Abstract
Study design:
Retrospective study.
Objective:
To investigate the progression of spinal tract lesions in cervical spondylotic myelopathy (CSM) at C3-4 intervertebral level using spinal cord-evoked potensials (SCEPs).
Setting:
This study was conducted at the Department of Orthopaedic Surgery, Yamaguchi University Graduate School of Medicine, Japan.
Methods:
A total of 30 patients with CSM were investigated in this study. In all patients, only the C3-4 intervertebral level was symptomatic, as shown by examination of SCEPs. SCEPs were recorded following median nerve stimulation (MN-SCEPs), transcranial electric stimulation (TES-SCEPs) and spinal cord stimulation (spinal-SCEPs).
Results:
The incidence of abnormalities varied in the order of MN-SCEPs (100%), TES-SCEPs (90%) and spinal-SCEPs (67%). Patients were grouped into three types according to SCEPs: transverse (all SCEPs abnormal), post-erolateral (abnormalities in the MN-SCEPs and TES-SCEPs) and upper limbs sensory (abnormal only for MN-SCEPs). In all, 20 of the 30 patients (67%) were the transverse type, 7 (23%) the post-erolateral type and 3 (10%) the upper limbs sensory type.
Conclusion:
The present study showed the lateral part of the posterior funiculus mediating upper limb sensory function was more vulnerable than the lateral corticospinal tract, which is consistent with numbness tending to appear at an early stage of mild CSM.
Similar content being viewed by others
Introduction
Several reports have described the pathology of cervical spondylotic myelopathy (CSM) from autopsy specimens.1, 2 Most of these were obtained from patients with typical spastic paralysis, and involvement of the lateral corticospinal tract was a common finding. Ito3 reported the progression pattern and histological findings of the lesions in the spinal cord affected by CSM. Atrophy and neuronal loss in the anterior horn and intermediate zone developed first, followed by degeneration of the lateral and posterior funiculus. However, it is not clear which spinal tract is most vulnerable in CSM or how the spinal tract lesions progress from a mild to severe stage. Finger numbness is the most common initial symptom of most patients with mild stage CSM.4 However, the pathophysiology underlying this finger numbness is presently unclear. Spinal cord-evoked potensials (SCEPs) are useful for evaluating the functional integrity of spinal tracts.5 We have evaluated electrophysiologically the functions of three spinal tracts and reported in this study our investigation on the correlation between progression of spinal tract lesions in CSM at the C3-4 intervertebral level estimated by multi-modal SCEPs and neurological findings.
Materials and methods
A retrospective study of 122 CSM patients who underwent laminoplasty as well as SCEPs studies between April 1997 and July 2008 was conducted. Written informed consent with the approval of the Human Experimentation Ethics Committee of Yamaguchi University Graduate School of medicine was obtained for preoperative magnetic resonance imaging (MRI) investigation and electrophysiological studies in all patients, and those who fulfilled the following criteria were included in this study.
A diagnosis of myelopathy was established based on the presence of hyperreflexia including positive Hoffmann sign and upper extremity sensory disturbance as well as obvious MRI-documented cervical spinal cord compression. Sensory and motor nerve conduction velocities in peripheral nerves were within normal limits. We excluded the patients with myelopathy from other causes such as compression from the ossified posterior longitudinal ligament, disc herniation and trauma and those with radiculomyelopathy.
Of the 122 patients, 30 patients (15 men) selected on the basis of SCEPs abnormalities at the C3-4 intervertebral level were analysed. Their mean age was 71.8 years (range 47–87 years). The mean length of clinical history before admission was 16.5 months (range 2 months–16 years). The mean duration of postoperative follow-up was 1 year and 5 months (range 6 months–7 years and 6 months). The clinical symptoms and results of neurological examination are summarised in Table 1. The area of numbness was determined by asking patients the part of the upper extremity in which they felt numbness according to the dermatome proposed by Brain.6 Radiographic examinations included plain radiography and preoperative MRI. In all patients treated using laminoplasty, five intervertebral levels (C3-4 to C7-T1) were always decompressed according to the Hattori’s method.7
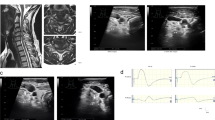
Abnormal SCEPs findings at the C3-4 intervertebral level were associated with damage of the long tract. SCEPs following median nerve stimulation (MN-SCEPs), transcranial electric stimulation (TES-SCEPs) and spinal cord stimulation (spinal-SCEPs) were recorded intraoperatively. The MN-SCEPs at the C3-4 intervertebral level are mediated by the lateral parts in the posterior columns (Burdach tract), TES-SCEPs by the lateral corticospinal tract and the N2 components of spinal-SCEPs by the medial parts in the posterior columns (Goll tract). The median nerves were stimulated (square wave pulse, 0.2 ms duration, 3-Hz rate) at the wrist. The stimulus intensity was set at 1.5 times that required to produce a thumb twitch in the awakened condition. TES was delivered as square pulses of 0.2 ms duration and at an intensity of 100 mA through needle electrodes (13R25, Dantec, Denmark) placed on the skull. The anode was placed 7 cm laterally to the right of the Cz position (10–20 International System) on a line joining the external auditory meatus. The cathode was placed on the opposite side. spinal-SCEP was delivered by an epidural catheter electrode inserted into the dorsal epidural space from the C7-T1 and T11-12 interlaminar space. Square wave pulses (0.2 ms duration, 3-Hz rate) were delivered at an intensity of 15–20 mA. Before laminoplasty, all SCEPs were recorded intraoperatively with recording electrodes (13R25) inserted in the ligamentum flavum at each interlaminar space. A reference electrode was inserted into the subcutaneous tissue in the posterior aspect of the neck for recording of MN-SCEPs and spinal-SCEPs. A bipolar recording method was used (active proximal and reference distal) for the recording of TES-SCEPs.
