Abstract
Study design:
Cross-sectional descriptive analysis of magnetic resonance imaging (MRI) and clinical outcome.
Objectives:
The aim of this study was to present anatomically consistent and independent spinal cord atrophy measures based on standard MRI material and analyze their specific relations to sensory and motor outcome in individuals with chronic incomplete spinal cord injury (SCI).
Setting:
Danish study on human SCI.
Methods:
We included 19 individuals with chronic incomplete SCI and 16 healthy controls. Participants underwent MRI and a neurological examination including sensory testing for light touch and pinprick, and muscle strength. Antero–posterior width (APW), left–right width (LRW) and cross-sectional spinal cord area (SCA) were extracted from MRI at the spinal level of C2. The angular variation of the spinal cord radius over the full circle was also extracted and compared with the clinical scores.
Results:
The motor score was correlated to LRW and the sensory scores were correlated to APW. The scores correlated also well with decreases in spinal cord radius in oblique angles in coherent and non-overlapping sectors for the sensory and motor qualities respectively.
Conclusion:
APW and LRW can be used to assess sensory and motor function independently. The finding is corresponding well with the respective locations of the main sensory and motor pathways.
Similar content being viewed by others
Introduction
Magnetic resonance imaging (MRI) of the spinal cord has become a routine tool for evaluation of the site and extent of spinal cord injury (SCI) but conventional imaging seems to have little prognostic value and it is not possible to extract parameters from the images that are well correlated to specific functional deficits.1, 2 More sensitive techniques could potentially have an important role for guiding and individualizing the rehabilitation and treatment of individuals with SCI. Diffusion tensor imaging and magnetization transfer have been suggested as MRI modalities for detection of lesions and evaluation of white matter integrity.3, 4 However, because of the diversity of lesions and demanding imaging conditions around the spinal cord, no clear quantitative correlations between lesion and clinical deficits have been reported using those techniques.
Another approach is to estimate the reduction in spinal cord width, area or volume, that is, atrophy, after lesion from conventional structural MRI. Spinal atrophy has been observed in groups of individuals with pathologies such as autosomal dominant leukodystrophy, amyotrophic lateral sclerosis and hereditary paraplegia.5, 6, 7 Good correlation between spinal cord area (SCA) and a number of global clinical scores has also been found in several studies of patients with multiple sclerosis in cross-sectional as well as longitudinal settings.8
It is not known whether a similar quantitative relation between spinal cord atrophy and the extent of disability is found in individuals with SCI. It is similarly not known whether a more clinically relevant and detailed information of specific spinal pathways affected by atrophy may be obtained by measuring decrease in spinal cord width in different directions. Sensory pathways are dominantly located in the posterior and anterior parts of the spinal cord white matter, whereas motor pathways are dominantly located more laterally.9, 10
Our hypothesis was that atrophy in the antero–posterior parts would be well correlated to sensory deficits, whereas atrophy in the left–right parts would be better correlated to motor deficits (see Figure 1a). Our aim was to introduce robust, sensitive and specific parameters to evaluate the functional state of individuals with SCI.
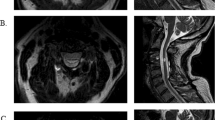
(a) Schematic illustration of some main spinal pathways. Motor tracts are mainly located in the lateral funiculi whereas the sensory tracts are found in the dorsal funiculus. It is hypothesized that atrophic changes in different directions of the spinal cord is correlated to specific clinical deficits. (b) A standard clinical T1 weighted magnetization prepared rapid gradient echo (MPRAGE) MRI is used for the analysis, a plane perpendicular to the spinal cord is defined at the upper level of the C2 process (arrow). (c) Resliced pseudo-axial volume at C2. Spinal cord (SC, solid) and the cerebrospinal fluid (CSF, dashed) are approximately outlined. The spinal cord boundary intensity is set to the mean of the CSF and SC regions. (d) SCA, LRW, APW and R(α) are extracted from the mask.
