Abstract
Study design:
Prospective study.
Objectives:
In a prospective study, 45 consecutive cases of cervical spinal cord injury without radiographic evidence of trauma (SCIWORET) who were treated non-operatively were analyzed to correlate the magnetic resonance image findings with the initial neurological deficit and the extent of neurological recovery at 2 years.
Setting:
University tertiary-care teaching hospital in South India.
Methods:
The neurological status of patients who did not have any radiographic or computerized tomographic abnormality at the time of admission was assessed by ASIA Impairment Scale (AIS) modification of Frankel’s grading. The spinal cord abnormality seen in the magnetic resonance imaging was noted. The neurological status at the end of 2 years was recorded.
Results:
Twenty-seven of the 45 patients (60%) had cord oedema, 8 (17.77%) had cord contusion, 8 (17.77%) patients had a normal cord and 2 (4.44%) patients had cord swelling on the magnetic resonance image. Out of 27 patients who presented with cord oedema, 14 (31.11%) patients recovered from AIS D to AIS E and 6 (13.33%) patients did not recover and remained at AIS D. Seven (15.55%) patients who had a normal cord recovered completely to AIS E. Five (11.11%) patients who had contusion of the cord recovered up to AIS D.
Conclusion:
The initial neurological status correlates with magnetic resonance imaging findings. Subsequent neurological recovery is dependent on the type of cord damage and initial neurological status. The rate of recovery and the final motor outcome are inversely related to the length of cord involvement.
Similar content being viewed by others
Introduction
Spinal cord injury without radiological abnormality is characterized by acute traumatic myelopathy despite normal plain radiographs and normal computed tomographic studies.1 It is more common in children than in adults and represents a significant fraction of paediatric spinal cord injuries.1
SCIWORA can also occur in adults, but is considered to be less frequent than the syndrome of acute spinal cord injury without radiographic evidence of trauma (SCIWORET), which may be associated with cervical spondylosis, ossification of posterior longitudinal ligament, spinal canal stenosis, ankylosing spondylitis, disc herniation and other degenerative conditions.2, 3
The diagnosis can be difficult if the potential for its occurrence is not considered, as the injury is not identifiable in routine radiographs or computed tomographic scans. SCIWORET is most often caused by significant trauma and can result in serious and irreversible consequences for the patient. The most important challenge to the clinician is to recognize this syndrome as quickly as possible so that treatment can be instituted early. In the literature, there is no unified criterion to identify the patients with SCIWORET who are at risk of developing persistent neurological deficit and disabilities.
This study was undertaken to determine whether the initial neurological deficit and subsequent neurological recovery following SCIWORET of the cervical spine depend on the nature and extent of cord damage seen in magnetic resonance imaging (MRI) scan images.
Materials and methods
Three hundred and twenty-seven cases of cervical spinal injuries were treated at this centre between October 2005 and September 2009. Patients with (a) radiological evidence of trauma (fractures, dislocations or subluxations), (b) prevertebral soft tissue shadow of >5 mm thickness opposite C3 and >20 mm opposite C6, (c) presence of spinal cord injury at the thoracic or lumbar region and (d) pathological lesions in the spine were excluded from the study. Patients with degenerative changes were not excluded. Fifty-four cases had SCIWORET on plain radiographs or computed tomographic scans. All of them underwent MR imaging to look for neural and extraneural injury. Six patients with incomplete data and three patients who were lost to follow-up were excluded from the study. The remaining 45 patients formed the basis of this study.
The mean age at the time of injury was 40.6 years (range 16–70 years) and the male to female ratio was 8:1. The most common mechanism of injury was road traffic accident (46.7%), whereas 40% patients had a history of a fall. Eighteen patients presented within 6 h following the injury, 21 patients were admitted within 24 h, whereas the rest were seen between 24 and 48 h.