All SCEP signals were amplified and filtered with a bandpass of 20–3000 Hz using a standard evoked potential/electromyography instrument (Nicolet Viking, Nicolet Biomedical USA, Middleton, WI, USA). Averages from 100 to 200 MN-SCEP, 40–60 TES-SCEP and 20–30 spinal-SCEP responses were obtained. Two different averaged responses were superimposed and displayed.
In MN-SCEPs, abnormality was determined from the amplitude ratio of spinal responses at each intervertebral level to that recorded at the C6-7 intervertebral level as reported earlier.8 The lower limits of the amplitude ratio were 0.4 for C3-4 intervertebral level. In TES-SCEPs and spinal-SCEPs, intervertebral levels with a marked reduction in size of the negative peak (reduction of >50%) were considered as significant.9, 10
SCEPs findings were compared with clinical symptoms and signs. It was reported that weakness of shoulder abduction, hyperreflexia of biceps tendon reflex (BTR) and the area of numbness were useful factors related to longitudinal level diagnosis in CSM at the C3-4 intervertebral level.11, 12
Statistical analysis was performed using the Mann–Whitney U test. P<0.05 was considered statistically significant.
Results
As shown in Table 2, the incidence of abnormalities varied in the order of MN-SCEPs (100%), TES-SCEPs (90%) and spinal-SCEPs (67%). The patients were grouped into three types according to SCEPs results. All SCEPs were abnormal in the transverse type (TT) (Figure 1), abnormalities in MN-SCEPs and TES-SCEPs were observed in the post-erolateral type (PT) (Figure 2), while only MN-SCEPs were abnormal in the upper limbs sensory type (UT) (Figure 3). In all, 20 of 30 patients (67%) were classified as the TT, 7 (23%) as the PT and 3 (10%) as the UT (Table 3). Hyperreflexia of BTR was correlated with involvement of the lateral corticospinal tract. A total of 21 of 30 patients (70%) showed hyperreflexia of BTR and this was observed in 78% (21/27) of the TT and PT but in none of the three UT. Between two types with and without involvement of the lateral corticospinal tract, the incidence of hyperreflexia of BTR showed significant difference (P<0.03).
In all, 17 of 30 patients (57%) showed weakness of shoulder abduction and this feature was again restricted to patients with the TT and PT. Hyperreflexia of BTR and weakness of shoulder abduction were closely correlated with involvement of the lateral corticospinal tract. All patients felt numbness in all fingers, but only one patient had numbness around the shoulder of the C5 segmental region. Preoperative sagittal T2-weighted MRI showed intramedullary, high signal intensity changes at the C3-4 level in 17 of 30 (57%) patients. High-signal intensity areas were observed in 55% (11/20) of the TT, 57% (4/7) of the PT and 66% (2/3) of the UT. Preoperative axial T2-weighted MRI revealed that small symmetric intramedullary high-signal intensity areas, a so-called snake eyes appearance were observed in 35% (7/20) of the TT. The presence or absence of high-intensity areas on MRI did not correlate with the severity of myelopathy before surgery (P>0.05). For clinical assessment, the Japanese Orthopaedic Association (JOA) scoring system for cervical myelopathy was employed (Table 4).13, 14 The JOA scores were 7.7 points before surgery (UT: 13points, PT: 9.8 points, TT: 6.2 points) and 11.2 points at the final follow-up (UT: 14.8 points, PT: 13.2 points, TT: 10.1 points).
Between UT and PT, preoperative JOA score revealed significant difference (P<0.04). Between UT and TT, preoperative and postoperative JOA score revealed significant difference (P<0.006 and P<0.007, respectively). Between PT and TT, preoperative and postoperative JOA score revealed significant difference (P<0.004 and P<0.008, respectively). Between UT and PT, postperative JOA score revealed no significant difference (P>0.05).
