Abstract
Study design:
Case report.
Objective:
To describe the clinical benefit of a spinal cordectomy with the aim of limiting neurological deterioration related to the development of a subacute posttraumatic ascending myelopathy (SPAM) supporting previously described mechanism for SPAM formation.
Setting:
National Spinal Injuries Centre, Stoke Mandeville Hospital, UK.
Method and results:
A 38-year old patient presented 6 months after spinal cord injury substantial neurological deterioration expanding from the initial T4-injury level through C4. Magnetic resonance imaging revealed intra-medullary haemorrhage at the site of injury and subsequent-ascending cord oedema. A cordectomy was performed leading to neurological stabilisation and complete resolution of SPAM.
Conclusion:
Cordectomy can be an effective intervention in case of rapid progressive neurological deterioration.
Similar content being viewed by others
Introduction
Delayed neurological deterioration following established traumatic spinal cord injury is rare but increasingly recognised. It has acquired different descriptive terms including progressive posttraumatic myelomalacic myelopathy, presyrinx state1 and subacute posttraumatic ascending myelopathy (SPAM). These conditions describe a complication where a neurological deficit may progress usually weeks or months after the original spinal cord insult. A common feature involves cord and function changes four or more segments above the initial level of injury some time after their initial assessment.
The condition was initially described in 1969 by Frankel2 but the exact mechanism of deterioration is still unknown. Several hypotheses have been postulated including secondary injury, venous thrombosis, congestive ischaemia, infection, apoptosis, obstruction of the cerebrospinal fluid (CSF) pathways and impaired venous drainage of the cord.
The prognosis is variable but may be neurologically devastating or even fatal if the level of cord involved ascends to the brainstem.
A number of different treatments have been employed in treating this condition including decompression, anticoagulation, steroids and osmolar therapy with no benefit. Duroplasty and cord untethering have been shown to be effective.3
In this article we report magnetic resonance imaging (MRI) appearances before and after spinal cordectomy as a last resort to manage ascending neurological deterioration, yielding a favourable outcome leading to the complete resolution of SPAM which we suggest supports a pathogenesis based on altered CSF dynamics within the cord suggested previously by other authors.1
Case report
A 38-year old male involved in a motorcycle accident sustained a thoracic spine fracture-dislocation at the T4/T5 level. The patient was admitted 19 days post injury and his neurological examination confirmed T4 American Spinal Cord Injury grade A paraplegia with motor score of 50, light touch score of 48 and pin prick score of 45.
The spinal fracture-dislocation was fixed 4 days after injury. Pedicle screws were placed two levels above (T3/T4) and two levels below (T6/T7) the decompressive laminectomy done at T5 with resulting good alignment and spinal canal clearance.
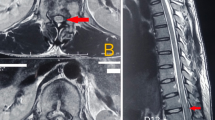
Initial MRI scan demonstrated cord oedema extending from T1/2 disc level to the T7 vertebral body level (Figure 1a). Small focal low-signal-intensity spots on the axial and sagittal T2-weighted fast spin echo scans indicated likely foci of intra-medullary microhaemorrhage both at the T4/5 injury level itself and proximally within the central canal at T1/2 (Figures 1b and c).
(a) Sagittal T2-weighted fast spin echo (FSE) scan at 1 day post injury demonstrates cord oedema extending from the T1/2 disc level to the T7 vertebral body level around the T4/5 dislocation level. Small low signal foci at the T4/5 injury level are in keeping with areas of intra-medullary microhaemorrhage (arrow). (b) Axial T2-weighted FSE scan at the T4/5 injury level demonstrates focal areas of intra-medullary low signal in keeping with microhaemorrhage (arrows).
A follow-up MRI was performed 23 days after the initial injury demonstrating progression of the previously noted fluid signal within the cord, which now extended from C4 vertebral body level to T7 with some mild cord expansion (Figure 2). The patient did not demonstrate neurological deterioration and was scheduled for outpatient follow-up and repeat scan.
(a) Sagittal T2-weighted fast spin echo (FSE) scan of the cervicothoracic levels at 6 months post injury demonstrates further extension of the abnormal signal to the level of the cervicomedullary junction. There is mild cord expansion. (b) Sagittal T2-weighted FSE scan of the thoracolumbar levels demonstrates further extension of the abnormal signal to the level of the conus. There is mild cord expansion. (c) Axial T2-weighted FSE scan at T9/10 level demonstrates fluid signal within the central cord. There is associated mild cord expansion.
3 months after injury the patient complained of weakness affecting his left hand. There was grade four power in the C8/T1 myotomes. There were no sensory changes. At subsequent follow-up, 6 months post injury, further neurological deterioration in the cervical myotomes involving the right side at C8/T1 levels was noted. There was sensory impairment to pinprick sensation up to the C4 dermatome. Reflexes in the upper limb were absent. The MRI demonstrated further progression of the abnormal cord signal which now extended from the cervicomedullary junction to the conus. There was mild cord expansion and central increased T2 cord signal in keeping with developing myelitis (Figure 2).
In view of these imaging findings and the patient's deteriorating neurological status, a T3 thoracic spine laminectomy and T4 level cordectomy with untethering of the cord was performed.