To test our hypothesis we used the method introduced by Losseff et al.11 to segment the cross-sectional shape of the spinal cord. We calculated the area and widths of the spinal cord in the anterior–posterior and left–right directions in individuals with SCI and correlated it with preserved sensory function and muscle strength in the upper and lower extremities. Furthermore, the angular variation in spinal cord radius was extracted and the functional correlations to atrophy in oblique directions were also analyzed.
Materials and methods
Participants
We enrolled 16 healthy volunteers with no known history of neurological disorders (mean (s.d.) 39 (14) years, gender: 1 female/15 males) and 19 individuals with chronic SCI (mean (s.d.) 46 (12) years, gender: 1 female/18 males) including lesions on cervical (n=15), thoracic (n=3) and lumbar (n=1) spinal cord levels. Only patients with clinically incomplete lesions and regained or partly regained locomotion were included in the study corresponding to ASIA (American Spinal Injury Association) Impairment Scale D.12 One participant was categorized as ASIA Impairment Scale A because of the lack of sacral sparring but could walk and was in all other aspects an ASIA Impairment Scale D. The etiology of the spinal cord lesion was non-traumatic in 3 (syringomyelia and angioblastorm, transverse myelitis, and meningites) and traumatic in 16 (traffic related in 6, sports related in 6 and falls in 4). Time from injury spanned from 1 to 28 years (mean 13 years). We certify that all applicable institutional and governmental regulations regarding the ethical use of human volunteers were followed during the course of this research.
Clinical investigation
The individuals with SCI were neurologically examined in accordance with the International Standards for Neurological Classification of SCI.12 This includes sensory testing of all segments from C2 to S4–5 on the right and left side for light touch (LT) and pinprick (PP), with scores of 0 (absent sensibility), 1 (impaired sensibility) and 2 (normal sensibility). A total sensory score was calculated for LT (LTSS) and PP (PPSS) with maximum values of 112 when all segments are normal. A motor score was calculated on the basis of muscle strength testing of five key muscle functions in each upper and lower extremity respectively on the 0–5 Medical Research Council scale, that is, a maximum score of 5 × 5 × 4=100.
MRI protocol
The images were acquired on a Siemens Trio 3 tesla system using a 1 channel birdcage head coil (Siemens, Erlangen, Germany). A T1 weighted magnetization prepared rapid gradient echo sequence (TR=1540 ms, TE=3.93 ms, flip angle 9°, sagital image matrix 192 × 256 × 256 and isometric image resolution 1 mm3) was used giving a good contrast between the spinal cord and the cerebrospinal fluid. The sequence was a part of a standard brain protocol and is used for general morphological analysis and as structural reference in functional MRI studies, but the field of view covered the whole head and the upper cervical cord. To check the reproducibility of the method, two of the healthy volunteers were scanned 7 and 12 times respectively at different days spread over 18 months. Linear rigid body realignment of the images was performed using SPM5 (http://www.fil.ion.ucl.ac.uk/spm) to evaluate inter-scan difference in subject placement.
Image correction
Image items located far from the center of the scanner during acquisition, such as the cervical spinal cord in our brain sequence, are prone to geometrical distortions caused by nonlinearities in the scanner's gradient fields that may confound quantitative analysis.13 To remove those image warping effects, correction was performed off-line using in-house software implemented in Matlab (The MathWorks, Inc., Natick, MA, USA) as described earlier.14 The gradient field map for the gradient system of the MR scanner was used as provided by the vendor.
Area and width estimates
A mid-sagital slice was chosen for defining anatomical landmarks (Figure 1b). A plane perpendicular to the spinal cord was drawn from the superior edge of the C2-process and formed the mid-plane in an interpolated axial data set with isometric resolution 0.1 mm3 and 21 slices. The increased resolution was used to account for partial volumes of cerebrospinal fluid and spinal cord. Two regions of interest were drawn manually in the mid-plane covering the spinal cord and the cerebrospinal fluid space (Figure 1c). The mid-value of the mean intensities in the two regions of interests was used as boundary threshold to extract a spinal cord mask as described earlier.11 The area of the mask was used as SCA, and antero–posterior width (APW) and left–right widths (LRWs) were extracted (Figure 1d). APW and LRW only reflect the width in two orthogonal directions, but atrophy will affect measures in all directions. To assess the effect of atrophy in oblique directions, the radius from the cord shape center of mass to its border, R(α), was measured over the whole circle with an angular resolution of 6°, (Figure 1d). The mean measures of all 21 axial slices was used for the statistics to avoid random effects in single slices from nerve roots, noise, and so on. The same observer performed all manual steps blinded to the identity of the individual participants.
