Abstract
Study design:
Retrospective 9-year survey.
Objectives:
Clinical presentation of acute myelitis syndromes is variable, and neuroimaging and laboratory findings are not specific enough to establish the diagnosis with certainty. We evaluated the spectrum clinical features and paraclinical findings encountered during diagnostic workup and aiding the diagnosis.
Setting:
Department of Neurology, Inselspital Bern, Switzerland.
Material:
Charts and magnetic resonance imaging (MRI) of 63 patients discharged with the diagnosis of acute transverse myelitis.
Results:
The diagnosis was supported by abnormal MRI and cerebrospinal fluid (CSF) findings in 52 patients (82.5%) and suspected in the remaining either because of a spinal cord MRI lesion suggestive of myelitis (n=5), or abnormal CSF findings (n=4), or electrophysiological evidence of a spinal cord dysfunction (n=2). Clinical impairment was mild (ASIA D) in the majority. All patients had sensory disturbances, whereas motor deficit and autonomic dysfunction were less frequent. Neurological levels were mainly located in cervical or thoracic dermatomes. Spinal cord lesions were visualized by MRI in 90.4% of the patients and distributed either in the cervical or thoracic cord, or both. Multiple lesions were present in more than half of the patients, and lateral, centromedullary and posterior locations were most common. A high percentage of multiple sclerosis (MS)-typical brain lesions and CSF findings suggested a substantial number of MS-related myelitis in our cohort.
Conclusion:
The diagnostic workup of acute myelitis discloses a broad spectrum of CSF or MRI findings, and may be associated with diagnostic uncertainty due to lack of specific CSF or MRI features, or pathological findings.
Similar content being viewed by others
Introduction
The diagnosis of acute transverse myelitis syndromes is based on the clinical symptoms and signs of a spinal cord syndrome in conjunction with evidence of inflammation within the spinal cord such as cerebrospinal fluid (CSF) abnormalities or lesions visible on magnetic resonance imaging (MRI).1
Clinical symptoms of acute transverse myelitis may include motor, sensory and autonomic dysfunction.2, 3 As transverse myelitis may be the initial manifestation of multiple sclerosis (MS),2, 3, 4 often the expanded disability status scale is used for rating the extent of clinical impairment.5 For comparison with other myelopathies, the American Spinal Injury Association (ASIA) score has proven reliable,6 but has not been applied in large series of patients with acute transverse myelitis. Neuroradiological examinations including spinal cord MRI have become an important tool in the workup of patients with acute myelopathy. However, spinal cord pathologies detected on MRI are not specific for transverse myelitis.7, 8 Evidence for an inflammatory pathogenesis can also be derived from CSF analysis, which may show mild pleocytosis, elevated protein levels or oligoclonal bands (OCB).2, 4 Initial workup will focus on the exclusion of central nervous system infections and secondary myelitis syndromes, for example systemic lupus erythematosus, Sjögren's syndrome, primary antiphospholipid syndrome, sarcoidosis and various forms of vasculitis.9 However, the lack of a specific marker, the broad variability of clinical presentation and of MRI and laboratory findings account for diagnostic uncertainty in clinical practice.
The goal of this retrospective study was to describe the spectrum of clinical presentations together with CSF and neuroradiological findings in a large series of patients discharged with the diagnosis of acute transverse myelitis.
Materials and methods
Inclusion/exclusion criteria
This study was approved by the Institutional Review Board. Charts of patients from the Department of Neurology, Inselspital, Bern, who had been discharged with the diagnosis of acute transverse myelitis from January 1999 to December 2007 were studied. All patients met the following inclusion criteria: (i) presentation of symptoms and signs of acute non-compressive myelopathy between the age of 16 and 65 years without prior history of symptoms suggestive of a central nervous system demyelinating event, (ii) evolution of symptoms for no more than 4 weeks, sustained for at least 48 h and maximum peak reached in more than 4 h and (iii) no evidence of symptoms or signs suggestive of cranial involvement. Patients who did not have MRI scans of the whole spinal cord for re-analysis were not selected. Excluded were patients with a history of recent malignancy, abdominal surgery, radiation therapy, epidural spinal block, arteriovenous malformation of the spinal cord, paraneoplastic syndromes, patients with immune-mediated rheumatic diseases and infectious myelopathy according to serological testing, and patients with a CSF cell count of >100 μl−1. Patients with neuromyelitis optica were excluded as well, but aquaporin-4 antibody testing was not possible, yet, when most of the patients were seen.