Neurological evaluation
After admission, neurological examination was carried out every 2 h for the next 24 h. The neurological deficit noted after the return of the bulbocavernous reflex was considered as the initial neurological status. The neurological examination at the time of presentation and subsequent follow-up was carried out by a single assessor (MB) who did not have access to the radiological findings. The neurological status was graded according to ASIA Impairment Scale (AIS) classification and AIS motor and sensory scores according to International Standards for the Neurologic and Functional Classification of Spinal Cord Injury patients.4 Incomplete spinal cord injuries were further categorized as central cord, Brown Sequard, anterior cord and posterior cord syndromes.
Radiological evaluation
Anteroposterior, lateral roentgenograms and computed tomography scans were performed in all cases to rule out any skeletal injuries. All patients who did not have any radiographic evidence of trauma (SCIWORET) underwent magnetic resonance imaging (0.5 T). A consultant radiologist who was blinded to clinical details reviewed all MRI data. The type of cord injury was categorized as described by Ramon et al.5 Mid-saggital MR images were analysed for spinal cord changes and were classified as normal cord, type I pattern (cord haemorrhage), type II pattern (cord oedema), type III pattern (contusion or mixed), compression pattern and transection pattern.5
The extent of cord damage and the extent of prevertebral hyperintensity (PVH)3 was measured as the number of segments involved with respect to the cervical vertebrae.6 Each vertebral body was divided into two parts: the upper half or segment 1 and the lower half or segment 2. The intervertebral disc below each vertebral body was defined as segment 3. An anatomic location was expressed as the portion of the closest vertebral body that intersected a horizontal line drawn through the lesion. For example, a lesion located at the lower half of the fifth cervical body was expressed as C5.3. Each segment location from C2 to T1 was rank ordered, which yielded 22 possible locations.6
Treatment
All the patients were treated non-operatively by halter traction with minimal weight for 3 weeks. Intravenous methyl prednisolone was given for 48 h. They were then immobilized in a polyethylene-moulded extended cervical collar and were taught muscle strengthening and joint mobilization exercises.
Follow-up
The neurological status was reassessed at regular intervals of 3 months. Cervical spine stress views were taken at 3 months follow-up to rule out any cervical spine instability; none of these patients had cervical spine instability. At the end of first 3 months, the patients were ambulated depending on the neurological status. Neurological recovery was recorded on the basis of the neurological level, motor and sensory scores and AIS grade at 3 months, 1 year and 2 years. The recovery rates of the motor and sensory ratios were calculated to compare the degree of improvement based on the method described by Lucas and Ducker:7

Statistical analysis
Statistical analysis was performed using SPSS Version 17.0 for Windows. The difference in the median recovery rate and final motor score was compared between patients with normal and abnormal spinal cords using the Mann–Whitney U-test. Pearson’s correlation coefficient was computed to test the relationship between the length of cord changes, extent of PVH, recovery rate and final motor score.
Results
Neurological status
The neurological status of the patients at admission and follow-up is depicted in Table 1. Forty-three patients had incomplete neurological deficit, whereas two had complete neurological deficit. Out of 45 patients, 22 (48.9%) had central cord syndrome, 2 patients had complete cord injury, whereas 1 patient had a Brown–Sequard syndrome. Twenty patients had incomplete neurological deficit; however, they did not fall into any of the above neurological groups.
Twenty-seven patients (60%) presented with AIS D on admission, followed by AIS C (24.4%). Only two patients presented with complete motor and sensory deficit (AIS A). As per the neurological presentation, 38 (84.4%) patients had mid-cervical segment (C3–C5) injury, whereas the upper and lower cervical spine regions were involved in 2 (4.4%) and 5 (11.1%) cases, respectively.
Neurological impairment and MRI findings
MR images showed spinal cord oedema in 27 (60%) cases, whereas spinal cord contusion and normal cord were seen in 8 (17.8%) cases each. Two patients showed cord swelling, whereas no patient had cord haemorrhage or cord transection. The association between the neurological deficit and cord changes seen in MRI at the time of admission is shown in Table 2. PVH was observed in 38 cases (84.4%) and it extended over a mean of 10.69±6.4 vertebral segments. PVH associated with cord swelling, cord contusion, cord oedema and normal cord extended over 17±1.4, 16.5±6.09, 11.19±4.19 and 1.6±3.1 vertebral segments, respectively (P<0.001).
