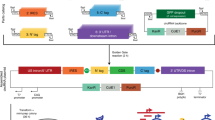
Abstract
Genetic engineering to improve the capabilities of plants is essential given climate change and population growth pressures. Current manipulation methods are laborious and species dependent, which limits advances in agriculture and molecular farming. Therefore, new approaches and tools are needed to broaden the range of transformable species and increase the throughput at which transformation is achieved. Nanotechnology has revolutionized delivery, sensing and imaging in microbial and animal systems, but its application in plants remains scant. However, reports of nano-mediated delivery for the genetic manipulation of plants have emerged, including direct germline editing as well as plastid and mitochondrial genome modification. Here, we review the application of nanotechnology to plant genetic manipulation, including the development of nanocarriers for the delivery of genetic cargos and advances in nano-mediated plant regeneration. Particular focus is given to understanding structure–function relationships for the rational design of nanocarriers, and how these developments can catalyse progress in nucleic acid and protein delivery for plant biotechnology applications.
Key points
-
Population growth and climate change pose serious challenges to plant-based systems, requiring improvement through genetic manipulation to ensure their maintenance.
-
Current manipulation methods are amenable to a limited range of species and with low throughput. Nanotechnology-based strategies could overcome these limitations.
-
Advances in understanding nanomaterial structure–function relationships enable the development of first-principle models of the cellular fate of carriers and design heuristics related to size, charge and shape.
-
Nanotechnology-mediated delivery of site-specific nucleases and large cargos, such as transcription factors, is a promising strategy to improve the efficiency of genetic manipulation in plants.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Cunningham, F. J., Goh, N. S., Demirer, G. S., Matos, J. L. & Landry, M. P. Nanoparticle-mediated delivery towards advancing plant genetic engineering. Trends Biotechnol. https://doi.org/10.1016/j.tibtech.2018.03.009 (2018).
Buyel, J. F. Plants as sources of natural and recombinant anti-cancer agents. Biotechnol. Adv. 36, 506–520 (2018).
Chung, Y. H. et al. Integrating plant molecular farming and materials research for next-generation vaccines. Nat. Rev. Mater. 7, 372–388 (2022).
Waring, B. et al. Forests and decarbonization — roles of natural and planted forests. Front. For. Global Change 3, 58 (2020).
Dasgupta, S. & Robinson, E. J. Z. Attributing changes in food insecurity to a changing climate. Sci. Rep. 12, 4709 (2022).
FAO. The State of Food Security and Nutrition in the World 2022 (FAO, 2022).
Fischer, R. & Buyel, J. F. Molecular farming — the slope of enlightenment. Biotechnol. Adv. 40, 107519 (2020).
Schillberg, S. & Finnern, R. Plant molecular farming for the production of valuable proteins - critical evaluation of achievements and future challenges. J. Plant Physiol. 258–259, 153359 (2021).
Mora, C. et al. Over half of known human pathogenic diseases can be aggravated by climate change. Nat. Clim. Chang. https://doi.org/10.1038/s41558-022-01426-1 (2022).
Pingali, P. L. Green revolution: impacts, limits, and the path ahead. Proc. Natl Acad. Sci. USA 109, 12302–12308 (2012).
Breseghello, F. & Coelho, A. S. G. Traditional and modern plant breeding methods with examples in rice (Oryza sativa L.). J. Agric. Food Chem. 61, 8277–8286 (2013).
Bevan, M. Binary agrobacterium vectors for plant transformation. Nucleic Acids Res. https://doi.org/10.1093/nar/12.22.8711 (1984).
Klein, T. M., Wolf, E. D., Wu, R. & Sanford, J. C. High-velocity microprojectiles for delivering nucleic acids into living cells. Nature 327, 70–73 (1987).
McNulty, M. J. et al. Molecular pharming to support human life on the moon, mars, and beyond. Crit. Rev. Biotechnol. 41, 849–864 (2021).
Ku, H.-K. & Ha, S.-H. Improving nutritional and functional quality by genome editing of crops: status and perspectives. Front. Plant Sci. 11, 577313 (2020).
Sanzari, I., Leone, A. & Ambrosone, A. Nanotechnology in plant science: to make a long story short. Front. Bioeng. Biotechnol. 7, 120 (2019).
Buschmann, M. D. et al. Nanomaterial delivery systems for mRNA vaccines. Vaccines 9, 65 (2021).
Patra, J. K. et al. Nano based drug delivery systems: recent developments and future prospects. J. Nanobiotechnol. 16, 71 (2018).
Willner, M. R. & Vikesland, P. J. Nanomaterial enabled sensors for environmental contaminants. J. Nanobiotechnol. 16, 95 (2018).
