Abstract
Study design:
Retrospective study.
Objectives:
To identify factors associated with the development of early onset post-traumatic syringomyelia within 5 years of spinal cord injury.
Setting:
Department of Rehabilitation Medicine, Pusan National University School of Medicine, Korea.
Methods:
We retrospectively examined the records of 502 patients with traumatic cervical or thoracic spinal cord injury who underwent follow-up magnetic resonance imaging (MRI) examinations more than once a year for at least 5 years. Patients were assessed in terms of the neurological level of injury, the severity of initial spinal cord injury, the use of surgery and the extent of spinal canal involvement. The latter was evaluated by calculating the shortest antero-posterior diameter of the injured vertebral canal and the spinal reserve capacity as shown on MRI at the time of trauma onset and at the time of diagnosis of syringomyelia.
Results:
Syringomyelia developed within 5 years in 37 (7.3%) of the 502 patients. The mean age of these 37 patients was 44.6 years (range, 17–67 years) and the mean interval from spinal cord injury to onset of syringomyelia was 38.8 months (range, 2–54 months). The development of post-traumatic syringomyelia within 5 years was not significantly related to the severity or level of injury, the use of spinal surgery or the extent of spinal canal encroachment (P≥0.05 for each comparison).
Conclusion:
Early onset syringomyelia occurring within 5 years after spinal cord injury was not associated with neurological injury level, severity of injury, the use of spinal surgery or canal encroachment.
Similar content being viewed by others
Introduction
Post-traumatic syringomyelia can occur after traumatic spinal cord injury, and is a major cause of delayed aggravated neurological symptoms. Post-traumatic syringomyelia is characterized by the formation of a cavity filled with cerebrospinal fluid (CSF), which causes symptoms including severe pain, motor weakness and changes in spasticity. Syringomyelia was first described by Bastian in 1867,1 and, in 1880, Strümpell et al.2 reported instances of syringomyelia following spinal cord injury.3
The clinical incidence of post-traumatic syringomyelia in patients with spinal cord injury has been estimated at 0.3–3.2%, although the incidence recorded radiologically and at autopsy appears to be higher than the clinical incidence.4, 5 The increased use of magnetic resonance imaging (MRI) for diagnosis has caused post-traumatic syringomyelia to be reported in 12–22% of patients with spinal cord injury.6
The mechanisms underlying the development of post-traumatic syringomyelia have yet to be established. Processes postulated to be involved include: ‘slosh and suck’ at the site of injury, liquefaction, development of hematoma, a malacic change in the spinal cord, necrosis, enzymatic lysis, tissue loss and arachnoiditis.7, 8
Several studies have been sought to identify clinical and radiological factors associated with post-traumatic syringomyelia. Factors examined include: age at onset, gender, cause of injury, site of injury, severity of injury, type of vertebral fracture, treatment method and the degree of encroachment into the vertebral canal.6, 9 Complete spinal cord injury and arachnoiditis have been associated with an increased incidence of post-traumatic syringomyelia, and increasing age, damage at the cervical and thoracic levels, dislocated spinal fracture and the use of spinal instrumentation without decompression have all been identified as risk factors for the early development of post-traumatic syringomyelia.6 To date, however, none of those factors have been established as being definitively causative.
The present study have been sought to identify factors associated with the development of early-onset syringomyelia within 5 years following traumatic spinal cord injury. Patients were followed up for at least 5 years post-injury. Factors investigated included the site of neurological injury (that is, cervical vs thoracic), the severity of the spinal cord injury, the type of treatment (that is, surgery vs non-surgery), and the degree of spinal canal involvement. Data were obtained using MRI.
Materials and methods
This retrospective study examined the records of 502 patients with traumatic spinal cord injury who had undergone follow-up MRI examinations more than once a year for at least 5 years following the injury. Patients with spinal cord injuries received clinical follow-up, in the interval since 1993. Patients with a concurrent brain injury at the time of trauma onset, with lumbar or cauda equina injury, or who were pediatric, were excluded. The present study used the criteria for post-traumatic syringomyelia as a cystic cavity to extend at least two spinal segments.
Each patient was assessed in terms of the cervical or thoracic spinal cord injury, and the extent of injury was measured using the ASIA Impairment Scale (AIS), whereby 225 patients were of grade AIS A (complete injury), 28 of AIS B, 159 of AIS C and 90 of AIS D (the latter three classes had incomplete injuries). Finally, each patient was assessed in terms of whether the treatment involved surgery (decompression laminectomy and vertebral fixation; the surgery group) or not (the non-surgery group).
