Abstract
Study design:
Experimental study.
Objective:
A recently characterized CatSper genes, encodes for unique Ca2+ channels in the testes, where they play essential roles in sperm motility. The aim of this research is to evaluate potential changes in the expression of CatSper genes, sperm parameters and testis histology following spinal cord injury (SCI).
Setting:
Department of Anatomical Sciences, Tarbiat Modares University, Tehran, Iran.
Methods:
A total of 75 adult NMRI mice were divided into three groups (25 in each group) of SCI, sham and control. Following laminectomy, SCI group was subjected to injury at the ninth thoracic vertebra. The epididymal sperm parameters were studied at day 1 and at weeks 1, 2, 4 and 6 after injury. One testis was removed for morphological study and the other testis were used for molecular study. Reverse transcription-PCR was performed at different time in all the groups.
Results:
Our results revealed that SCI affects spermatogenesis as well as sperm quality and quantity. In gene expression analysis, there was a significant downregulation of CatSpers 1 and 2 at 4 and 6 weeks following contusion. There were no differences between the semen parameters and CatSper genes expression at the different time points in the sham and control groups.
Conclusions:
Our data indicated that the SCI mouse model causes a significant reduction in all sperm parameters, along with deleterious effects on spermatogenesis as well as the expression of CatSpers 1 and 2.
Similar content being viewed by others
Introduction
Spinal cord injury (SCI) could elevate abnormal sperm morphology, decrease sperm motility and impair male fertility.1, 2 The poor sperm motility of SCI patients may attribute to abnormal sperm maturation and transport in the epididymis. The examination of testicular biopsies from SCI patients has shown various abnormalities in spermatogenesis including spermatogenic arrest, reduced spermatogenic cell number or regression of the seminiferous epithelium.1, 2 Recent reports, however, suggest that alterations in cAMP might cause the poor fertility following SCI.3
Ion channels are multipass transmembrane proteins that efficiently catalyze ion transport through the nonconducting lipid bilayers. Environmental cues activate sperm motility through transduction events involving ion channels located on spermatozoa.4 Mammalian spermatozoa have to further mature within the female reproductive tract through a process called capacitation.5 Although millions of sperm are released, only a few reach the oocyte.6 The mechanism underlying the sperm motility and its importance in fertility still remains poorly understood.7
Sperm motility is dependent on the presence of external Ca2+. The most convincing evidence for the role of ion channels in sperm motility comes from characterizing a novel class of Ca2+ channels, CatSper family, whose expression is confined to the testes. Four members of the family have been described so far, and two of them, CatSpers 1 and 2, localize to the flagella.8, 9 The knockout mice lacking CatSper 1 gene product are infertile, mostly due to a lack of sperm motility and hyperactivation.8 Furthermore, CatSpers 1- and 2-knockout mice have identical phenotypes, apparently because all four members of the family collaborate with each other to form a complex functional channel.10
Despite the vital role that CatSper gene family plays in sperm motility and in the male fertility, there has been no study so far to determine any potential correlation between SCI and CatSper gene expression regulation. In this study, we have examined the changes in CatSper genes expression, sperm parameters and testis histology, following contusion in a mouse model of SCI.
Materials and methods
Animals
A total of 75 NMRI male mice were obtained from Razi institute (Tehran, Iran) and divided into three different groups (25 in each group) of SCI, sham and control. The animals were housed in small groups under standard lighting conditions with free access to water and food. They were allowed to adapt for at least 1 week in the animal room, before they were subjected to the surgery. Animals were also maintained and handled according to the protocols approved by the Tarbiat Modares University Animal Care and Use Committee. At specific time intervals after the surgery (day 1 and weeks 1, 2, 4 and 6), the SCI as well as the sham and control mice were killed by chloroform and both testes were removed. One testis was fixed in Boins fixative and processed for histological examination, and the other one was frozen in liquid nitrogen and stored at −80 °C for subsequent reverse transcription-PCR analysis.
SCI model
We surgically induced cord injury in mice, based on the protocol previously described by Wrathall et al.11 Briefly, the mice were anesthetized with Ketamine (90 mg kg−1) and Xylazine (10 mg kg−1) and the spinal cord was exposed at the T8–T10 vertebra level by laminectomy. Spinal cords were contused with a rod (2.5 g weight) dropped from 2 cm height. The muscle and skin layers were then sutured. The mice belonging to the sham group received only laminectomy, whereas the control group mice were not exposed to any type of injury or medication.