Li, H., Merkl, P., Sommertune, J., Thersleff, T. & Sotiriou, G. A. SERS Hotspot engineering by aerosol self‐assembly of plasmonic Ag nanoaggregates with tunable interparticle distance. Adv. Sci. 9, 2201133 (2022).
El-Shetehy, M. et al. Silica nanoparticles enhance disease resistance in arabidopsis plants. Nat. Nanotechnol. https://doi.org/10.1038/s41565-020-00812-0 (2020).
Zarattini, M. et al. LPMO-Oxidized cellulose oligosaccharides evoke immunity in arabidopsis conferring resistance towards necrotrophic fungus B. cinerea. Commun. Biol. 4, 727 (2021).
Demirer, G. S. et al. High aspect ratio nanomaterials enable delivery of functional genetic material without DNA integration in mature plants. Nat. Nanotechnol. 14, 456–464 (2019).
Kwak, S.-Y. et al. Chloroplast-selective gene delivery and expression in planta using chitosan-complexed single-walled carbon nanotube carriers. Nat. Nanotechnol. 14, 447–455 (2019).
Odahara, M. et al. Nanoscale polyion complex vesicles for delivery of cargo proteins and Cas9 ribonucleoprotein complexes to plant cells. ACS Appl. Nano Mater 4, 5630–5635 (2021).
Schwartz, S. H., Hendrix, B., Hoffer, P., Sanders, R. A. & Zheng, W. Carbon dots for efficient small interfering RNA delivery and gene silencing in plants. Plant Physiol 184, 647–657 (2020).
Zhang, H. et al. Nanoparticle cellular internalization is not required for RNA delivery to mature plant leaves. Nat. Nanotechnol. 17, 197–205 (2022).
Krens, F. A., Molendijk, L., Wullems, G. J. & Schilperoort, R. A. In vitro transformation of plant protoplasts with Ti-plasmid DNA. Nature 296, 72–74 (1982).
Burnett, M. J. B. & Burnett, A. C. Therapeutic recombinant protein production in plants: challenges and opportunities. Plants People Planet 2, 121–132 (2020).
Marillonnet, S., Thoeringer, C., Kandzia, R., Klimyuk, V. & Gleba, Y. Systemic agrobacterium tumefaciens–mediated transfection of viral replicons for efficient transient expression in plants. Nat. Biotechnol. 23, 718–723 (2005).
Niazian, M., Sadat Noori, S. A., Galuszka, P. & Mortazavian, S. M. M. Tissue culture-based agrobacterium-mediated and in planta transformation methods. Czech J. Genet. Plant Breed 53, 133–143 (2017).
Nyaboga, E., Tripathi, J. N., Manoharan, R. & Tripathi, L. Agrobacterium-mediated genetic transformation of Yam (Dioscorea rotundata): an important tool for functional study of genes and crop improvement. Front. Plant Sci. 5, 463 (2014).
Song, G., Prieto, H. & Orbovic, V. Agrobacterium-mediated transformation of tree fruit crops: methods, progress, and challenges. Front. Plant Sci. 10, 226 (2019).
Gelvin, S. B. Integration of agrobacterium T-DNA into the plant genome. Annu. Rev. Genet. 51, 195–217 (2017).
Lowe, K. et al. Morphogenic regulators Baby Boom and Wuschel improve monocot transformation. Plant Cell 28, 1998–2015 (2016).
Kong, J. et al. Overexpression of the transcription factor growth-regulating factor5 improves transformation of dicot and monocot species. Front. Plant Sci. 11, 572319 (2020).
Cheng, M., Lowe, B. A., Spencer, T. M., Ye, X. & Armstrong, C. L. Factors influencing agrobacterium-mediated transformation of monocotyledonous species. In Vitro Cell. Dev. Biol. Plant 40, 31–45 (2004).
Tie, W. et al. Reasons for lower transformation efficiency in Indica rice using agrobacterium tumefaciens-mediated transformation: lessons from transformation assays and genome-wide expression profiling. Plant Mol. Biol. 78, 1–18 (2012).
De Saeger, J. et al. Agrobacterium strains and strain improvement: present and outlook. Biotechnol. Adv. 53, 107677 (2021).
Ikeuchi, M. et al. Molecular mechanisms of plant regeneration. Annu. Rev. Plant Biol. 70, 377–406 (2019).
Lian, Z. et al. Application of developmental regulators to improve in planta or in vitro transformation in plants. Plant Biotechnol. J. 20, 1622–1635 (2022).
Singh, R. K. & Prasad, M. Advances in agrobacterium tumefaciens-mediated genetic transformation of graminaceous crops. Protoplasma 253, 691–707 (2016).