Laboratory examination
The workup included serum analyses with routine laboratory tests. The following serologies were routinely performed: syphilis, lyme borreliosis, HIV, cytomegalovirus, herpes-simplex virus, varicella-zoster virus, enterovirus, Coxsackie virus A and B, adenovirus and Epstein–Barr virus. Immune-mediated disorders were checked by analysis of blood-sedimentation rate, C-reactive protein, rheumatoid factor, antinuclear antibodies, antineutrophil cytoplasmic antibodies and antibodies against cardiolipin. When indicated, ancillary test for exclusion of metabolic disorders such as vitamin B12/folic acid deficiency and copper deficiency were performed. The CSF analysis included cell count (normal ⩽4 cells μl−1), protein levels and electrophoresis with silver staining for detection of OCB. Calculation of albumin quotient (normal <7.4) and IgG index (<0.57) was performed.
Demographic data and clinical examination
The clinical features of spinal cord dysfunction were classified according to the International Standards for Neurological and Functional Classification of Spinal Cord injury proposed by the American Spinal Injury Association (ASIA).6 The initial degree of impairment was measured by the ASIA impairment scale. ASIA score grade D was regarded as mild, C as moderate and B and A as severe disability.
The neurological level was defined by the most caudal segment of the spinal cord with normal sensory and motor function on both sides. Cervical, thoracic or lumbosacral neurological levels were distinguished. The distribution of the neurological syndrome was rated as segmental, quadrant, hemicorporal, para- and tetrasymptomatic.
MRI examination
All patients underwent a spinal cord MRI on a 1.5-T MR scanner for diagnostic workup. Sagittal T1- and T2-weighted and axial T2-weighted fast spin echo sequences were performed routinely, and usually also sagittal/axial T1-weighted images after DTPA gadolinium administration. Lesion characteristics were evaluated by a rater blinded to symptoms and neuroradiological examination reports. The analysis parameters included the extension of the lesion on sagittal images and classification of the axial distribution. Axial lesion distribution was scored as lateral, centromedullary, anterior, posterior and complete (Figure 2). When a lesion expanded over two segments, the segment involved by the largest part of the lesion was taken for analysis of axial lesion topography. Brain MRI was performed in 62/63 patients and scans were normal in 16 patients (25.8%). MS-typical distribution of brain lesions was found in 39 patients (62.9%) whereas unspecific lesions were detected in 7 patients (11.3%).
Results
Patient demographics
We identified 63 patients (43 women, 68.3%). Mean age (s.d.; range) was 37.8 years (10.6; 17.0–61.9).
Neurological level and syndrome
Most frequently the neurological level was located within the cervical (n=29 (46%)) and thoracic spinal cord (n=26 (41.3%)). A lumbosacral level was less frequent (n=8 (12.7%)).
Different clinical syndromes were determined at presentation (Table 1). The most frequent neurological syndrome was a para-symptomatic involvement (46%), followed by hemi- and tetra-symptomatic (27% and 10%, respectively). Rarely a quadrant (6%) or a segmental (1.6%) distribution of symptoms was present.
Presenting symptoms
All of our patients had sensory disturbances at presentation (n=63; (100%)), as shown in Table 2. Dysfunction of the motor system was observed in 30 patients (47.6%), whereas autonomic failure was found in 12 patients (19.0%).
Clinical impairment
The majority of our cohort suffered from mild impairment (ASIA D; n=59 (93.8%)), whereas impairment was moderate (ASIA C; n=3 (4.8%)) or severe (ASIA A and B; A, n=1 (2.6%)) only in a few patients (Figure 1).
Laboratory findings
Data on cell count were available in 59 patients, and for albumin quotient, IgG index and OCB in 61 patients. Pleocytosis was present in 29/59 patients (49.2%), the mean cell count (s.d., range) was 9.0 cells μl−1 (14.8, 1–90). Pathological albumin indices were found in 11/61 patients (18.0%) and abnormal IgG indices in 51/61 patients (82.0%). Mean albumin index was 5.4 (2.2, 1.6–11.8) and mean IgG index was 1.1 (0.9, 0.4–5.9). OCBs were present in the CSF of 51/61 patients (83.6%).
MRI lesion characteristics
A spinal cord lesion suggestive of myelitis was present in 57 patients (90.4%). A total of 110 lesions were detected. Among these 57 patients with detectable MRI lesions, 56 patients underwent contrast imaging with DTPA–gadolinium. Contrast enhancement of one or more lesion(s) was found in 35 patients (62.5%). A repeat MRI was not performed in patients in whom a spinal cord lesion could not be detected on the initial scan. Among patients without a visible spinal cord lesion on MRI, two patients had pure sensory deficits whereas in the remaining four patients a combination of sensory and motor/autonomic impairment was ascertained.