Statistics
Student's t-test was used to evaluate difference in the spinal cord measures between groups. Pearson's linear correlation coefficient, r, was used to test the correlation between the atrophy measures and the functional measures from the clinical investigation.
Results
Reproducibility and shape extraction
SCA was estimated in two healthy individuals scanned at multiple times before and after gradient nonlinearity correction. Realignment of the data sets showed inter-scan rotations and translations spanning 13.3° and 31 mm for the same individual representative to the variation in subject positioning in a clinical workflow. As a consequence of the gradient nonlinearity correction, the coefficient of variance was reduced from 8.05 to 1.01% for the SCA, 4.33 to 0.95% for the LRW and 4.03 to 0.93% for the APW. The area of interest was visualized in all data sets. Central edemas were visible in two individuals and the edema was included in the shape. Those individuals did not affect the overall statistics and the data were included in the analysis for the interest of a broad applicability of the technique.
Spinal cord injured vs control
The SCI group showed a significant decrease in SCA compared with the control group. Furthermore, significant decreases were also observed in both APW and LRW, with decreases in APW being more pronounced, (Table 1). One individual with SCI did not participate in all clinical tests and was excluded in the functional correlations. Several deficit-specific relations were found (Table 2). SCA and APW correlated to the two sensory scores LTSS and PPSS (Figure 2). The sensory scores were more strongly correlated to APW than to SCA (APW-PPSS r=0.75 (P=0.0003) vs SCA-PPSS r=0.72 (P=0.0008)) and APW was more strongly correlated to PPSS than LTSS (APW-PPSS r=0.75 (P=0.0003) vs APW-LTSS r=0.66 (P=0.002)). None of the sensory scores correlated to changes in LRW. The motor score correlated both to SCA and LRW, but not to APW. No correlations with patient age, time from lesion, weight or height were observed, but a weak correlation to lesion level was found (Table 2).
Angular relations
A polar plot showing the correlation coefficient between R(α) and LTSS, PPSS and the motor score is presented in Figure 3. Correlation coefficients are only shown for angles with a strong significance (P<0.01). The sensory scores correlated to radii in two broad sectors in the anterior and posterior parts. The motor score correlated to radii in more narrow sectors in the lateral parts with a slight ventral displacement. Both motor and sensory correlations were stronger on the right side.
The directional correlations between spinal cord radius and the motor score (solid), PPSS (dark gray) and LTSS (bright gray). Correlations are plotted for directions with high significance (P<0.01). The radial axis shows the correlation coefficient and the spinal cord drawing illustrates the main directions in the plot. A, anterior; L, left; P, posterior; R, right.
Discussion
In our sample of spinal cord injured individuals, atrophy in APW and LRW, derived from standard clinical T1 weighted head MRI, were independently correlated to sensory and motor scores.
Our method for extracting the axial shape of the spinal cord differs slightly from the original method of Losseff et al.11 The C2 vertebral process was used as caudal landmark instead of the C2/C3 intravertebral disc. This was conducted, first to obtain better signal from the head coil, second, to improve the LRW measurement by avoiding contribution from nerve roots and third to get closer to the level in which the spinal canal is the largest giving better prerequisites for the segmentation with lower cerebrospinal fluid flow speeds, less cord motion and better separation between the spinal cord and the canal. Gradient nonlinearity correction was found crucial for reducing the effect of subject positioning and vendor-specific gradient design geometry. Geometrical distortions were effectively reduced on multiple images acquired over 18 months on the same two subjects. The improvement in parameter reproducibility also included our additional width measures LRW and APW. By using a sequence with a high isotropic resolution a better estimate in right–left direction will be obtained, compared with earlier implementations of the method using sagital images with large slice thickness.