Boynton, J. E. et al. Chloroplast transformation in Chlamydomonas with high velocity microprojectiles. Science 240, 1534–1538 (1988).
Liu, J. et al. Genome-scale sequence disruption following biolistic transformation in rice and maize. Plant Cell 31, 368–383 (2019).
Ahmad, N., Michoux, F., Lössl, A. G. & Nixon, P. J. Challenges and perspectives in commercializing plastid transformation technology. J. Expt. Bot. 67, 5945–5960 (2016).
Burris, K. P., Dlugosz, E. M., Collins, A. G., Stewart, C. N. & Lenaghan, S. C. Development of a rapid, low-cost protoplast transfection system for switchgrass (Panicum virgatum L.). Plant Cell Rep. 35, 693–704 (2016).
Vanitharani, R., Chellappan, P. & Fauquet, C. M. Short interfering RNA-mediated interference of gene expression and viral DNA accumulation in cultured plant cells. Proc. Natl Acad. Sci. USA 100, 9632–9636 (2003).
Woo, J. W. et al. DNA-free genome editing in plants with preassembled CRISPR-Cas9 ribonucleoproteins. Nat. Biotechnol. 33, 1162–1164 (2015).
Kang, B.-C. et al. Chloroplast and mitochondrial DNA editing in plants. Nat. Plants 7, 899–905 (2021).
Reed, K. M. & Bargmann, B. O. R. Protoplast regeneration and its use in new plant breeding technologies. Front. Genome Ed. 3, 734951 (2021).
Altpeter, F. et al. Advancing crop transformation in the era of genome editing. Plant Cell https://doi.org/10.1105/tpc.16.00196 (2016).
Anjanappa, R. B. & Gruissem, W. Current progress and challenges in crop genetic transformation. J. Plant Physiol. 261, 153411 (2021).
Leva, A. & Rinaldi, L. (eds) Recent Advances in Plant in Vitro Culture (IntechOpen, 2012).
George, E. F., Hall, M. A. & Klerk, G.-J. D. Plant Propagation by Tissue Culture: Volume 1. The Background (Springer Science & Business Media, 2007).
Vasil, I. K. & Vasil, V. Totipotency and embryogenesis in plant cell and tissue cultures. In Vitro 8, 117–125 (1972).
Altpeter, F. et al. Particle bombardment and the genetic enhancement of crops: myths and realities. Mol. Breeding 15, 305–327 (2005).
Gao, C. Genome engineering for crop improvement and future agriculture. Cell 184, 1621–1635 (2021).
Saha, P. & Blumwald, E. Spike‐dip transformation of Setaria Viridis. Plant J. 86, 89–101 (2016).
Soman, J., Hema, J. & Subramanian, S. In Advances in Plant Transgenics: Methods and Applications (eds Sathishkumar, R., Kumar, S. R., Hema, J. & Baskar, V.) 3–22 (Springer Singapore, 2019).
Thorpe, T. A. History of plant tissue culture. Mol. Biotechnol. 37, 169–180 (2007).
Erland, L. A. E., Shukla, M. R., Glover, W. B. & Saxena, P. K. A simple and efficient method for analysis of plant growth regulators: a new tool in the chest to combat recalcitrance in plant tissue culture. Plant Cell Tiss. Organ Cult. 131, 459–470 (2017).
Kothari, S. L., Joshi, A., Kachhwaha, S. & Ochoa-Alejo, N. Chilli peppers — a review on tissue culture and transgenesis. Biotechnol. Adv. 28, 35–48 (2010).
Yaqoob, U., Kaul, T. & Nawchoo, I. A. In vitro plant regeneration of some recalcitrant Indica rice (Oryza sativa L.) varieties. Vegetos 34, 102–106 (2021).
Han, Y., Jin, X., Wu, F. & Zhang, G. Genotypic differences in callus induction and plant regeneration from mature embryos of barley (Hordeum vulgare L.). J. Zhejiang Univ. Sci. B 12, 399–407 (2011).
Che, P. et al. Developing a flexible, high-efficiency agrobacterium-mediated Sorghum transformation system with broad application. Plant Biotechnol. J. 16, 1388–1395 (2018).
Azhakanandam, K. & Zhang, Z. J. In Recent Advancements in Gene Expression and Enabling Technologies in Crop Plants (eds Azhakanandam, K., Silverstone, A., Daniell, H. & Davey, M. R.) 291–312 (Springer, 2015).
Nguyen, T.-V., Thanh Thu, T., Claeys, M. & Angenon, G. Agrobacterium-mediated transformation of Sorghum (Sorghum bicolor (L.) moench) using an improved in vitro regeneration system. Plant Cell Tiss. Organ. Cult 91, 155–164 (2007).