Sperm preparation
Spermatozoa were obtained from mature NMRI mice. The animals were killed by chloroform and spermatozoa were obtained from cauda epididymis. Spermatozoa were released by mincing the epididymis in the collection medium. The phosphate-buffered saline was incubated at 37 °C in 5% CO2 for 45 min. After the incubation, the sperm suspension was examined for sperm parameters. The sperm parameters including viability, motility, abnormal morphology rates and sperm count were assessed according to the World Health Organization criteria.12
Testicular histology
To examine the testicular histology, tissue sections in 5-μm thickness from the Bouin's fixed tissues were stained with hematoxylin, before being observed under light microscope.
RNA extraction and reverse transcription
Total RNA was isolated from frozen tissues using the RNX plus solution (Cinnagen, Tehran, Iran), according to the manufacturer's instructions as described previously.10 The purity and integrity of the extracted RNA was evaluated by the optical density measurement (260/280 nm ratios) and by visual observation of the samples that were electrophoresed on agarose gels. Both methods indicated the integrity of the extracted RNA with little or no protein carryover. cDNA synthesis reactions were performed according to the manufacturer's instructions (Fermentas, cDNA Synthesis Kit no. K1622, Opelstrasse, Germany).
PCR
The PCR primers for mouse CatSpers 1–4 and mouse β-2 microglobulin (β-2m, as an internal control) were designed using Genrunner software (version 3.02; Hastings Software Inc.). The features of the primers and the expected sizes of the amplified products are summarized in Table 1. PCR was performed using 2 μl of synthesized cDNA with 0.25 μl of Taq polymerase (Cinnagen, Iran). The number of PCR cycles was optimized for both CatSpers (35 cycles) and β-2m (30 cycles) cycles. The cycling conditions were as the following: denaturation at 94 °C for 30 s, annealing at 58 °C for 30 s, extension at 72 °C for 1 min, with a final extension at 72 °C for 10 min.
Semiquantitative reverse transcription-PCR
The PCR products were separated on a 1.5% agarose gel and subsequently were visualized by ethidium bromide staining. The amount of DNA was quantified by measuring the intensity of light emitted from corresponding bands under UV light using UVDoc software (version 12; Kapelan GmbH Co., Germany). The results were expressed as the ratio of the intensity of the CatSper genes band to that of β-2m amplification to account for any differences in the starting amounts of RNA.
Statistical analysis
All data were analyzed to determine whether they were normally distributed. The data then were evaluated using ANOVA and Duncan's test (95% confidence level and significant P<0.05).
Results
Changes in sperm parameters after SCI
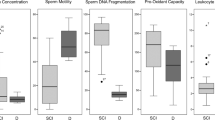
The changes in the sperm parameters at specific time points after SCI in mouse are summarized in Table 2. As shown in the table, the sperm motility rate of SCI group is gradually decreased from its almost unchanged level at day 1 to around 40% of its control level at 6 weeks of injury. The same observation was made for abnormal morphology rate, where it is reduced gradually to almost half of its value by the end of experiment (at the 6 week). Similar conclusion was made for the viability rate and sperm count (Table 2).
Morphological changes of the testis following SCI
In the SCI group, the size of the testes was reduced compared to that of the control and sham groups after 2 weeks of surgery. Furthermore, the number of superficial blood vessels in the surface of the testis of SCI group showed significant reduction compared to the control and sham groups (data not shown).
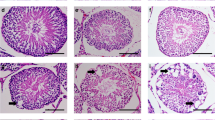
In the control mice, the testes contained well-organized seminiferous epithelium, along with the presence of mature spermatids in the luminal edge, demonstrating the normal process of spermatogenesis (Figure 1a).
Photomicrograph of morphological appearances of the mouse seminiferous tubules of (a) control group with normal tubules (IC, intestinal cells; L, lumen and I, intestinal space). (b) Spinal cord injury (SCI) group at 14 days after the injury, note that the integrity of the seminiferous tubules is lost and the tubules are almost without any sperm. (c) SCI group at 28 days after injury, note that the seminiferous basal lamina and the integrity of the tubules are further diminished. Several vacuoles ( → ) are also formed within the seminiferous tubules, which are without any sperm. (d) SCI group at 42 days after injury. Several changes are evident in seminiferous tubules and the number and the size of vacuoles ( → ) are increased compared to previous group (Hematoxylin and eosin staining).
In the SCI group, noticeable degenerative changes in the appearance of seminiferous tubules began by 2 weeks after the induction of injury. At this time point, the integrity of the tubules was somewhat impaired and some of the seminiferous tubules had apparently none or few spermatozoa (Figure 1b). By 28 days after surgery, the integrity of the basal lamina of seminiferous tubules was lost and the tubes showed some vacuoles formed inside the tubes (Figure 1c). Seminiferous tubules of a mouse showed total regression of spermatogenesis. Four weeks after SCI, abnormalities in spermatogenesis including delay or failure in spermiation (as indicated by the presence of mature spermatids in the lumen, vacuolization of spermatid nuclei or incomplete spermatogenesis) were observed in most of the SCI animals (Figure 1c). After 6 weeks since the surgery, the morphological changes of the seminiferous tubules were more dramatic compared to the earlier post-injury times (Figure 1d).