At the level corresponding to T3 and T4 there was cyst formation, which was opened and drained. The spinal cord was found to be adherent to the dural sac anteriorly. The spinal cord was then transected at T5 level. A feeding catheter was inserted into the collapsed cyst but it was not possible to pass the catheter rostrally into the central spinal canal beyond the cyst. These findings were in keeping with initial imaging appearance of ascending myelitis or SPAM.
The cord was further transected at T3/T4 intervertebral disc level and completely untethered. The dura was closed and T4/T6 pedicle screw fixation was performed to stabilise the segment.
Cord histology revealed a loss of myelin with vacuolation of the remaining myelin. There was no parenchymal B or T-cells cord inflammatory change but there was perivascular cuffing by foamy macrophages within the cord parenchyma and signs of mild T-cell inflammation and fibrosis around the nerve roots.
After 4 weeks of surgery neurological examination revealed normal power in all myotomes, normal reflexes in both upper limbs and residual impaired sensation to pinprick up to the C4 dermatome.
An MRI scan performed 6 months after the cordectomy demonstrated complete resolution of the abnormal cervical and mid/lower thoracic cord signal with the cord now appearing normal in signal and size above and below the cordectomy level (Figure 3). The patient's neurology has been unchanged at 6 and 12 month follow-up.
(a) Sagittal T2-weighted fast spin echo (FSE) 6 months following cordectomy procedure demonstrates resolution of the previously noted abnormal cervical and upper thoracic cord signal. (b) The cordectomy defect is noted as a fluid signal gap at the T4/T5 level. (c) Axial T2-weighted FSE at the T1/2 disc demonstrates resolution of the previously noted central cord signal change. (d) Axial T2 FSE at the T7/8 disc level demonstrates resolution of the previously noted central cord signal change.
Discussion
Subacute neurological deterioration following traumatic cord injury was originally described by Frankel in 1969 who estimated that the condition affected ∼1% of patients.2 Lee et al.3, 4 described 40 cases with neurological deterioration where all patients had dural tethering and made a good response following untethering and duraplasty.They postulated a multifactorial cause to the condition including altered CSF dynamics. Fischbein et al.1 described a presyrinx state and hypothesised that obstructed CSF flow leads to prolonged parenchymal T2 and that the condition was reversible with intervention.
A number of other case reports and small series have since been reported where the authors describe similar progressive deterioration in neurological level of patients, usually but not always with subsequent improvement. Suppositions include second injury, arterial or venous infarction, fibrocartilage embolisation, infection, apoptosis, autoimmune injury and cord oedema secondary to altered CSF drainage.
The case described demonstrates intra-medullary haemorrhage at the site of injury and subsequent ascending cord oedema. It is reasonable to postulate that debris following injury may occlude the spinal cord canal drainage. This will not cause imbalance in interstitial cord fluid if the arachnoid and pia mater allow alternative CSF pathways communicating with the subarachnoid space. If significant adhesion ensues and the spinal canal is blocked, then interstitial fluid builds in the cord interstitium and cord oedema ensues in conditions described as progressive posttraumatic myelomalacic myelopathy, presyrinx or SPAM. The delayed presentation of these conditions probably reflects time for tethering of the arachnoid to develop and cause imbalance in CSF drainage.
Described interventions include dural untethering1 and cordectomy.3 Both of these interventions would allow drainage of interstitial fluid into the epidural space if the central spinal canal is not patent. We suggest that the improvement on MRI of the cord appearances were because of this mechanism.
We suggest that our observations of microhaemorrhage in the cord canal and the response to cordectomy supports the proposal of Lee3 and Fischbein1 that SPAM is secondary to altered CSF drainage. When neurology progresses rapidly, surgical intervention with untethering or cordectomy should be considered.
References
Fischbein NJ, Dillon WP, Cobbs C, Weinstein PR . The “presyrinx” state: a reversible myelopathic condition that may precede syringomyelia. AJNR Am J Neuroradiol 1999; 20: 7–20.
Frankel HL . Ascending cord lesion in the early stages following spinal injury. Paraplegia 1969; 7: 111–118.
Lee TT, Arias JM, Andrus HL, Quencer RM, Falcone SF, Green BA . Progressive posttraumatic myelomalacic myelopathy: treatment with untethering and expansive duraplasty. J.Neurosurg 1997; 86: 624–628.
Stoodley MA, Jones NR, Yang L, Brown CJ . Mechanisms underlying the formation and enlargement of noncommunicating syringomyelia: experimental studies. Neurosurg Focus 2000; 8: E2.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Meagher, T., Belci, M., López de Heredia, L. et al. Resolution of SPAM following cordectomy: implications for understanding pathophysiology. Spinal Cord 50, 638–640 (2012). https://doi.org/10.1038/sc.2011.133
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sc.2011.133
Keywords
This article is cited by
-
A rare cause of neurological deterioration to complete paraplegia after surgery for thoracic myelopathy: a case report
Spinal Cord Series and Cases (2019)
-
Subacute posttraumatic ascending myelopathy: a literature review
Spinal Cord (2017)
-
Subacute post-traumatic ascending myelopathy after T12 burst fracture in a 32-year-old male: case report and surgical result of cervical durotomy
Spinal Cord Series and Cases (2016)
-
Subacute delayed ascending myelopathy: not just a post-traumatic disorder
Spinal Cord (2014)