Discussion
Kokubun and Hirabayashi11, 12 described neurogenic findings related to longitudinal level diagnosis of CSM at the C3-4 level (Table 5). Clinical symptoms were different according to the degree of spinal cord compression. The progression of spinal tract lesions was investigated in this study using SCEPs. We could not, however, describe the involvement of anterior funiculus and spinothalamicus tracts in this study. Kameyama15 reported that anterior spinal tracts with severe CSM showed almost no pathological features.
The UT was observed in mild CSM at early stages. The mean preoperative symptom duration was 16.5 months (the UT: 6 months, the PT: 9.4 months, the TT: 19.4 months). Duration of symptoms and preoperative neurological function may commonly affect surgical outcome. These results demonstrate that the most frequent initial symptom in CSM is finger numbness.4 All three patients with the UT had finger numbness and clumsiness of hands. Concomitant abnormality of MN-SCEPs correlated well with these symptoms. However, it is still uncertain whether the cause of numbness is involvement of the posterior horn or posterior funiculus.16 If the cause of numbness is involvement of the posterior horn, most patients with CSM at the C3-4 level would be expected to show numbness at the C5 dermatome. However, this was observed in only one of 30 patients (3%). All 30 patients showed numbness in all five fingers. Involvement of the posterior horn cannot explain the cause of numbness. Finger numbness could be associated with involvement of the lateral parts of the posterior funiculus. Chang17 reported that high cervical cord compression might produce dysfunction of the dorsal column caudal to the direct compressive sites, and the funiculus cuneatus of C6-8 cord was most affected in high cervical myelopathy. Median nerve is formed by the union of the lower cervical nerve roots (C6-C8). MN-SCEPs at the C4-5, C5-6 and C6-7 intervertebral levels include synapse-dependent component. However, MN-SCEPs at the C3-4 intervertebral level are synapse-independent probably originating from the lateral parts of the posterior funiculus (C6-8), which could explain that all patients felt numbness in all fingers (C6, C7 and C8 segmental regions)
Ogino18 reported clinicopathological correlations with neurological findings in CSM at the terminal stage. They described that the lateral corticospinal tracts were more vulnerable than the posterior funiculus. Ito3 reported a common pattern of lesion progression in CSM that involved initial atrophy and neuronal loss in the anterior horn and intermediate zone, followed by degeneration of the lateral and posterior funiculus. Marked atrophy eventually developed throughout the entire grey matter and severe degeneration occurred in the lateral funiculus. Patients in these reports showed moderate to severe CSM, however, those grouped into the UT were mild CSM. The current results are therefore different to these previous pathological findings.
Mizuno19 reported that patients with single-level snake eyes appearance had significant upper-limb motor weakness. Damage of the anterior horn will cause muscle weakness in restricted region of upper extremities. In all, 35% (7/20) of the TT with snake eyes appearance revealed muscle weakness on shoulder abduction.
Hyperreflexia of BTR was frequently seen and was observed in 78% of patients with involvement of lateral corticospinal tracts. Mild involvement of lateral corticospinal tracts was associated with normal BTR. Weakness of shoulder abduction was observed in 63% of patients with involvement of lateral corticospinal tracts. We considered that involvement of long tracts in CSM occurred initially in the lateral parts of the posterior funiculus (abnormalities in MN-SCEPs only), followed by the lateral corticospinal tract (abnormalities in the TES-SCEPs) and eventually the medial parts of the posterior funiculus (abnormalities in spinal-SCEPs).
For further work, we will analyse patients selected on the basis of SCEPs abnormalities at the C4-5 intervertebral level. MN-SCEPs recorded at the C4-5 intervertebral level are post-synaptic potentials. Posterior horns are estimated by MN-SCEPs recorded at the C4-5 intervertebral level. In addition, SCEPs following ulnar nerve stimulation (UN-SCEPs) are recorded intraoperatively. The UN-SCEPs at the C4-5 intervertebral level are mediated by the lateral parts of the posterior columns. We will evaluate electrophysiologically the functions of three spinal tracts and posterior horns at the C4-5 intervertebral level.
Conclusions
We have presented 30 patients with CSM at the C3-4 intervertebral level determined by SCEPs findings. Although ivolvement of the anterior spinal tract was unknown, this study showed the lateral parts of the posterior funiculus mediating upper limb sensory function was more vulnerable than the lateral corticospinal tract. We confirmed that the lateral parts of the posterior funiculus were most vulnerable in three spinal tracts in this study.
Finger numbness in mild, early stage CSM was associated with this pathology.