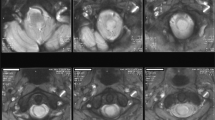
The extent of spinal canal involvement was evaluated by calculating the shortest anterior–posterior (A–P) diameter of the vertebral canal and by estimating the spinal reserve capacity on MRI scans taken at the time of trauma onset and at the time of diagnosis of syringomyelia. The percentage value of the shortest A–P diameter of the vertebral canal (A–P%) was calculated using the following formula proposed by Hashimoto et al.10 and Keene et al.11:
A–P% (at the level of skeletal injury)=(1−X/Y) × 100, where X represents the shortest A–P diameter on the mid-sagittal plane at the level of the vertebral injury, and Y represents the mean value of the longest A–P diameter measured in the mid-sagittal planes of the regions of the spinal canal proximal and distal to the injured vertebra (Figure 1).
Magnetic resonance image, used to measure the ratio of the narrowest antero-posterior diameter in the spinal canal (A–P%=[1-x/(a+b)/2] × 100), where x is the minimum diameter at the site of injury; a is the maximum diameter above the site of injury; and b is the maximum diameter below the site of injury.
The spinal reserve capacity was defined as the ratio of the area of spinal canal surrounded by the dura mater to the overall area measured on the horizontal plane of the damaged region of spinal cord, and was calculated using a formula proposed by Weisz et al.12:
Space reserve capacity (%)=C2/C1 × 100, where C1 represents the area of the spinal canal and C2 represent the area of the neural content (the spinal cord) (Figure 2).
An independent samples’ t-test was used to analyze associations between the development of syringomyelia and the factors investigated. Statistical analysis was performed using SPSS for Windows (Version 10.1; SPSS Inc., Chicago, IL, USA).
Results
The 502 study patients were of mean age 46.2 years, and were composed of 407 men and 95 women. In terms of injury location, 237 patients had cervical injuries and 265 had thoracic injuries. With regard to the severity of injury, 225 patients had complete injuries and 277 had incomplete injuries (Table 1).
Syringomyelia developed within 5 years after injury in 37 (7.3%) of the 502 patients. Only eight patients of 37 had symptomatic clinical presentations suggesting syringomyelia including pain of the hands, alteration of sensation or motor strength. Twenty (8.4%) of the 237 patients with cervical injury and 17 (6.4%) of the 265 patients with thoracic injury developed syringomyelia within 5 years after injury (P⩾0.05). Eighteen (8.0%) complete injury patients and 19 (6.9%) incomplete injury patients developed syringomyelia (P⩾0.05). The mean age of syringomyelia patients was 44.6 years (range, 17–67 years). The mean time of onset of syringomyelia following occurrence of the spinal cord injury was 38.8 months (range, 2–54 months) (Table 2). Fifteen (6.7%) of the 223 surgery patients and 22 (7.9%) of the 279 non-surgery patients developed syringomyelia (P⩾0.05).
In syringomyelia patients, the percentage value of the shortest A–P diameter of the spinal canal at the time of trauma onset (A–P %) was 38%, and the spinal reserve capacity was 71%. In non-syringomyelia patients, these values were 40 and 65%, respectively. The two groups did not differ in terms of these measures of spinal canal involvement (P⩾0.05).
The mean interval between spinal cord injury and diagnosis of syringomyelia was 38.8 months. A–P% and spinal reserve capacity were compared between syringomyelia and non-syringomyelia patients at that timepoint. At the time of injury and at the time of diagnosis of syringomyelia, mean A–P% and spinal reserve capacity were 25% and 62%, respectively, in patients with syringomyelia, and 24% and 58%, respectively, in patients without syringomyelia; the figures were thus similar in the two groups (P⩾0.05) (Figure 3)
Discussion
Although diagnosis of post-traumatic syringomyelia has been suggested to require the presence of a cystic cavity extending to more than two spinal segments, other authors have indicated that syringomyelia should be diagnosed on the basis of clinical and radiological findings, with no attention paid to cavity size.3 MRI has been found to be the most sensitive diagnostic imaging technique, and also yields information on the morphology of spinal cord cysts. On all imaging sequences, a spinal cord cyst should have a signal intensity equivalent to that of CSF. If necessary, gadolinium administration may help differentiate a cyst from a scar or tumor, because a post-traumatic cyst should not show nodular or mass-like enhancement.13
The time from spinal cord injury to the development of post-traumatic syringomyelia ranged widely in previous reports.5, 6 We assessed only those patients who developed early post-traumatic syringomyelia, thus within 5 years after spinal cord injury, because of difficulties in conducting long-term follow-up including regular MRI examinations in a general clinical setting. Post-traumatic syringomyelia has been reported to occur anywhere between 3 months and 34 years following spinal cord injury.3, 14 In the present study, we found that syringomyelia was diagnosed as early as within 2 months following spinal cord injury, and that the mean time for diagnosis was 38.8 months.