A single lesion was detected in 29/57 patients (50.9%) (Figure 2). The mean number of lesions was 1.93 (s.d. 1.28, range 0–6). MRI lesions were detected within the cervical cord (27/57, 47.4%), thoracic cord (14/57, 24.6%), or both in cervical and thoracic cord (16/57, 28.1%).
One hundred and one of 110 (91.8%) lesions were smaller than one vertebral segment. Four lesions (3.6%) extended between the length of one and two vertebral bodies, and five (4.5%) over more than two vertebral bodies (Figure 2). No lesion was longer than three vertebral bodies. The mean lesion length was 13.3 mm (s.d. 7.4, range 3.0–52.8). Lesions within the cervical cord had a mean length of 13.3 mm (6.4; 3.0–30), and within the thoracic cord 13.0 mm (8.7; 4.0–52.8). CSF variables did not differ between patients with lesions <1 vs >1 vertebral segment.
Axial MRI lesion topography
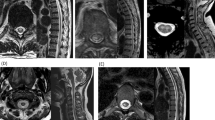
One hundred and ten lesions of 57 patients were evaluated for topography on T2-weighted axial scans (Figure 3). The most frequent localizations were lateral (n=39; 35.5%), centromedullary (n=29; 26.4%) and posterior (n=27; 24.5%). Anterior lesion localizations (n=12; 10.9%) and lesions covering the entire axial section (n=3; 12%) were less frequent. In patients with thoracic lesions only, no anterior lesion was seen.
Axial topography of 110 spinal cord lesions from 57 patients with acute transverse myelitis. (a–c) Examples of the most frequent axial lesion distributions on T2-weighted scans are shown (A lateral, B dorsal and C centromedullary). (d) Schematic presentation of different lesions types. (e) Frequencies of different lesion types.
Diagnosis of acute transverse myelitis
The diagnosis of acute transverse myelitis was supported by both MRI lesion detection within the spinal cord and at least one pathological CSF finding (cell count, IgG index, albumin quotient or OCB) in 52 patients (85.2%). In a few patients the diagnosis was supported either by a spinal cord lesion visible on MRI (n=5; 7.9%) or abnormal CSF findings (n=4; 8.2%) alone. In the remaining two patients, transcranial magnetic stimulation indicated a spinal cord lesion by detection of central motor conduction deficits and the diagnosis of transverse myelitis could only be assumed after ruling out other etiologies.
Discussion
A broad range of neurological disturbances and neuroradiological findings can be encountered in acute transverse myelitis. However, signs and symptoms are mostly mild and loss of walking ability is rare. With an ASIA score D rating in 93.7% of our patients, impairment was not as severe as in spinal cord ischemia (ASIA D 42%) or traumatic spinal cord injury (ASIA C 57%) (Figure 1).10, 11 In all our patients, sensory deficits were present at presentation. Motor dysfunction was found in about half (47.5%), and pareses exceeding grade 3 were found in four patients only (⩽ASIA C). In contrast, in spinal cord ischemia motor deficits were reported in 35–100%.7, 10 High frequencies of bowel and bladder dysfunction are particularly seen in patients with traumatic conus medullaris syndrome, but may also be present in transverse myelitis.12, 13 Bladder dysfunction was less frequently present in our cohort (19%), which might be because of an overrepresentation of milder and preferably MS-related cases seen at our tertiary care center.
Neurological examination including assessment of neurological levels is a key step for obtaining evidence of a spinal dysfunction and decision on further examinations. Admittedly, only a moderate agreement between neurological levels and neuroimaging findings is reported in the literature. For instance the concordance rate in cervical compressive myelopathy is only 65%.14 The spinal levels were mostly located within the cervical and thoracic dermatomes (46% and 41.3%, respectively). This finding is distinct from spinal cord ischemia, where neurological levels are predominantly thoracal and lumbosacral (39% and 40%, respectively).10 Neurological dysfunction occurred most frequently with para-syndromatic features (46%). Other manifestations were hemicorporal and tetra-syndromatic (27% and 15.9%, respectively). Single segment or quadrant distributions of symptoms were rare but may not be easily recognized as a spinal cord syndrome.