Initial neurological status and neurological recovery
The mean AIS motor scores at the time of admission, at 3 months, 1 year and 2 years follow-up are shown in Table 3. The AIS motor score improved at each follow-up, which was statistically significant (P<0.001) (Figure 1). When the mean AIS motor score between different MRI groups were analyzed using analysis of variance, the differences were found to be statistically significant (P<0.001). The median AIS motor scores at the time of presentation, at 3 months, 1 year and 2 years follow-up were 62, 92, 96 and 98 respectively.
Cases with normal cords showed a mean recovery rate of 95.56±12.54 at 2 years, followed by spinal cord oedema (recovery rate: 87.70±21.67), cord contusion (recovery rate: 48.72±42.08) and cord swelling (recovery rate: 39.42±1.68). Differences in the recovery rates between different spinal cord changes were statistically significant (P<0.05).
The initial upper limb motor score showed a significant correlation with the initial lower limb motor score (P<0.001) as well as AIS score at 2 years (P<0.05) and the recovery rate (P<0.05), but showed no significant correlation with the rostral extent of cord changes.
A negative correlation was found between the length of cord changes and the recovery rate (−0.026) as well as the final motor score (−0.042), which was of statistical significance (P<0.05). Similarly, there was a significant negative correlation between the length of PVH and AIS at the time of presentation (P<0.001), final follow-up (P<0.001) and the rate of recovery (P<0.001).
Discussion
In this study, SCIWORET accounted for 16.5% of all cervical spine injuries. This clearly indicates that it is not an uncommon cause of traumatic myelopathy in adults.8, 9, 10
Excessive non-physiological movement of the cervical spine involving hyperextension, hyperflexion or torsion can distract or compress the cord and damage it. Although the forces generated by such motion in SCIWORET are sufficient to injure the cord, they are below the critical threshold needed to disrupt discocorporeal or discoligamentous structures and cause fractures or dislocations.
MR imaging is essential to determine the nature of injury to the cord and should be carried out in all cases of suspected SCIWORET to differentiate neurological deficit caused by extrinsic compression and those caused by haemorrhage, oedema and other forms of cord injury.5 In a recent study, Machino et al.3 correlated the MRI findings seen in SCIWORET with the severity of neurological deficit and surgical outcome. They divided the MRI findings into intramedullary high-signal intensity and PVH. Intramedullary high-signal intensity and PVH were found in more than 90% of their patients. The range of intramedullary high-signal intensity significantly reflected preoperative AIS and prognosis for neurological outcome.3
In this study, spinal cord oedema was the most common (60%) change noted in patients with SCIWORET and cord contusion was encountered in 18%. This differs from observations of Tewari et al.11 who reported a frequency of cord contusion of 32% and cord oedema in only 5%. One possible reason for this difference may be the variability in interpretation of the more subtle MRI changes in the spinal cord by different radiologists. This highlights the need for further studies evaluating intra- and interobserver agreement in the diagnosis of the spectrum of cord changes in MR images in SCIWORET.
Previous reports suggest that in the vast majority of instances SCIWORET is associated with incomplete cord injury;11, 12, 13 we also noted incomplete cord lesions in 95% of cases with central cord syndrome in half of these patients.
The nature of cord damage appears to determine the severity of initial neurological deficit; in this study, a large proportion (44%) of patients who had AIS D neurological deficit had cord oedema, whereas patients with cord contusion had more severe neurological damage (AIS A in two patients).
The extent of recovery too appears to be related to the pattern of cord damage.11, 14, 15 At 2 years, among those who had complete neurological recovery, a large proportion were those with no demonstrable changes in the cord on the MRI or those with cord oedema, whereas incomplete recovery was more frequently seen in patients with cord contusion.
