Abstract
Study design:
Case report.
Objectives:
To report on the respiratory and kinematic changes associated with body weight supported treadmill training (BWSTT) in an individual with an incomplete cervical spinal cord injury (SCI).
Setting:
Human Locomotion Research Laboratory, Vancouver, BC, Canada
Methods:
A 38-year-old man with an incomplete SCI at the C1/C2 level, graded on the American Spinal Injury Association Impairment Scale (AIS) as AIS D, participated in this case study. He performed three BWSTT sessions (45 min) per week for 12 weeks. Before and after training, respiration was measured at the mouth using a pneumotachograph while treadmill walking at 1.6 km h−1.
Results:
Completion of 12 weeks of BWSTT resulted in a lowering of minute ventilation during treadmill exercise. A coupling of locomotion and respiration was observed during treadmill walking after the training program, whereas the relationship was initially absent.
Conclusion:
This case illustrates that BWSTT can result in a reduced ventilatory demand and promote locomotor–respiratory coupling during walking in an individual with an incomplete cervical SCI.
Similar content being viewed by others
Introduction
Body weight supported treadmill training (BWSTT) is a rehabilitation intervention used to promote the recovery of walking in individuals with incomplete spinal cord injury (SCI). It is hypothesized that locomotor-related afferent feedback is a key contributor to neuroplastic changes associated with improved walking. Peripheral afferent feedback also contributes to the ventilatory response to exercise, as shown in neonatal rats in whom stimulation of cervical and lumbar dorsal roots can entrain the respiratory rhythm.1, 2 It is not known whether persons with SCI show entrainment of movement and breathing during treadmill walking or whether BWSTT can modulate this relationship.
Case report
A 38-year-old male with an incomplete SCI, graded on the American Spinal Injury Association Impairment Scale (AIS) as AIS D, participated in this case study. He incurred a traumatic SCI at the C1/C2 level 4 years ago. Informed written consent was obtained and the procedures received institutional ethical approval. He performed three BWSTT sessions per week for 12 weeks using the Lokomat robotic gait trainer (Hocoma AG, Volketswil, Switzerland). During each session, he completed 45 min of treadmill walking. Treadmill speed during each session was set to the maximum tolerated by the participant and progressed from 1.0 km h−1 at the beginning of the training program up to 2.0 km h−1 by the end. Resting spirometry was performed pre- and post-training. Before and after training, respiration was measured at the mouth using a pneumotachograph while treadmill walking at 1.6 km h−1. Breath-by-breath values of minute ventilation (VE), tidal volume (VT), breathing frequency (fb) and inspiratory duty cycle (TI/TTOT) were determined. Consecutive incidences of peak hip extension (recorded by position sensors at the hip) were used to define the step cycles.
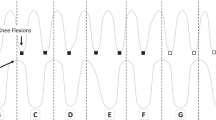
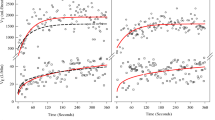
After training, overground walking speed measured over 10 m improved from 0.87 to 0.96 m s−1 (+10.3%). The maximum overground walking distance covered within 6 min improved from 221 to 267 m (+20.8%). Resting spirometric values did not change after BWSTT (Table 1). During treadmill walking, VE was reduced by 10 l min−1 after BWSTT, which was mediated by reductions in both VT and fb (Table 2). Similar values for TI/TTOT were observed pre- (0.43) and post- (0.44) training. Pre-training, there was a 1:1 relationship between breathing frequency and limb movement. The onset of inspiration occurred across the gait cycle indicating no apparent relationship between the onset of inspiration and the gait cycle phase (Figure 1). Post-training, the 1:1 ratio was maintained; however, 95% of all inspiratory efforts began at a distinct stepping phase (0–20% of gait cycle).
Discussion
This case study shows two findings. First, BWSTT resulted in a lowering of VE during treadmill exercise in a person with an incomplete cervical SCI. We interpret this to mean that BWSTT resulted in a reduced ventilatory demand, and presumably metabolic cost of breathing, during treadmill walking. Second, locomotor–respiratory coupling was stronger after BWSTT.
