Abstract
Study design:
Focus group study
Objective:
To investigate cell-specific endoplasmic reticulum (ER) stress reactions in contusive spinal cord by evaluating the expression of the glucose-regulated protein 78 (GRP78) and C/EBP homologous transcription factor protein (CHOP) using immunohistochemical staining.
Setting:
Data were analysed at Tokai University School of Medicine in Japan.
Methods:
The authors generated rat spinal cord injury (SCI) models using an IH-Impactor (100 kdyne(LI), 200 kdyne (HI)). Rats were killed at 1, 3, 5, 7 and 14 days post operation (dpo). Spinal cord sections were prepared and the expression ratio of GRP78 and CHOP was evaluated in oligodendrocyte precursor cells (OPCs) (NG2+), oligodendrocytes (OLs) (APC+), neurons (NeuN+) and astrocytes (GFAP+) using double immunohistochemical staining. We examined an area 8 mm distal from SCI-epicenter.
Results:
Compared with the sham group, both injured groups had higher GRP78 expression ratio in contused spinal cord at 1 dpo. GRP78 expression ratio was highest in GFAP+ cells of both groups, and lowest in NG2+ cells. Although GRP78 was expressed strongly immediately after SCI in the both groups, increased CHOP expression was observed only in the HI group. The CHOP expression in NG2+ cells was significantly higher than that observed in GFAP+ cells at 5 dpo.
Conclusion:
Although the ER stress response contributes to cell survival in the low-stress SCI conditions, the ER stress response induces an apoptotic cascade in high-stress SCI conditions. The ER response varies according to cell type, with the highest observed in astrocytes, and the lowest observed in oligodendrocyte precursor cells.
Similar content being viewed by others
Introduction
Spinal cord injury (SCI) has a major impact on the daily living activities of patients. The severity of neural damage depends on the combination of the initial mechanical injury and the subsequent secondary damage caused by a self-destructive cascade that enlarges the damaged area.1, 2, 3 Free radicals, excitatory amino acids and the arachidonic acid cascade from injured cell membranes have been reported as factors related to secondary damage.1, 4
Recently, there has been growing interest in protein-modification disorders related to endoplasmic reticulum (ER) stress during delayed neuronal cell death in various central nervous system conditions, such as cerebral ischemia, Alzheimer's disease, Parkinson’s disease, brain injury and SCI.5, 6, 7 ER stress causes protein-modification blockage, which spurs the accumulation of abnormal proteins. This accumulation in turn induces the expression of glucose-regulated protein 78 (GRP78), which hydrolyses ATP to promote protein folding.8 Since the accumulation of unfolded proteins leads to apoptosis, the enhancement of GRP78 is a possible strategy to inhibit apoptotic cell death following neurotrauma and neurodegenerative disease.5, 9, 10 Excessive accumulation of abnormal proteins leads to the expression of C/EBP homologous transcription factor protein (CHOP), which is a prominent proapoptotoc protein of the ER stress response.11, 12
Understanding of the precise mechanism of ER stress-mediated apoptosis in SCI may lead to the development of novel treatment strategies. In this study, the expression of ER stress response proteins was immunohistologically examined in the various cell types of the injured spinal cord, specifically focusing on the ER chaperone GRP78, which has a protective function in ER stress, and the transcription factor CHOP, which promotes ER-mediated apoptosis.12
Materials and methods
Animal population
Male Sprague Dawley rats (age, 8–10 weeks; weight, 280–320 g; n=75) were procured from Nippon Crea (Kanagawa, Japan). All experiments were performed according to the approved protocol of Tokai University School of Medicine Animal Experiment and Research Support Center. The rats were placed under general anaesthesia through inhalation of 4% isoflurane and a laminectomy of the 10–11th thoracic levels were performed under sterile conditions to expose the dura mater. SCI was induced using an Infinite Horizon SCI device (IH Impactor; Precision Systems & Instrumentation, Lexington, KY, USA). Three groups of animals were created: a low-degree injury group (100 kdyne: LI group), a high-degree injury group (200 kdyne: HI group)13 and a sham group that received laminectomies without SCI.