The spatial extent of cord damage (expressed as the length of cord involved) is also clearly related to both the extent of neurological damage and the rate of recovery; this was noted both in this study and in earlier reports (Figure 2).16, 17 This knowledge will help the surgeon to give the patient meaningful information regarding the prognosis of SCIWORET.
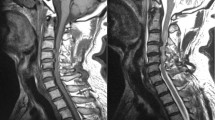
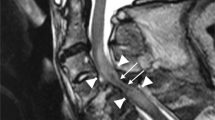
(a–c) MRI findings at admission. (a) Patient with Cord oedema who presented with AIS C and recovered to AIS E, (b) patient with cord contusion who presented with AIS B and recovered to AIS D and (c) patient with cord contusion involving 14 segments who presented with AIS B and recovered to AIS C at 2 years. This patient had features of OPLL (ossification of posterior longitudinal ligament).
In this study, PVH was observed in 84.4% patients. However, we believe that PVH is not the cause of neurological deficit but their presence signifies the injury caused to the spinal column, which is not visible in the radiographs. The presence of PVH signifies the severity of the injury to the cervical spine, whereas intramedullary high-signal intensity signifies the severity of the spinal cord injury, which is not only responsible for the initial neurological deficit but also determines the neurological outcome.
The management of SCIWORET remains controversial. Although most authors believe that significant improvement of the neurological status will occur irrespective of the nature of treatment,13 there are those who have undertaken surgical intervention.3, 18 In this series, we opted to treat all the patients non-operatively and the final outcome was comparable to those who treated patients with SCIWORET surgically.3, 18
There are some limitations in this study. First, nine patients were lost to follow-up. Second, patients should have also been evaluated in terms of functional outcome, which may not correlate well with the neurological recovery. Further studies need to be carried out to correlate the AIS with functional outcome. We acknowledge that there is little evidence to justify the use of methyl prednisolone in spinal cord injuries and we have since abandoned its use. The rationale of using halter traction for a short period of time was simply to ensure rest till soft tissue healing occurred. Finally, the results of this study support earlier observations1, 3, 5, 11, 12, 13, 14 that MRI changes are consistently seen in patients with SCIWORET. In the light of these observations, the continued use of the term ‘SCIWORET’ may be questioned.
Conclusion
The incidence of SCIWORET is not uncommon in adults. The initial neurological status correlates well with MR imaging findings. Patients with minimal or no cord changes on MR imaging have the best outcome followed by those who show cord oedema. Patients with cord contusions have a poor prognosis. The recovery rate and the final motor outcome are inversely related to the length of cord involvement.
Data archiving
There were no data to deposit.
References
Pang D, Wilberger JE Jr . Spinal cord injury without radiographic abnormalities in children. J Neurosurg 1982; 57: 114–129.
Tator CH . Clinical manifestations of acute spinal cord injury. In: Benzel EC, Tator CH (eds). Contemporary Management of Spinal Cord Injury. American Association of Neurological Surgeons: Park Ridge, IL, USA. 1995 pp 15–26.
Machino M, Yukawa Y, Ito K, Nakashima H, Kanbara S, Morita D et al. Can magnetic resonance imaging reflect the prognosis in patients of cervical spinal cord injury without radiographic abnormality? Spine 2011; 36: E1568–E1572.
Maynard FM Jr, Bracken MB, Creasey G, Ditunno JF Jr, Donovan WH, Ducker TB et al. International Standards for Neurological and Functional Classification of Spinal Cord Injury. American Spinal Injury Association. Spinal Cord 1997; 35: 266–274.
Ramón S, Domínguez R, Ramírez L, Paraira M, Olona M, Castelló T et al. Clinical and magnetic resonance imaging correlation in acute spinal cord injury. Spinal Cord 1997; 35: 664–673.
Flanders AE, Schaefer DM, Doan HT, Mishkin MM, Gonzalez CF, Northrup BE . Acute cervical spine trauma: correlation of MR imaging findings with degree of neurologic deficit. Radiology 1990; 177: 25–33.