References
Kameyama T, Ando T, Yanagi T, Hasizume Y . Neuroimaging and pathology of the spinal cord in compressive cervical myelopathy. Rinsho Byori 1995; 43: 886–890.
Hashizume T . Pathology of spinal cord damage due to cervical spondylosis. Igaku no ayumi 2008; 226: 1127–1130.
Ito T, Oyanagi K, Takahashi H, Takahashi H, Ikuta F . Cervical spondylotic myelopathy, clinicophathologic study on the progression pattern and thin myelinated fibers of the lesions of seven patients examined during complete autopsy. Spine 1996; 21: 827–833.
Kokubun S, Sati T, Ishii Y, Tanaka Y . Cervical myelopathy in the Japanese. Clin Orthop 1996; 323: 129–138.
Tsuyama N, Tsuzuki N, Kurokawa T, Imai T . Clinical application of spinal cord action potentials measurement. Int Orthop 1978; 2: 39–46.
Brain L, Walton J . Brain’s Disease of the Nervous System, 7th ed Oxford University Press: London, 1969, 40–43.
Kawai S, Sunago K, Doi K, Saika M, Taguchi T . Cervical laminoplasty(Hattori’s method). Procedure and follow-up results. Spine 1988; 13: 1245–1250.
Kaneko K, Kawai S, Taguchi T, Fuchigami Y, Ito T, Morita H . Correlation between spinal cord compression and abnormal patterns of median nerve somatosensory evoked potentials in compressive cervical myelopathy: comparison of surface and epidurally recorded responses. J neurol Sci 1998; 158: 193–202.
Kanchiku T, Taguchi T, Kaneko K, Fuchigami Y, Yonemura H, Kwai S . A correlation between magnetic resonance imaging and electrophysiological findings in cervical spondylotic myelopathy. Spine 2001; 26: E294–E299.
Tani T, Ishida K, Ushida T, Yamamoto H . Intraoperative electroneurography in the assessment of the level of operation for cervical spondylotic myelopathy in elderly. J Bone Joint Surg Br. 2000; 82: 269–274.
Kokubun S . Neurological localization of the symptomatic level of lesion in cervical spondylotic myelopathy. Rinsho Seikeigeka 1984; 19: 417–424.
Hirabayashi K, Satomi K, Wakano K . Level diagnosis neurology of cervical spondylotic myelopathy-retrospective observation in cases treated by anterior spinal fusion at a single level. Rinsho Seikei Geka. 1984; 19: 409–415.
Yonenobu K, Abumi K, Nagata K, Taketomi E, Ueyama K . Interobserver and intraobserver reliability of the Japanese orthopaedic association scoring system for evaluation of cervical compression myelopathy. Spine 2001; 26: 1890–1895.
Holly LT, Matz PG, Anderson PA, Groff MW, Heary RF, Kaiser MG et al. Functional outcomes assessment for cervical degenerative disease. J. Neurosurg Spine 2009; 11: 238–244.
Kameyama T, Hashizume Y . Pathology of cervical spondylotic myelopathy. Shinkeinaika 2001; 55: 335–345.
Yamazaki Y . Relationship between the dysethesic digits and the level of disc protrusion. Spine spinal cord 2000; 13: 841–846.
Chang MH, Liao KK, Cheung SC, Kong KW, Chng SP . Clumsy hands’ and tactile agnosia secondary to high cervical spondylotic myelopathy: a clinical and electrophysiological correlation. Acta Neurol Scand 1992; 86: 622–625.
Ogino H, Tada K, Okada K, Yonenobu K, Yamamoto T, Ono K et al. Canal diameter, anteroposterior compression ratio, and spondylotic myelopathy of the cervical spine. Spine 1983; 8: 1–15.
Mizuno J, Nakagawa H, Inoue T, Hashizume Y . Clinicopathological study of ‘snake eye appearance’ in compressive myelopathy of the cervical spinal cord. J Neurosurg (Spine) 2003; 99: 162–168.
Acknowledgements
The authors sincerely dedicate this study to the late Dr Kazuo Kaneko who originally devised this pathology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Imajo, Y., Kato, Y., Yonemura, H. et al. Relative vulnerability of various spinal tracts in C3-4 cervical spondylotic myelopathy: multi-modal spinal cord evoked potentials. Spinal Cord 49, 1128–1133 (2011). https://doi.org/10.1038/sc.2011.68
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sc.2011.68
Keywords
This article is cited by
-
Resting-state functional magnetic resonance imaging indices are related to electrophysiological dysfunction in degenerative cervical myelopathy
Scientific Reports (2024)
-
Characteristics of C6–7 myelopathy: assessment of clinical symptoms and electrophysiological findings
Spinal Cord (2016)
-
Preoperative diagnosis of the responsible level in CCM using CMAPs: comparison with SCEPs
Spinal Cord (2014)