CatSper genes expression alterations following SCI
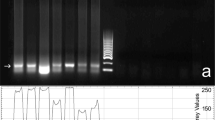
The expression of all members of the CatSper gene family (CatSpers 1–4) was detected in all time points of the experiments with the SCI as well as the control and the sham groups. The relative expression of the genes (compared to the changes in the expression of β-2m in each sample, as an internal control) was nearly unchanged from the first day and the first week after the surgery. However, at second week following the surgery, the expression of the genes started to decrease and at the fourth week there was statistically significant downregulation of the CatSpers 1 and 2 genes (Table 3 and Figure 2). In contrast, despite a slight downregulation, the relative expression of CatSpers 3 and 4 did not show statistically significant differences compared to that of control groups (Table 3).
Post-surgery time course analysis of CatSpers 1–4 gene expression alterations in a mouse model of spinal cord contusion. Total mRNA from mouse testis at different time points after contusion is reverse transcribed and amplified using specific primers for CatSpers 1–4. β-2 microglobulin (β-2m) is used in all samples as an internal control. (a) The control group, (b) 28 days post-surgery and (c) 42 days post-surgery.
Discussion
In men with SCI, fertility is severely impaired. These patients have a unique semen profile characterized by normal sperm concentration but low sperm motility.2 This suggest that the low sperm motility is probably the major cause of subfertility in these patients. Therefore, deciphering the underlying mechanism regulating sperm motility and the way they have been altered in SCI patients is of great importance. In this study, we have employed a mouse model of contusion to induce SCI; in doing so, we examined sperm parameters, testis morphological changes and the potential alterations in the expression of the genes involved in sperm motility.
Our data demonstrated that sperm parameters were significantly reduced in SCI group. This finding is in agreement with previous reports,1 including the study of Bilups et al., which they performed sympathectomy on rats and they investigated the epididymal sperm parameters.13 According to their findings, SCI have diminishing effects on the quality and quantity of sperms. Moreover, Linsemneyer et al. reported that the qualitative and quantitative impairment of spermatogenesis occurs during the acute phase of SCI in rats.14
In mammals, sperm must swim a long distance along the female reproductive tract before reaching and fertilizing the ovum. Thus, adequate sperm motility is vital for the correct timing and successful fertilization process. Recently, a novel class of Ca2+ channel, CatSpers 1–4, are cloned and characterized. The CatSper gene family is exclusively expressed in testis, and they have vital roles in sperm motility and male fertility in both mice8 and human.15 Furthermore, it has been hypothesized that the CatSper proteins may form a functional tetrameric channel.10 Our data reveal a significant downregulation of CatSpers 1 and 2 following SCI in the mouse model. Our findings could provide a clue to further elucidate the possible mechanism underlying the reduced sperm motility in SCI patients. Moreover, our results are in accord with our previous report in which we observed a significant reduction of CatSper 1 expression among patients who lack sperm motility as compared with patients whose infertility cannot be ascribed to a deficiency in sperm motility.15
Probably CatSper family is not the sole factor downregulated in our SCI model and other factors might be affected as well. For this reason, a global search of factors that are altered following SCI might be of great help to provide a molecular picture of subfertility problem following the SCI. Indeed, changes in the expression of some genes taking place in the early stage after SCI indicate the complexity of secondary SCI.16 Some investigators suggest that the low motility rate may be the result of defective transcription of some genes encoding proteins involved in sperm motility and other functions.17 One (or some) of these genes may be defectively transcribed in some spermatozoa of the low-motile fraction. The low-motile fraction may be composed of heterogeneous subsets in which different genes were defectively transcribed. Another explanation for the differential distribution of mRNAs between high- and low-motile sperm might lie in the translation occurring in sperm.17 Probably there are some mechanisms that increase reactive oxygen species (ROS) and in return these ROS affect sperm's DNA.17
The loss of the ability to swim forward and the failure of hyperactivation of CatSpers 1 and 2 null sperms could suggest that these proteins are involved in the sperm forward velocities, and that the proteins are essential for the generation of a hyperactivated form of motility.18, 19 The powerful asymmetric motion of hyperactivated spermatozoa requires Ca2+ entry into the sperm tail. Hyperactivation requires CatSpers 1 and 2 putative ion channel genes, but the function of two other related genes (CatSpers 3 and 4) is not well understood.