Lucas JT, Ducker TB . Motor classification of spinal cord injuries with mobility, morbidity and recovery indices. Am Surg 1979; 45: 151–158.
Hendey GW, Wolfson AB, Mower WR, Hoffman JR . Spinal cord injury without radiographic abnormality: results of the National Emergency X-Radiography Utilization Study in blunt cervical trauma. J Trauma 2002; 53: 1–4.
Crooks F, Birkett AN . Fractures and dislocations of the cervical spine. Br J Surg 1944; 31: 252–265.
Regenbogen VS, Rogers LF, Atlas SW, Kim KS . Cervical spinal cord injuries in patients with cervical spondylosis. Am J Roentgenol 1986; 146: 277–284.
Tewari MK, Gifti DS, Singh P, Khosla VK, Mathuriya SN, Gupta SK et al. Diagnosis and prognostication of adult spinal cord injury without radiographic abnormality using magnetic resonance imaging: analysis of 40 patients. Surg Neurol 2005; 63: 204–209.
Gupta SK, Rajeev K, Khosla VK, Sharma BS, Paramjit, Mathuriya SN et al. Spinal cord injury without radiographic abnormality in adults. Spinal Cord 1999; 37: 726–729.
Saruhashi Y, Hukuda S, Katsuura A, Asajima S, Omura K . Clinical outcomes of cervical spinal cord injuries without radiographic evidence of trauma. Spinal Cord 1998; 36: 567–573.
Takahashi M, Harada Y, Inoue H, Shimada K . Traumatic cervical cord injury at C3–4 without radiographic abnormalities: correlation of magnetic resonance findings with clinical features and outcome. J Orthop Surg 2002; 10: 129–135.
Hayashi K, Yone K, Ito H, Yanase M, Sakou T . MRI findings in patients with a cervical spinal cord injury who do not show radiographic evidence of a fracture or dislocation. Paraplegia 1995; 33: 212–215.
Flanders AE, Spettell CM, Tartaglino LM, Friedman DP, Herbison GJ . Forecasting motor recovery after cervical spinal cord injury: value of MR imaging. Radiology 1996; 20: 649–655.
Selden NR, Quint DJ, Patel N, d’Arcy HS, Papadopoulos SM . Emergency magnetic resonance imaging of cervical spinal cord injuries: clinical correlation and prognosis. Neurosurgery 1999; 44: 785–792.
Kawano O, Ueta T, Shiba K, Iwamoto Y . Outcome of decompression surgery for cervical spinal cord injury without bone and disc injury in patients with spinal cord compression: a multicenter prospective study. Spinal Cord 2010; 48: 548–553.
Acknowledgements
We thank Professor Rajgopal Kadavigere, Professor and Head of Department of Radiodignosis and Imaging, for his support in evaluation of the MRI images. We also thank Professor Benjamin Joseph, Manipal, for his valuable suggestions to improve the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Mohanty, S., Bhat, N., Singh, K. et al. Cervical spinal cord injuries without radiographic evidence of trauma: a prospective study. Spinal Cord 51, 815–818 (2013). https://doi.org/10.1038/sc.2013.87
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sc.2013.87
Keywords
This article is cited by
-
Spinal cord injury without radiologic abnormality: an updated systematic review and investigation of concurrent concussion
Bulletin of the National Research Centre (2023)
-
The influence of timing of surgery in the outcome of spinal cord injury without radiographic abnormality (SCIWORA)
Journal of Orthopaedic Surgery and Research (2020)
-
A geriatric patient with spinal cord injury without radiographic abnormality: outcomes and causes
Spinal Cord Series and Cases (2018)
-
Spinal cord injury without radiographic abnormality (SCIWORA) in adults: MRI type predicts early neurologic outcome
Spinal Cord (2016)
-
Cervical spinal cord injuries without radiographic evidence of trauma: a prospective study
Spinal Cord (2014)