Immunohistochemical staining
5 rats from each group at 1, 3, 5, 7, and 14 days post-operation (dpo) were anesthetized and transcardially perfused with 2% paraformaldehyde in 0.1 M phosphate-buffered saline. The spinal cords were removed, post-fixed overnight in 2% paraformaldehyde in phosphate-buffered saline, dehydrated for 3 days in sucrose and embedded in optimal cutting temperature compound (OCT Compound; Sakura Finetek; Japan). Frozen serial continuous sections with a thickness of 14 μm were prepared from an area 8-mm caudal to the epicenter,14 blocked in phosphate-buffered saline with 5% goat normal serum and 0.3% Triton-X at room temperature for 1 h and stained overnight at 4 °C with the following primary antibodies.14
mouse anti-APC (Genetex, Irvine, CA, USA, 1:100, a marker of oligodendrocytes), mouse anti-NeuN (Chemicon, Billerica, MA, USA, 1:100, a marker of neurons), mouse anti-NG2 (Chemicon, 1:100, a marker of OPCs), mouse anti-GFAP (Abcam, Cambridge, MA, USA, 1:400, a marker of astrocytes), rabbit anti-GRP78 BiP antibody (Abcam, 1:200) and rabbit anti-CHOP/GADD153 antibody (Millipore, Billerica, MA, USA, 1:100). Sections were rinsed in phosphate-buffered saline, and the following secondary antibodies were applied for 2 h at room temperature: a goat anti-mouse Alexa Fluor 488 (1:800) antibody to label primary antibodies of each cell marker and a goat anti-rabbit Alexa Fluor 594 (1:800) antibody to label GRP78 and CHOP. Sections were finalised by staining the nucleus with 4′,6-diamino-2-phenylindole (DAPI, VECTA). The stained sections were observed using a confocal microscope (LSM710; Carl Zeiss, Oberkochen, Germany) and imaging software (Zen 2009 Lite Edition, Carl Zeiss).
Analysis
We performed manual counting of five adjacent sections for each analysis. To quantify the GRP78 and CHOP expression ratios, the number of cells with positive staining of GRP78 or CHOP in the nucleus was counted and was divided by the total number of nuclei in the dorsal column white matter. To quantify the GRP78 and CHOP expression ratios in each cell type, the number of cells double positive for each cell marker and GRP78 or CHOP was counted and was divided by the total number of cells positive for each cell marker. Astrocytes, oligodendrocytes (OLs) and oligodendrocyte precursor cells (OPCs) were examined in the dorsal cord white matter, while neurons were examined in the anterior horn area. Both ratios were denoted in percentages. Mann–Whitney U test was applied for statistical analysis. Significance was set at P<0.05.
Statement of ethics
We certify that all applicable institutional and governmental regulations concerning the ethical use of animals were followed during the course of this research.
Results
Immunohistochemical staining and cell count
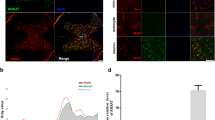
Although the counting of astrocytes, OLs and OPCs in the dorsal cord was performed without difficulty, the neurons in the dorsal cord were limited and had poor cytoplasms. We therefore elected to perform the counting of neurons in the anterior horn, in which cellular integrity was higher. We observed GRP78 staining in the nucleus and along the cytoplasm of the dendrites, while CHOP was observed in the cell nucleus (Figure 1). Double-stained images confirmed GRP78 immunoreactivity in the nucleus and the cell cytoplasm. NG2+ cells had narrower cytoplasmic surfaces than did those of other cell types (Figure 2). Neurons, OLs and OPCs showed uniform positive CHOP staining mainly in the nucleus. Granular staining inside the cell cytoplasm as well as the nucleus was confirmed in astrocytes (Figure 3).