We found that the incidence of syringomyelia developing within 5 years after spinal cord injury was 7.3%. The incidence of clinical post-traumatic syringomyelia has been reported to range from 1.1–3.4%,3, 4, 5, 14 whereas the incidence of symptomatic syringomyelia has ranged from 1–9%.5, 13, 14 In contrast, autopsy and MRI studies have reported an incidence of approximately 22%.6
The present study examined whether the site of neurological injury was predictive of syringomyelia development. We found that the incidence of syringomyelia was similar in both cervical and thoracic spinal cord injury patients. Abel et al.15 and Klekamp et al.16 reported similar findings. However, other studies suggest that syringomyelia development may be associated with the injury site. Sgouros and Williams17 found that 78% of syringomyelia patients had a thoracolumbar spinal injury, whereas El Masry et al.5 and Perrouin-Verbe et al.9 concluded that cavities form more commonly in thoracic compared with cervical spinal cord injury patients. In contrast, Rossier et al.,14 Lyons et al.,18 and Vannemreddy et al.6 found that syringomyelia was more commonly associated with cervical spinal cord injury. Such observations are consistent with theories suggesting that the development of syringomyelia is associated with an increase in CSF pressure, because the mid-cervical spinal canal and the subarachnoid space are both relatively narrower than the thoracolumbar canal, as shown by Yarkony et al.19
We also investigated whether syringomyelia development correlated with the severity of neurological injury. We found that syringomyelia rates were similar in the complete and incomplete injury groups. However, other studies have concluded that the prevalence of syringomyelia in complete injury patients was approximately double of that found in incomplete injury patients.6, 14
The present study examined whether syringomyelia development differed when conservative or surgical treatment was given. We found that the incidence of syringomyelia was similar in both the surgery and non-surgery groups. In a study of post-traumatic spinal cord injury patients, Vannemreddy et al.6 reported that syringomyelia occurred earlier in patients who underwent fixation surgery or spinal instrumentation compared with non-surgery patients. In addition, syringomyelia occurred later in patients who underwent posterior laminectomy compared with those who underwent open reduction and internal fixation rather than laminectomy. Thus, local narrowing of the spinal canal may occur in patients who undergo open reduction and internal fixation without decompression surgery. Moreover, post-traumatic inflammatory responses in the separated soft tissue and bone may cause changes in the kinetics and dynamics of the CSF, accompanying fibrosis of the pia mater and arachnoiditis, in turn leading to the development of syringomyelia.8
The current study explored whether a link existed between the severity of spinal canal encroachment and the occurrence of post-traumatic syringomyelia. We found that the severity of encroachment at the time of spinal cord injury did not differ between syringomyelia and non-syringomyelia patients. Consistent with these findings, Wang et al.20 found no correlation between the severity of spinal stenosis and the occurrence of post-traumatic syringomyelia. However, others have suggested that an association may exist between the severity of spinal stenosis and syringomyelia. A study in 20 patients found a correlation between the development of post-traumatic syringomyelia and spinal cord compression accompanied by kyphosis and syringomyelia.3 Moreover, spinal canal compromise and formation of a scar in the dura mater and subarachnoid membrane at the site of injury were reported to be the probable key factors in the formation of a spinal cord cavity.7 Using an animal model, Cho et al.8 showed that trauma-induced adhesive arachnoiditis caused blockage of the subarachnoid space; this interfered with normal CSF circulation. In the test animals, syringomyelia was caused by CSF influx into the dorsal root entry point at the site of the injury, in turn attributable to an increased pressure within the subarachnoid space. Perrouin-Verbe et al.9 reported that development of post-traumatic spinal stenosis correlated with syringomyelia, and that syringomyelia was less frequent in patients with severe spinal stenosis who underwent laminectomy. It should be noted that a number of factors may affect correlations between spinal stenosis severity and syringomyelia; these include (for example) the type of surgery employed and the resultant anatomical changes.
The current study involved patients who were followed up for at least 5 years post-injury. However, other studies have shown that post-traumatic syringomyelia can develop as late as 34 years after spinal cord injury.5 This may explain why some of our present findings differ from those of long-term studies. In the present study, patients were classified simply into surgery and non-surgery groups, with no attention being paid to the surgical methods used. Also, neither patient age/gender nor MRI cord findings after injury was evaluated as a potential risk factor; this may be considered a limitation of our study. In addition, the validity of measurements of spinal canal compromise should be further evaluated. A future study should consider measurement bias in this regard.
Conclusions
The present study investigated whether a range of factors were predictive of syringomyelia development within 5 years following post-traumatic spinal cord injury. We found that 7.3% of patients developed syringomyelia within 5 years of injury. We also found that syringomyelia development was not associated with the site of neurological injury, the severity of the injury, a history of spinal surgery or the extent of spinal canal involvement. Therefore, it appears that such variables are of no predictive value in terms of syringomyelia development. Although the present findings are consistent with the reports of others, not all authors who work in the field are in agreement. Therefore, further meticulous long-term studies are required before the present findings may be considered clinically applicable.