It is known that breathing frequency changes as a function of exercise and that 1:1 coupling can be observed. A neural mechanism underlying this coordination could be afferent feedback. Electrical stimulation of lumbar dorsal roots in neonatal rats can entrain and reset the respiratory rhythm, which supports the concept of pathways between lumbar afferents, the medullary respiratory network and phrenic motoneurons.1 Feedforward mechanisms are also thought to underlie the coordination of respiration and locomotion. In a decorticate cat model, the increase in respiration precedes the onset of locomotion in response to stimulation of brainstem locomotor centers, indicating that central neural commands can drive locomotor–respiratory coordination.3 Our observations suggest that there may be plasticity in the neural mechanisms mediating locomotor–respiratory coupling, which is possibly mediated by these feedback and feedforward pathways.
Respiratory complications remain one of the leading causes of morbidity and mortality in SCI and this is particularly true of cervical injuries in which interruptions of the descending respiratory pathways are often present. What might be the clinically relevant benefits to locomotor–respiratory coupling? Synchronization would enable one to combine the ventilatory and postural functions of the respiratory muscles with a reduction in energy cost. There is some evidence to suggest that as the degree of entrainment increases, whole-body oxygen consumption is reduced.4 Reducing the cost of breathing and minimizing the fraction of cardiac output directed to respiratory muscles could allow for greater blood flow to the working limb.5 This may be relevant in SCI in which a ‘hypokinetic’ circulatory response to exercise is almost universally observed. This possibility needs to be tested and may be important in understanding the therapeutic potential of BWSTT for persons with SCI.
References
Morin D, Viala D . Coordinations of locomotor and respiratory rhythms in vitro are critically dependent on hindlimb sensory inputs. J Neurosci 2002; 22: 4756–4765.
Giraudin A, Cabirol-Pol MJ, Simmers J, Morin D . Intercostal and abdominal respiratory motoneurons in the neonatal rat spinal cord: spatiotemporal organization and responses to limb afferent stimulation. J Neurophysiol 2008; 99: 2626–2640.
Eldridge FL, Millhorn DE, Waldrop TG . Exercise hyperpnea and locomotion: parallel activation from the hypothalamus. Science 1981; 211: 844–846.
Garlando F, Kohl J, Koller EA, Pietsch P . Effect of coupling the breathing- and cycling rhythms on oxygen uptake during bicycle ergometry. Eur J Appl Physiol 1985; 54: 497–501.
Dempsey JA, Sheel AW, St Croix CM, Morgan BJ . Respiratory influences on sympathetic vasomotor outflow in humans. Respir Physiol Neurobiol 2002; 130: 3–20.
Acknowledgements
We thank Kathryn Luttmann and Katherine Pauhl for valuable assistance with the body weight supported treadmill training program. This study was supported by the British Columbia Medical Services Foundation, the Christopher and Dana Reeve Foundation, the Canada Foundation for Innovation, and the Natural Sciences and Engineering Research Council of Canada (NSERC). MFB Sherman was supported by graduate scholarships from NSERC and the Michael Smith Foundation for Health Research (MSFHR). AW Sheel was supported by a Scholar Award from the MSFHR and a New Investigator Award from the Canadian Institutes of Health Research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sherman, M., Lam, T. & Sheel, A. Locomotor–respiratory synchronization after body weight supported treadmill training in incomplete tetraplegia: a case report. Spinal Cord 47, 896–898 (2009). https://doi.org/10.1038/sc.2009.50
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sc.2009.50
Keywords
This article is cited by
-
Locomotor-respiratory coupling in ambulatory adults with incomplete spinal cord injury
Spinal Cord Series and Cases (2022)
-
Effect of repeated locomotor training on ventilatory measures, perceived exertion and walking endurance in persons with motor incomplete spinal cord injury
Spinal Cord Series and Cases (2020)
-
Attentional focus does not impact locomotor–respiratory coupling in trained runners
European Journal of Applied Physiology (2020)
-
Effects of overground locomotor training on the ventilatory response to volitional treadmill walking in individuals with incomplete spinal cord injury: a pilot study
Spinal Cord Series and Cases (2017)
-
Does locomotor training improve pulmonary function in patients with spinal cord injury?
Spinal Cord (2015)