The diagnostic criteria proposed by the Working Group for Idiopathic Transverse Myelitis require either the evidence of inflammation in gadolinium-enhanced MRI or CSF.15 The frequency of detectable spinal cord MRI lesions was high (90.4%) but contrast enhancement was not always present (60.3%). Different MR sequences can be used for the detection of spinal cord lesions. Short-tau inversion recovery fast spin echo sequences and T2-weighted fast spin echo sequences were reported to be most accurate.16 This might be considered particularly in patients with minimal deficits in which only minor lesions may be anticipated. Interestingly, pure sensory deficits were only present in one third of our patients without visible lesions. Similar numbers of negative spinal cord MR examination have been reported in the literature and it is believed that a combination of both, the technical progress of spinal cord MRI and examinations at an earlier time point will lower this percentage.9 Indeed, the more subacute development of transverse myelitis and limited expansion of lesions may also account for this shortcoming. Particularly post-contrast MRI might be helpful for distinguishing spinal cord neoplasm and inflammation in case of normal CSF examination.17
A mean lesion number of 1.27 (range 1–6) was reported in a French cohort of patients with transverse myelitis and the number of spinal cord lesions was found to be associated with poor outcome in MS-related myelitis.2 About half of our patients had a single MRI lesion (50.9%), and in the remaining multiple and up to 6 lesions were detected (Figure 2). MRI lesions were found in accordance to neurological levels most frequently within the cervical cord, resembling findings from earlier studies of transverse myelitis in children and adults.18, 19 Longitudinal lesion expansion usually did not exceed the height of one vertebral body (91.8%), but a few patients showed lesions expanding over the height of two to three vertebral bodies. It is believed that patients with extensive lesions may suffer from more severe disability and have an unfavorable prognosis.20 Most frequently lateral (35.5%), centromedullary (26.4%) and posterior (24.5%) lesions were detected on axial scans (Figure 3). Interestingly, posterior-lateral lesions were reported to be associated with increased risk for development of MS.2
Cerebrospinal fluid diagnostics are the mainstay for ruling out infectious causes,21 and may support the diagnosis of transverse myelitis by providing evidence for inflammation. Abnormal CSF findings were less frequent than evidence for MRI lesions during workup. OCB (83.6%) and abnormal IgG index (82.0%) were the most common pathological CSF findings. Normal WBC counts were detected in CSF in about 50% of the patients, a similar frequency was reported in childhood transverse myelitis.19
Assessment of the risk for conversion to MS is of major importance due to the option of early initiation of immunomodulatory treatment. The performance of a brain MRI and detection of MS-typical brain lesions may be helpful for defining a subgroup at high risk for conversion to MS.2, 3 In a cohort of patients with acute transverse myelitis, MS-typical MRI lesions may be detected in more than two third of the patients.2, 22 The diagnostic criteria for idiopathic transverse myelitis demand a negative brain MRI.15 Applying this criterion, the diagnosis of idiopathic transverse myelitis was made in only 15.6% of all patients with non-compressive myelopathies.18 In our series, patients with negative brain MRI made up 11.3%, and we also detected unspecific brain MRI lesions in 25.8%. Our patients had a mean age of 37.8 years, which was just between the mean age of studies by French investigators on idiopathic acute transverse myelitis and acute partial transverse myelitis (mean age 38.3 and 35 years, respectively).2, 18 However, transverse myelitis during childhood which has peaks between 0–2 and 5–8, and >10 years of age was not covered by the present study.19 None fulfilled the criteria of neuromyelitis optica but testing for aquaporin-4 antibodies was not available when most of the patients were seen.
This study confirms and expands earlier findings in acute transverse myelitis. In our cohort with a high percentage of presumably MS-related myelitis, most patients presented with sensory deficits and mild functional impairment, but the clinical presentation can be highly variable. Signal abnormalities on spinal cord MRI that mostly extend less than the height of one vertebral body, are rarely located anteriorly and rarely cover the entire cord cross section. Oligoclonal bands are the most frequent abnormal CSF finding. Nevertheless, in a few patients ancillary investigations will rule out most spinal cord disorders and leave the clinician with a suspicion but no proof of an inflammatory lesion. In the future, new serological markers may help to distinguish between specific myelitis subtypes and assist in the diagnostic workup.
References
Krishnan C, Kaplin AI, Pardo CA, Kerr DA, Keswani SC . Demyelinating disorders: update on transverse myelitis. Curr Neurol Neurosci Rep 2006; 6: 236–243.
Cordonnier C, de Seze J, Breteau G, Ferriby D, Michelin E, Stojkovic T et al. Prospective study of patients presenting with acute partial transverse myelopathy. J Neurol 2003; 250: 1447–1452.