Immunostaining for GRP78, CHOP, and cellular markers in the injured spinal cord. Representative immunostaining results of the injured spinal cord from the HI group (200 kdyne), at 5 dpo (nuclear stain in blue, scale bars: 50 μm). GRP78 immunoreactivity is localized in the nucleus and cytoplasm (a, b), while CHOP immunoreactivity is localized in the nucleus (c, d). Double immunostaining for GRP78 and the following cellular markers: NeuN (e), GFAP (f), APC (g) and NG2 (h). Double immunostaining for CHOP and the following cellular markers: NeuN (i), GFAP (j), APC (k) and NG2 (l).
Immunostaining for GRP78 in each cell type. Double immunostaining for GRP78 (red) and the following cellular markers (green) on sections of the injured spinal cord from the HI group (200 kdyne) at 5 dpo (nuclear stain in blue, scale bars: 10 μm): (a) NeuN+ neurons, (b) GFAP+ astrocytes, (c) APC+ OLs, (d) NG2+ OPCs. NG2+ cells had narrower cytoplasmic surfaces than other cell types. GRP78 staining was observed in the nucleus and the cytoplasm of dendrites.
Immunostaining for CHOP in each cell type. Double immunostaining for CHOP (red) and the following cellular markers (green) on sections of the injured spinal cord from the HI group (200 kdyne) at 5 dpo (nuclear stain in blue, scale bars: 10 μm): (a) NeuN+ neurons, (b) GFAP+ astryocytes, (c) APC+ OLs, and (d) NG2+ OPCs. Neurons, OLs and OPCs showed uniform positive CHOP staining mainly in the nucleus (a, c, d). Granular staining inside the cell cytoplasm as well as the nucleus was confirmed in astrocytes (b).
Differences in GRP78 and CHOP expression ratio based on the degree of SCI
Both the HI and LI groups exhibited a significantly higher GRP78 expression ratio compared with that observed in the sham group at 1 dpo (HI group: 35.32±4.2%, LI group: 27.40±5.5%, sham group: 11.97±1.35%, P=0.0368), but the differences were not significant between the HI and LI groups.
GRP78 expression decreased gradually over time from the peaks observed at 1 dpo in the HI and LI groups. Although the HI group exhibited a significantly higher ratio compared with the sham group at 3 dpo, there was no significant difference in the GRP78 expression ratio between the LI and the sham groups (HI group: 22.22 ±3.15%, LI group: 19.36±5.00%, sham group: 11.48±4.24%). Significant differences were not observed between the three groups at 7 dpo and 14 dpo.
In contrast, the CHOP expression ratio increased gradually over time, from the very low values observed in the LI and HI groups immediately after injury. There were initially no significant differences in the ratio between the LI, HI and sham groups initially. Subsequently, the CHOP expression ratio increased gradually and both groups showed a significantly higher ratio compared with the sham group at 5 dpo and 7 dpo. The HI group exhibited a significantly higher ratio compared with the LI group at 7 dpo (LI group: 28.32±3.05%, HI group: 18.56±3.30%, P=0.0061). Although the HI group showed a trend towards a higher ratio compared with the LI group at the other time points, the differences were not statistically significant (Figure 4).
GRP78 and CHOP expression ratio in contused spinal cord structure injury. The expression ratio of GRP78 was high immediately after injury, but exhibited a gradual decreasing trend, until there were no significant differences in the ratio between the high-degree injury (HI), low-degree injury (LI) injury and sham groups after 5 dpo. The CHOP expression ratio increased in the subacute phase of the injuries and was significantly higher in the HI group than in the LI group at 7 dpo. Data are the mean±s.d. (n=5, *P<0.05).
Changes in the GRP78 expression ratio in each cell type
Maximum GRP78 expression ratio was observed at 1 dpo in all cell types, with the exception of NG2+ OPCs, in which the peak was observed at 3 dpo.
