespective of site, and hence any confounding data. Another potential limitation is that we have not undertaken any analysis to validate that the composition of PRP did not differ from case to case. Further future research is needed to test the efficacy of PRP in PrUs healing, taking into consideration the methodological limitations of this study.
Conclusions
This is the first study in human beings that has demonstrated potential efficacy of PRP both clinically and histopathologically in enhancement of healing in chronic PrUs in SCI patients. Early granulation tissue formation and neovascularization between first and third week, and subsequent maturation of the granulation tissue and epithelization adds weight to the theory of various factors present in PRP causing neovascularization, cell proliferation and epithelialization. Advanced wound therapy using local applications of PRP seems to be a promising alternative to standard saline dressings in PrU healing. With the advantages of simple preparation, biocompatible safety, low cost and significant clinical effectiveness, it may be beneficial to study the effects of PRP in large-scale trials to validate it as an ideal therapy for enhanced wound healing in PrUs.
Data Archiving
There were no data to deposit.
References
Singh R, Dhankar SS, Rohilla R . Quality of life of people with spinal cord injury in northern India. Int J Rehabli Res 2008; 31: 247–251.
Drigotaite N, Krisciunas A . Complications after spinal cord injuries and their influence on the effectiveness of rehabilitation. Medicina (Kaunas) 2006; 42: 877–880.
Sell SA, Ericksen JJ, Reis TW, Droste LR, Bhuiyan MB, Gater DR . A case report on the use of sustained release platelet rich plasma for the treatment for chronic pressure ulcers. J Spinal Cord Med 2011; 34: 122–127.
Hunt TK . Basic principles of wound healing. J Trauma 1990; 30 (12 Suppl): S122–S128.
Rappl LM . Effect of platelet rich plasma gel in a physiologically relevant platelet concentration on wounds in persons with spinal cord injury. Int Wound J 2011; 8: 187–195.
Whitney J, Phillips L, Aslam R, Barbul A, Gottrup F, Gould L et al. Guidelines for the treatment of pressure ulcers. Wound Repair Regen 2006; 14: 663–679.
Reese RJ . Autologous platelet rich plasma (PRP): what do we know? Important concepts relevant to hair restoration surgery. Hair Transplant Forum Int 2010, 14–17.
Hunt T, Van FL . Enhancement of wound healing by growth factors. New Engl J Med 1989; 321: 11–12.
Eppley BL, Pietrzak WS, Blanton M . Platelet-rich plasma: a review of biology and applications in plastic surgery. Plast Reconstr Surg 2006; 118: 147e–159e.
Anitua E, Aguirre JJ, Algorta J, Ayerdi E, Cabezas AI, Orive G et al. Effectiveness of autologous preparation rich in growth factors for the treatment of chronic cutaneous ulcers. J Biomed Mater Res B Appl Biomater 2008; 84: 415–421.
Frykberg RG, Driver VR, Carman D, Lucero B, Borris-Hale C, Fylling CP et al. Chronic wounds treated with a physiologically relevant concentration of platelet-rich plasma gel: a prospective case series. Ostomy Wound Manage 2010; 56: 36–44.
Mazzucco L, Medici D, Serra M, Panizza R, Rivara G, Orecchia S et al. The use of autologous platelet gel to treat difficult- to-heal wounds: a pilot study. Transfusion 2004; 44: 1013–1018.
Gurgen M . Treatment of chronic wounds with autologous platelet-rich plasma. EWMA J 2008; 8: 5–11.
Scevola S, Nicoletti G, Brenta F, Isernia P, Maestri M, Faga A . Allogenic platelet gel in the treatment of pressure sores: a pilot study. Int Wound J 2010; 7: 184–190.
European Pressure Ulcer Advisory Panel. Pressure Ulcer Treatment Guidelines. EPUAPwww.EPUAP.org1999.
Pressure Ulcer Scale for Healing (PUSH) PUSH Tool Version 3.0:9/15/98 ©National Pressure Ulcer Advisory Panel http://www.npuap.org.
Knox RL, Hunt AR, Collins JC, DeSmet M, Barnes S . Platelet-rich plasma combined with skin substitute for chronic wound healing: a case report. J Extra Corpor Technol 2006; 38: 260–264.
Yuan T, Zhang CQ, Tang MJ, Guo SC, Zeng BF . Autologous platelet-rich plasma enhances healing of chronic wounds. Wounds 2009; 21: 280–285.
Crovetti G, Martinelli G, Issi M, Barone M, Guizzardi M, Campanati B et al. Platelet gel for healing cutaneous chronic wounds. Transfus Apher Sci 2004; 30: 145–151.
Gardner SE, Frantz RA, Bergquist S, Shin CD . A prospective study of the Pressure Ulcer Scale for Healing (PUSH). J Gerontol Med Sci 2005; 60: 93–97.
Hon J, Lagden K, McLaren A, O'Sullivan D, Orr L, Houghton PE et al. A prospective, multicenter study to validate use of the pressure ulcer scale for healing (PUSH) in patients withdiabetic, venous, and pressure ulcers. Ostomy Wound Manage 2010; 56: 26–36.
Werner S, Grose R . Regulation of wound healing by growth factors and cytokines. Physiol Rev 2003; 83: 835–870.
Pietramaggiori G, Kaipainen A, Czeczuga JM, Wagner CT, Orgill DP . Freeze-dried platelet-rich plasma shows beneficial healing properties in chronic wounds. Wound Repair Regen 2006; 14: 573–580.
Lynch SE, Nixon JC, Colvin RB, Antoniades HN . Role of platelet-derived growth factor in wound healing: synergistic effects with other growth factors. ProcNatl Acad Sci USA 1987; 84: 7696–7700.
Singh R, Singh R, Rohilla RK, Siwach R, Verma V, Kaur K . Surgery for pressure ulcers improves general health and quality of life in patients with spinal cord injury. J Spinal Cord Med 2010; 33: 396–400.
Fuoco U, Scivoletto G, Pace A, Vona VU, Castellano V . Anaemia and serum protein alteration in patients with pressure ulcers. Spinal Cord 1997; 35: 58 60.
Acknowledgements
We certify that all applicable institutional and governmental regulations concerning the ethical use of human in our country were followed during the course of this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Singh, R., Rohilla, R., Dhayal, R. et al. Role of local application of autologous platelet-rich plasma in the management of pressure ulcers in spinal cord injury patients. Spinal Cord 52, 809–816 (2014). https://doi.org/10.1038/sc.2014.144
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sc.2014.144
This article is cited by
-
“Platelet-Rich Fibrin Membrane-as a novel biomaterial for pressure injury healing in a person with spinal cord injury: A case report”
Spinal Cord Series and Cases (2022)