It was hypothesized that the location of sensory and motor pathways would determine axis-specific atrophy of the spinal cord. The motor score, evaluated as the ability to voluntarily activate different muscle groups, is mainly determined by the integrity of the corticospinal tract. The observation that the motor score was well correlated to LRW is well in line with the main location of this tract in the lateral funiculi. The sensory scores are based on conscious perceptions of touch, which are mediated mainly by the dorsal column and this is also in line with the correlation between the sensory score and APW.
The polar plots of the correlation coefficients for the clinical scores and the radius in individual directions show little overlap between significant atrophy components of sensory and motor qualities, supporting the complementary descriptive values of LRW and APW (Figure 3). The stronger correlations on the right side may be explained by asymmetry in the patient group. PPSS was lowest on the right side in nine patients, on the left side in six patients and the same in four patients. The motor score was lowest on the right side in 10 patients, on the left side in 4 patients and the same in 5 patients. Asymptomatic asymmetric spinal cord shape is common and the right half is larger than the left side in three quarters of the asymmetric cords. This observation is independent of handedness and is mainly attributed to a larger portion of the corticospinal tract descending on the right side.9 This difference in tract redundancy on the left and right side may also explain asymmetry in the angular relations.
Only a weak relation was found between lesion level and atrophy. This is likely explained by local effects progressing from the lesion, such as Wallerian degeneration, but global axonal loss because of deactivation of larger subsystems, mirrored in the decreases in clinical scores in our case, show larger significance. To fully understand this interaction, area measurements conducted at several segments would be necessary. This was not possible in our study because a full scan of the spinal cord was not performed. However, it is of interest for the purpose of clinical applicability of the technique that measurements at one specific high cervical level may provide clinically useful information regarding lesions throughout the cervical and thoracic spinal cord. This has also the advantage that the measurements at such a high level in most cases will not be confounded by edema or image artifacts caused by possible implants around the site of the lesion.
The fact that it was possible to use standard clinical head MRI means that it may be possible to include the measures as part of routine clinical evaluations within the tight time frames of radiological exams or to retrospectively analyze existing data. Obviously, more data are needed to evaluate the full clinical value of those measures. Particularly, the longitudinal morphological development must be studied in the sub-acute stage and this data may provide useful diagnostic or prognostic value as shown for the SCA in multiple sclerosis.15 Medical and surgical procedures to promote axonal regrowth are also subject to considerable research.16 To fully implement those treatments for clinical use on individuals with SCI, non-invasive quantitative markers of regional tract integrity are needed.
Conclusively, we have shown that it is possible to obtain specific correlations between different clinical deficits and independent geometrical components of spinal cord atrophy after SCI detected with a semi-automatic technique using standard structural T1 weighted head MRI. These measures may be of great importance for evaluation of spinal cord integrity as part of routine MR scanning after SCI. The broad usability of SCA in earlier studies suggest that LRW and APW may provide functional-specific atrophy for other neurological disorders affecting the spinal cord and thereby assist treatment and rehabilitation.
References
Lammertse D, Tuszynski MH, Steeves JD, Curt A, Fawcett JW, Rask C et al. Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: clinical trial design. Spinal Cord 2007; 45: 232–242.
Schwartz ED, Shumsky JS, Wehrli S, Tessler A, Murray M, Hackney DB . Ex vivo MR determined apparent diffusion coefficients correlate with motor recovery mediated by intraspinal transplants of fibroblasts genetically modified to express BDNF. Exp Neurol 2003; 182: 49–63.
Maier S . Examination of spinal cord tissue architecture with magnetic resonance diffusion tensor imaging. Neurotherapeutics 2007; 4: 453–459.
Smith SA, Golay X, Fatemi A, Jones CK, Raymond GV, Moser HW et al. Magnetization transfer weighted imaging in the upper cervical spinal cord using cerebrospinal fluid as intersubject normalization reference (MTCSF imaging). Magn Reson Med 2005; 54: 201–206.