Sellner J, Luthi N, Buhler R, Gebhardt A, Findling O, Greeve I et al. Acute partial transverse myelitis: risk factors for conversion to multiple sclerosis. Eur J Neurol 2008; 15: 398–405.
de Seze J, Stojkovic T, Breteau G, Lucas C, Michon-Pasturel U, Gauvrit JY et al. Acute myelopathies: Clinical, laboratory and outcome profiles in 79 cases. Brain 2001; 124 (Pt 8): 1509–1521.
Kurtzke JF . Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983; 33: 1444–1452.
Maynard Jr FM, Bracken MB, Creasey G, Ditunno Jr JF, Donovan WH, Ducker TB et al. International Standards for Neurological and Functional Classification of Spinal Cord Injury. American Spinal Injury Association. Spinal Cord 1997; 35: 266–274.
Nowak DA, Mutzenbach S, Fuchs HH . Acute myelopathy. Retrospective clinical, laboratory, MRI and outcome analysis of 49 cases. J Clin Neurosci 2004; 11: 145–152.
Scott TF, Kassab SL, Singh S . Acute partial transverse myelitis with normal cerebral magnetic resonance imaging: transition rate to clinically definite multiple sclerosis. Mult Scler 2005; 11: 373–377.
Scott TF . Nosology of idiopathic transverse myelitis syndromes. Acta Neurol Scand 2007; 115: 371–376.
Nedeltchev K, Loher TJ, Stepper F, Arnold M, Schroth G, Mattle HP et al. Long-term outcome of acute spinal cord ischemia syndrome. Stroke 2004; 35: 560–565.
Pagliacci MC, Celani MG, Zampolini M, Spizzichino L, Franceschini M, Baratta S et al. An Italian survey of traumatic spinal cord injury. The Gruppo Italiano Studio Epidemiologico Mielolesioni study. Arch Phys Med Rehabil 2003; 84: 1266–1275.
McKinley W, Santos K, Meade M, Brooke K . Incidence and outcomes of spinal cord injury clinical syndromes. J Spinal Cord Med 2007; 30: 215–224.
Kalita J, Shah S, Kapoor R, Misra UK . Bladder dysfunction in acute transverse myelitis: magnetic resonance imaging and neurophysiological and urodynamic correlations. J Neurol Neurosurg Psychiatry 2002; 73: 154–159.
Matsumoto M, Ishikawa M, Ishii K, Nishizawa T, Maruiwa H, Nakamura M et al. Usefulness of neurological examination for diagnosis of the affected level in patients with cervical compressive myelopathy: prospective comparative study with radiological evaluation. J Neurosurg Spine 2005; 2: 535–539.
Transverse Myelitis Consortium Working Group (TMCW). Proposed diagnostic criteria and nosology of acute transverse myelitis. Neurology 2002; 59: 499–505.
Campi A, Pontesilli S, Gerevini S, Scotti G . Comparison of MRI pulse sequences for investigation of lesions of the cervical spinal cord. Neuroradiology 2000; 42: 669–675.
Spirovski M, Kozic D, Kopitovic A, Ostojic J . Importance of axial postcontrast images in the differential diagnosis between inflammatory and neoplastic spinal cord enlargement. Clinical neurology and neurosurgery 2007; 109: 931–933.
de Seze J, Lanctin C, Lebrun C, Malikova I, Papeix C, Wiertlewski S et al. Idiopathic acute transverse myelitis: application of the recent diagnostic criteria. Neurology 2005; 65: 1950–1953.
Pidcock FS, Krishnan C, Crawford TO, Salorio CF, Trovato M, Kerr DA . Acute transverse myelitis in childhood: center-based analysis of 47 cases. Neurology 2007; 68: 1474–1480.
Scott T, Weikers N, Hospodar M, Wapenski J . Acute transverse myelitis: a retrospective study using magnetic resonance imaging. Can J Neurol Sci 1994; 21: 133–136.
Sellner J, Ebinger F, Jacobi C, Meyding-Lamadé U . Viral Meningoenzephalitis. Intensivmed 2005; 42: 136–145.
Bruna J, Martinez-Yelamos S, Martinez-Yelamos A, Rubio F, Arbizu T . Idiopathic acute transverse myelitis: a clinical study and prognostic markers in 45 cases. Mult Scler 2006; 12: 169–173.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sellner, J., Lüthi, N., Schüpbach, W. et al. Diagnostic workup of patients with acute transverse myelitis: spectrum of clinical presentation, neuroimaging and laboratory findings. Spinal Cord 47, 312–317 (2009). https://doi.org/10.1038/sc.2008.143
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sc.2008.143
Keywords
This article is cited by
-
Acute transverse myelitis in Lyme neuroborreliosis
Infection (2010)
-
Radiologisch-isoliertes Syndrom
Der Nervenarzt (2010)