GFAP+ astrocytes exhibited a significantly higher GRP78 expression ratio in the HI and LI groups at 1 dpo compared with other cell types (GFAP+ cells in HI group: 46.92±3.76%, P<0.05). Although the GFAP+ cells showed a statistically higher GRP78 expression ratio compared to NG2+ cells in the HI group at 3 dpo (GFAP+cells, 38.20±4.36%, NG2+cells, 27.38 ±3.03%, P=0.0215), no significant differences were observed between cell types in the LI group. There were no significant differences in the GRP78 expression ratio between cell types in the LI and HI groups at 5 and 7 dpo. Although the GRP78 expression ratio in the NG2+ cells was significantly lower than other cell types at 14 dpo in the HI group, no difference was observed between cell types in the LI group (Figure 5).
Comparison of the GRP78 expression ratio between all cell types. The highest GRP78 expression ratio was observed at the acute phase of injury followed by a gradual declining trend in all cells. GFAP+ cells exhibited a significantly higher GRP78 expression ratio in the HI and LI groups at 1 dpo compared with other cell types. Data are the mean ± s.d. (n=5, *P<0.05, **P<0.01).
Changes in the CHOP expression ratio in each cell type
The CHOP expression ratio in NG2+ cells of the LI group was significantly higher than that observed in GFAP+ cells at 5, 7 and 14 dpo with a peak at 7 dpo (NG2: 22.81 ±8.37%, GFAP: 13.73±3.42, P=0.0473).
GFAP+ cells of the LI group exhibited a trend towards lower CHOP expression ratios compared with other cell types. The CHOP expression ratio gradually increased after injury in all cell types of the HI group (Figure 6).
Comparison of the CHOP expression ratio between all cell types. In the HI group, there was no difference observed in CHOP expression in all cell types. In the LI group, the CHOP expression ratio in NG2+ cells was significantly higher than GFAP+ cells at 5, 7 and 14 dpo. Data are the mean ± s.d. (n=5, *P<0.05, **P<0.01).
In addition, all cell types of the HI group showed a significantly higher CHOP expression ratio compared with that observed in the sham group. However, the difference in the CHOP ratios of GFAP+ cells in the LI and sham groups did not reach significant levels (Figure 7).
Comparison of GRP78 and CHOP expression ratio in each cell type between sham, LI, and HI group. APC+ GRP78+ cell (a), NeuN+ GRP78+ cell (b), NG2+ GRP78+ cell (c), GFAP+ GRP78+ cell (d), APC+ CHOP+ cell (e), NeuN+ CHOP+ cell (f), NG2+ CHOP+ cell (g), and GFAP+ CHOP+ cell (h). The GRP78 expression ratio in the LI and HI groups was significantly higher in all cell types compared to the sham group, except for GFAP+ cells in which the difference in the GRP78 ratio between the LI and sham groups at 7 dpo and 14 dpo did not reach significant levels (a–d). The CHOP expression ratio in the HI group was significantly higher in all cell types compared to the sham group. However, no difference in the CHOP ratio was observed for GFAP+ cells between the LI and sham groups at any time point (e–h). (n=5, *P<0.05).
Discussion
The regeneration of the white matter tracts is extremely important in the functional prognosis of SCI; therefore, myelination is integral.15, 16 Although OPCs that are abundant in the adult spinal cord increase in number in response to such stimuli as demyelination and other external injuries,17 sufficient remyelination in not achieved in contusive SCI models due to differentiation blockage. This is in contrast to chemical demyelination models in which a replenishment of the lost OL pool is observed.18 A possible explanation for this phenomenon is cell death before OPC differentiation. If this is the case, alleviation of delayed cell death would not only decrease the spread of secondary damage, but would also improve the OPCs’ survival, amplification and differentiation into OLs, which would lead to increased remyelination.