Data archiving
There were no data to deposit.
References
Bastian HC . On a case of concussion-lesion with extensive secondary degeneration of the spinal cord. Proc Royal Med Chir Soc London 1867; 50: 499.
Strümpell A . Beitrage zur pathologie des ruckenmarks. Arch Psychiatry Nervenkr 1880; 10: 676–717.
Schurch B, Wichmann W, Rossier AB . Post-traumatic syringomyelia (cystic myelopathy): a prospective study of 449 patients with spinal cord injury. J Neurol Neurosurg Psychiatry 1996; 60: 61–67.
Umbach I, Heilporn A . Review article: post-spinal cord injury syringomyelia. Paraplegia 1991; 29: 219–221.
El Masry WS, Biyani A . Incidence, management, and outcome of post-traumatic syringomyelia. In mermory of Mr Bernard Williams. J Neurol Neurosurg Psychiatry 1996; 60: 141–146.
Vannemreddy SS, Rowed DW, Bharatwal N . Posttraumatic syringomyelia: predisposing factors. Br J Neurosurg 2002; 16: 276–286.
Williams B . Pathogenesis of posttraumatic syringomyelia. Br J Neurosurg 1992; 6: 517–520.
Cho KH, Iwasaki Y, Imamura H, Hida K, Abe H . Experimental model of posttraumatic syringomyelia: the role of adhesive arachnoiditis in syrinx formation. J Neurosurg 1994; 80: 133–139.
Perrouin-Verbe B, Lenne-Aurier K, Robert R, Auffray-Calvier E, Richard I, Mauduyt de la Grève I et al. Post-traumatic syringomyelia and posttraumatic spinal canal stenosis: a direct relationship: review of 75 patients with a spinal cord injury. Spinal Cord 1998; 36: 137–143.
Hashimoto T, Kaneda K, Abumi K . Relationship between traumatic spinal canal stenosis and neurologic deficits in thoracolumbar burst fractures. Spine 1988; 13: 1268–1272.
Keene JS, Fischer SP, Vanderby R, Drummond DS, Turski PA . Significance of acute posttraumatic bony encroachment of the neural canal. Spine 1989; 14: 799–802.
Weisz GM, Lee P . Spinal canal stenosis. Concept of spinal reserve capacity: radiologic measurements and clinical applications. Clin Orthop Relat Res 1983; 179: 134–140.
Schwartz ED, Falcone SF, Quencer RM, Green BA . Posttraumatic syringomyelia: pathogenesis, imaging, and treatment. AJR Am J Roentgenol 1999; 173: 487–492.
Rossier AB, Foo D, Shillito J, Dyro FM . Posttraumatic cervical syringomyelia. Incidence, clinical presentation, electrophysiological studies, syrinx protein and results of conservative and operative treatment. Brain 1985; 108: 439–461.
Abel R, Gerner HJ, Smit C, Meiners T . Residual deformity of the spinal canal in patients with traumatic paraplegia and secondary changes of the spinal cord. Spinal Cord 1999; 37: 14–19.
Klekamp J, Samii M . Syringomyelia: Diagnosis and Management 1st edn. Springer Berlin. 2002, 1–7.
Sgouros S, Williams B . A critical appraisal of drainage in syringomyelia. J Neurosurg 1995; 82: 1–10.
Lyons BM, Brown DJ, Calvert JM, Woodward JM, Wriedt CH . The diagnosis and management of posttraumatic syringomyelia. Paraplegia 1987; 25: 340–350.
Yarkony GM, Sheffler LR, Smith J, Chen D, Rayner SL . Early onset posttraumatic cystic myelopathy complicating spinal cord injury. Arch Phys Med Rehabil 1994; 75: 102–105.
Wang D, Bodley R, Sett P, Gardner B, Frankel H . A clinical magnetic resonance imaging study of the traumatised spinal cord more than 20 years following injury. Paraplegia 1996; 34: 65–81.
Acknowledgements
This study was partly supported for two years by Pusan National University Research Grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Ko, HY., Kim, W., Kim, S. et al. Factors associated with early onset post-traumatic syringomyelia. Spinal Cord 50, 695–698 (2012). https://doi.org/10.1038/sc.2012.35
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sc.2012.35
Keywords
This article is cited by
-
Post-traumatic syringomyelia resolution following surgical treatment: the moniliform syrinx with a better prognosis
Acta Neurologica Belgica (2023)
-
Silent post-traumatic syringomyelia and syringobulbia
Spinal Cord Series and Cases (2020)
-
The characteristics of posttraumatic syringomyelia
Spinal Cord (2016)