In conclusion, our work confirms the previous data regarding the poor quality of sperms in SCI animal models and the patients. It further revealed downregulations of two critical genes in sperm motility (CatSpers 1 and 2) following SCI. The latter finding could provide valuable information with potential clinical values on detailed molecular mechanisms underlying male subfertility in SCI patients.
References
Linsenmeyer TA, Perkash I . Infertility in men with spinal cord injury. Arch Phys Med Rehabil 1991; 72: 747–754.
Beretta G, Chelo E, Zanollo A . Reproductive aspects in spinal cord injured males. Paraplegia 1989; 27: 113–118.
Huang HFS, Li MT, Anesetti R, Pogach LM, Ottenweller JE . Alteration of cAMP signaling in rat testicular cells after spinal cord injury. J Spinal Cord Med 2003; 26: 69–78.
Darszon A, Nishigaki T, Wood C, Trevino CL, Felix R, Beltran C . Calcium channels and Ca2+þ fluctuations in sperm physiology. Int Rev Cytol 2005; 243: 79–172.
Visconti PE, Westbrook VA, Chertihin O, Demarco I, Sleight S, Diekman AB . Novel signaling pathways involved in sperm acquisition of fertilizing capacity. J Reprod Immunol 2002; 53: 133–150.
Ho HC, Suarez SS . Characterization of the intracellular calcium store at the base of the sperm flagellum that regulates hyperactivated motility. Biol Reprod 2003; 68: 1590–1596.
Eisenbach M . Mammalian sperm chemotaxis and its association with capacitation. Dev Genet 1999; 25: 87–94.
Ren D, Navarro B, Perez G, Jackson AC, Hsu S, Shi Q et al. A sperm ion channel required for sperm motility and male fertility. Nature 2001; 413: 603–609.
Quill T, Sugden SA, Rossi KL, Doolittle LK, Hammer RE, Garbers D . Hyperactivated sperm motility driven by required for fertilization. PNAS 2003; 100: 14869–14874.
Lobley A, Pierron V, Reynolds L, Allen L, Michalovich D . Genes characterisation identification of human and mouse CatSper3 and CatSper4 of a common interaction domain and evidence for expression in testis. Reprod Biol Endocrinol 2003; 1: 53.
Wrathall JR, Li W, Hudson LD . Myelin gene expression after experimental contusive spinal cord injury. J Neurosci 1998; 18: 8780–8793.
World Health Organization. Laboratory Manual for the Examination of Human Semen and Cervical Mucus Interaction, 3rd edn. Cambridge University Press: New York, 1999.
Billups KL, Tillman SL, Chang TS . Reduction of epididymal sperm motility after ligation of the inferior mesenteric plexus in the rat. Fertil Steril 1990; 35: 1076–1082.
Linsemneyer TA, Ottenweller JE, Huang HFS . Epididymal sperm transport in normal and recent spinal cord injured Sprague Dawley rats. J Spinal Cord Med 1999; 22: 102–106.
Nikpoor P, Mowla SJ, Movahedin M, Ziaee SA, Tiraihi T . CatSper gene expression in postnatal development of mouse testis and in subfertile men with deficient sperm motility. Hum Reprod 2004; 19: 124–128.
Liu CL, Jin AM, Tong BH . Detection of gene expression pattern in the early stage after spinal cord injury by gene chip. Chin J Traumatol 2003; 6: 18–22.
Gur Y, Breitbart H . Mammalian sperm translate nuclear-encoded proteins by mitochondrial-type ribosomes. Genes Dev 2006; 20: 411–416.
Carlson AE, Westenbroek RE, Quill T, Ren D, Clapham DE, Hille B et al. CatSper1 required for evoked Ca2+ entry and control of flagellar function in sperm. Proc Natl Acad Sci 2003; 100: 14864–14868.
Qi H, Moran M, Navarro B, Chong J, Krapivinsky G, Krapivinsky L et al. All four CatSper ion channel proteins are required for male fertility and sperm cell hyperactivated motility. PNAS 2007; 104: 1219–1223.
Acknowledgements
We thank Dr Hamid Reza Kalhor, for his advice. This work is supported by a TMU research grant.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rezaian, J., Movahedin, M. & Mowla, S. CatSper genes expression, semen characteristics and histology of testes in the contusive spinal cord-injured mice model. Spinal Cord 47, 76–81 (2009). https://doi.org/10.1038/sc.2008.81
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sc.2008.81
Keywords
This article is cited by
-
Prise en charge de l’infécondité dans les troubles de l’éjaculation: avis conjoints de l’andrologue, du biologiste et du gynécologue
Basic and Clinical Andrology (2009)