Recent studies reported that the ER stress response in the central nervous system is related to neurodegenerative changes.9, 6 Apoptosis after central nervous system damage occurs through an extrinsic pathway involving the Fas ligand, an intrinsic pathway through mitochondrial incompetence and the ER stress pathway. The ER stress response is a process that occurs to alleviate the accumulation of misfolded proteins in the ER that occurs as a result of cellular stress. In the ER stress response unfolded proteins are eliminated via three pathways involving the following factors: protein kinase-like ER kinase, activating transcription factor 6 and inositol-requiring kinase. When the unfolded protein response is activated within the ER in response to various cellular stresses, GRP78 is induced via the activating transcription factor 6 and IRE1α pathways to break down and eliminate unfolded proteins.8, 10, 19
In SCI, the expression of GRP78 has been histologically confirmed in the damaged tissue of the spinal cord at the acute stage.7, 15
It has been suggested that astrocytes have a unique ER stress response mechanism and a higher degree of response compared with other cell types.20 In this study, differences in the GRP78 expression ratio were observed between neurons, OPCs, OLs and astrocytes. The GRP78 expression ratio in astrocytes in the acute stage of SCI was significantly higher than that observed in other cell types, which histologically supports the probability of a stronger ER stress response mechanism in astrocytes. On the other hand, the peak GRP78 expression ratio in OPCs was significantly lower than that observed in the other cell types, which suggests weaker ER stress response in OPCs.
When the ER stress increases, the transcription factor CHOP induces apoptosis through a mechanism and targets genes that remain unknown.12 There was no significant difference in the peak CHOP expression ratio between cell types, although astrocytes exhibited a trend for the lowest ratio. The CHOP expression ratio at 7 dpo was significantly higher in the HI group than in the LI group, which suggests that during the subacute stage of SCI, the expression of CHOP increases significantly in response to the increasing severity of SCI.
Recent studies reported that cytoplasmic overexpression of CHOP might be correlated with non-apoptotic cascades.7, 12 In this study, cytoplasmic CHOP expression was observed only in astrocyte, in contrast to its nuclear expression in other cell types. Therefore, astrocytes appear to possess a unique stress response mechanism, that provides astrocytes with a means to control apoptosis within the damaged spinal cord.
Although no significant difference in the CHOP expression ratio was observed between OPCs and astrocytes in a high-damage environment, the CHOP expression ratio was significantly higher in OPCs than in astrocytes in a low-damage environment. This suggests that all cell types are equally susceptible to delayed cell death in cases of severe injury, but the tendency for delayed cell death is greater for OPCs compared with astrocytes in cases of less severe injury. Our results indicate that the ER stress response is weaker in OPCs compared to other cell types, implying that OPCs are more prone to delayed cell death.
In our previous in vitro study, the survival ratio of cells in an ER stress environment improved with increased expression of GRP78.7 This was confirmed in our in vitro study in which GRP78 expression protected cells by preventing the accumulation of unfolded protein in low-stress situations. However, the effects of GRP78 expression were insufficient in high-stress environments, and delayed cell death ensued via CHOP activation. To our knowledge, reports concerning the ER stress response in different cell types in SCI are limited. Our study indicates that the ER stress response varies according to cell types within injured spinal cord: the highest observed in astrocytes and the lowest observed in OPCs. Further studies are necessary to clarify ER stress-induced apoptosis, which will hopefully lead to a novel treatment to abrogate the functional deficit after SCI.
Data Archiving
There were no data to deposit.
References
Hall ED, Braughler JM . Free radicals in CNS injury. Res Publ Assoc Res Nerv Ment Dis 1993; 71: 81–105.
Tator CH, Fehlings MG . Review of the secondary injury theory of acute spinal cord trauma with emphasis on vascular mechanisms. J Neurosurg 1991; 75: 15–26.
Watanabe M, Fujimura Y, Nakamura M, Yato Y, Ohta K, Okai H et al. Changes of amino acid levels and aspartate distribution in the cervical spinal cord after traumatic SCI. J Neurotrauma 1998; 15: 285–293.
Park E, Velumian AA, Fehlings MG . The role of excitotoxicity in secondary mechanisms of SCI: a review with an emphasis on the implications for white matter degeneration. J Neurotrauma 2004; 21: 754–774.
Suyama K, Watanabe M, Sakabe K, Okada Y, Matsuyama D, Kuroiwa M . Overexpression of GRP78 protects glial cells from ER stress. Neurosci Lett 2011; 504: 271–276.
Lindholm D, Wootz H, Korhonen L . ER stress and neurodegenerative diseases. Cell Death Differ 2006; 13: 385–392.