Sundblom J, Melberg A, Kalimo H, Smits A, Raininko R . MR imaging characteristics and neuropathology of the spinal cord in adult-onset autosomal dominant leukodystrophy with autonomic symptoms. AJNR 2009; 30: 328–335.
Agosta F, Rocca MA, Valsasina P, Sala S, Caputo D, Perini M et al. A longitudinal diffusion tensor MRI study of the cervical cord and brain in amyotrophic lateral sclerosis patients. J Neurol Neurosurg Psychiatr 2009; 80: 53–55.
Krabbe K, Nielsen JE, Fallentin E, Fenger K, Herning M . MRI of autosomal dominant pure spastic paraplegia. Neuroradiology 1997; 39: 724–727.
Bar-Zohar D, Agosta F, Goldstaub D, Filippi M . Magnetic resonance imaging metrics and their correlation with clinical outcomes in multiple sclerosis: a review of the literature and future perspectives. Mult Scler 2008; 14: 719–727.
Nathan P, Smith M, Deacon P . The corticospinal tracts in man: course and location of fibres at different segmental levels. Brain 1990; 113: 303–324.
Nathan P, Smith M, Deacon P . Vestibulospinal, reticulospinal and descending propriospinal nerve fibres in man. Brain 1996; 119: 1809–1833.
Losseff NA, Webb SL, O’Riordan JI, Page R, Wang L, Barker GJ et al. Spinal cord atrophy and disability in multiple sclerosis. A new reproducible and sensitive MRI method with potential to monitor disease progression. Brain 1996; 119: 701–708.
Marino R, Barros T, Biering-Sørensen F, Burns SP, Donovan WH, Graves DE et al. International standards for neurological classification of spinal cord injury. J Spinal Cord Med 2003; 26: 50–56.
Haacke EM, Brown RW, Thompson MR, Venkatesan R . Magnetic Resonance Imaging: Physical Principles and Sequence Design. John Wiley & Sons: New York, 1999. p 614–616.
Jovicich J, Czanner S, Greve D, Haley E, van der Kouwe A, Gollub R et al. Reliability in multi-site structural MRI studies: effects of gradient non-linearity correction on phantom and human data. NeuroImage 2006; 30: 436–443.
Stevenson VL, Leary SM, Losseff NA, Parker GJ, Barker GJ, Husmani Y et al. Spinal cord atrophy and disability in MS - a longitudinal study. Neurology 1998; 51: 234–238.
Rossignol S, Schwab M, Schwartz M, Fehlings MG . Spinal cord injury: time to move? J Neurosci 2007; 27: 11782–11792.
Acknowledgements
We thank PT Hanne Knudsen for data collection during the clinical session. Henrik Lundell and Jens Bo Nielsen are supported by The Ludvig and Sara Elsass Foundation, Denmark. Dorothy Barthélemy is supported by The Canadian Institutes of Health Research (CIHR). Tim B Dyrby is supported by The Lundbeck Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Lundell, H., Barthelemy, D., Skimminge, A. et al. Independent spinal cord atrophy measures correlate to motor and sensory deficits in individuals with spinal cord injury. Spinal Cord 49, 70–75 (2011). https://doi.org/10.1038/sc.2010.87
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sc.2010.87
Keywords
This article is cited by
-
Optimized multi-echo gradient-echo magnetic resonance imaging for gray and white matter segmentation in the lumbosacral cord at 3 T
Scientific Reports (2022)
-
Magnetic resonance imaging features of dogs with incomplete recovery after acute, severe spinal cord injury
Spinal Cord (2018)
-
Ambulatory function in motor incomplete spinal cord injury: a magnetic resonance imaging study of spinal cord edema and lower extremity muscle morphometry
Spinal Cord (2017)
-
Subject-specific regional measures of water diffusion are associated with impairment in chronic spinal cord injury
Neuroradiology (2017)
-
Voxel-based analysis of grey and white matter degeneration in cervical spondylotic myelopathy
Scientific Reports (2016)