Penas C, Guzman MS, Verdu E, Fores J, Navarro X, Casas C . Spinal cord injury induces ER stress with different cell-type dependent response. J Neurochem 2007; 102: 1242–1255.
Lee AS . The ER chaperone and signalling regulator GRP78/BiP as a monitor of endoplasmic reticulum stress. Methods 2005; 35: 373–381.
Deslauriers AM, Afkhami-Goli A, Paul AM, Bhat RK, Acharjee S, Ellestad KK et al. Neuroinflammation and ER stress are coregulated by crocin to prevent demyelination and neurodegeneration. J Immunol 2011; 187: 4788–4799.
Haas IG . BiP (GRP78), an essential hsp70 resident protein in the ER. Experientia 1994; 50: 1012–1020.
Walter P, Ron D . The unfolded protein response: from stress pathway to homeostatic regulation. Science 2011; 334: 1081–1086.
Wang Z, Zhang C, Hong Z, Chen H, Chen W, Chen G . C/EBP homologous protein (CHOP) mediates neuronal apoptosis in rats with spinal cord injury. Experimental and Therapeutic Med 2013; 5: 107–111.
Scheff SW, Rabchevsky AG, Fugaccia I, Main JA, Lumpp JE Jr . Experimental modeling of SCI: characterization of a force-defined injury device. J Neurotrauma 2003; 20: 179–193.
Li GL, Farooque M, Holtz A, Olsson Y . Apoptosis of oligodendrocytes occurs for long distances away from the primary injury after compression trauma to rat spinal cord. Acta Neuropathol 1999; 98: 473–480.
Ohri SS, Maddie MA, Zhao Y, Qiu MS, Hetman M, Whittemore SR . Attenuating the endoplasmic reticulum stress response improves functional recovery after spinal cord injury. Glia 2011; 59: 1489–1502.
Nash B, Thomson CE, Linington C, Arthur AT, McClure JD, McBride MW et al. Functional duality of astrocytes in myelination. J Neurosci 2011; 31: 13028–13038.
McTigue D, Richa T, Ping W . NG2 Colocalizes With Axons and Is Expressed by a Mixed Cell Population in Spinal Cord Lesions. J Neuropathol Exp Neurol 2006; 65: 406–4207.
Watanabe M, Toyama Y, Nishiyama A . Differentiation of proliferated NG2-positive glial progenitor cells in a remyelinating lesion. J Neurosci Res 2002; 69: 826–836.
Schroder M, Kaufman RJ . ER stress and the unfolded protein response. Mutat Res 2005; 569: 29–63.
Saito A, Hino S, Murakami T, Kondo S, Imaizumi K . A novel ER stress transducer, OASIS, expressed in astrocytes. Antioxid Redox Signal 2007; 9: 563–571.
Acknowledgements
This work was supported in part by Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan (21591907).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Matsuyama, D., Watanabe, M., Suyama, K. et al. Endoplasmic reticulum stress response in the rat contusive spinal cord injury model-susceptibility in specific cell types. Spinal Cord 52, 9–16 (2014). https://doi.org/10.1038/sc.2013.118
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sc.2013.118
Keywords
This article is cited by
-
Carbon nanoparticles induce endoplasmic reticulum stress around blood vessels with accumulation of misfolded proteins in the developing brain of offspring
Scientific Reports (2020)
-
Novel Role of HAX-1 in Neurons Protection After Spinal Cord Injury Involvement of IRE-1
Neurochemical Research (2020)
-
Cellular and Subcellular Localization of Endoplasmic Reticulum Chaperone GRP78 Following Transient Focal Cerebral Ischemia in Rats
Neurochemical Research (2018)
-
Tissue-Engineered Regeneration of Hemisected Spinal Cord Using Human Endometrial Stem Cells, Poly ε-Caprolactone Scaffolds, and Crocin as a Neuroprotective Agent
Molecular Neurobiology (2017)
-
Necrostatin-1 Mitigates Endoplasmic Reticulum Stress After Spinal Cord Injury
Neurochemical Research (2017)