Casas, A. M. et al. Transgenic Sorghum plants via microprojectile bombardment. Proc. Natl Acad. Sci. USA 90, 11212–11216 (1993).
Zhao, Z. et al. Agrobacterium-mediated sorghum transformation. Plant Mol. Biol. 44, 789–798 (2000).
Liu, G. & Godwin, I. D. Highly efficient sorghum transformation. Plant Cell Rep. 31, 999–1007 (2012).
Wu, E. et al. Optimized agrobacterium-mediated sorghum transformation protocol and molecular data of transgenic sorghum plants. In Vitro Cell. Dev. Biol. Plant 50, 9–18 (2014).
Fus-Kujawa, A. et al. An overview of methods and tools for transfection of eukaryotic cells in vitro. Front. Bioeng. Biotechnol. 9, 701031 (2021).
Joersbo, M. & Brunstedt, J. In Electrical Manipulation of Cells (eds Lynch, P. T. & Davey, M. R.) 201–222 (Springer, 1996).
Neuhaus, G. & Spangenberg, G. Plant transformation by microinjection techniques. Physiol. Plant 79, 213–217 (1990).
Trick, H. N. & Finer, J. J. SAAT: sonication-assisted agrobacterium-mediated transformation. Transgenic Res. 6, 329–336 (1997).
Azencott, H. R., Peter, G. F. & Prausnitz, M. R. Influence of the cell wall on intracellular delivery to algal cells by electroporation and sonication. Ultrasound Med. Biol. https://doi.org/10.1016/j.ultrasmedbio.2007.05.008 (2007).
Vargason, A. M., Anselmo, A. C. & Mitragotri, S. The evolution of commercial drug delivery technologies. Nat. Biomed. Eng. 5, 951–967 (2021).
Li, C. et al. Recent progress in drug delivery. Acta Pharm. Sin. B 9, 1145–1162 (2019).
Roberts, T. C., Langer, R. & Wood, M. J. A. Advances in oligonucleotide drug delivery. Nat. Rev. Drug Discov. https://doi.org/10.1038/s41573-020-0075-7 (2020).
Zhang, Q., Ying, Y. & Ping, J. Recent advances in plant nanoscience. Adv. Sci. https://doi.org/10.1002/advs.202103414 (2022).
Mujtaba, M. et al. Nanocarrier-mediated delivery of miRNA, RNAi, and CRISPR-Cas for plant protection: current trends and future directions. ACS Agric. Sci. Technol. https://doi.org/10.1021/acsagscitech.1c00146 (2021).
Wu, H., Santana, I., Dansie, J. & Giraldo, J. P. In vivo delivery of nanoparticles into plant leaves: delivery of nanoparticles into plant leaves. Curr. Protoc. Chem. Biol. 9, 269–284 (2017).
Dong, X. Current strategies for brain drug delivery. Theranostics 8, 1481–1493 (2018).
Cosgrove, D. J. Assembly and enlargement of the primary cell wall in plants. Annu. Rev. Cell Dev. Biol. 13, 171–201 (1997).
Wang, P., Lombi, E., Zhao, F. J. & Kopittke, P. M. Nanotechnology: a new opportunity in plant sciences. Trends Plant Sci. https://doi.org/10.1016/j.tplants.2016.04.005 (2016).
Seelig, A., Gottschlich, R. & Devant, R. M. A method to determine the ability of drugs to diffuse through the blood-brain barrier. Proc. Natl Acad. Sci. USA 91, 68–72 (1994).
Terstappen, G. C., Meyer, A. H., Bell, R. D. & Zhang, W. Strategies for delivering therapeutics across the blood–brain barrier. Nat. Rev. Drug Discov. 20, 362–383 (2021).
Sahay, G. et al. Efficiency of siRNA delivery by lipid nanoparticles is limited by endocytic recycling. Nat. Biotechnol. https://doi.org/10.1038/nbt.2614 (2013).
Rajput, V. et al. Accumulation of nanoparticles in the soil-plant systems and their effects on human health. Ann. Agric. Sci. 65, 137–143 (2020).
Basha, G. et al. Influence of cationic lipid composition on gene silencing properties of lipid nanoparticle formulations of siRNA in antigen-presenting cells. Mol. Ther. https://doi.org/10.1038/mt.2011.190 (2011).
Zinger, A. et al. Enhancing inflammation targeting using tunable leukocyte-based biomimetic nanoparticles. ACS Nano 15, 6326–6339 (2021).
Guo, B. et al. Native protein delivery into rice callus using ionic complexes of protein and cell-penetrating peptides. PLoS ONE 14, e0214033 (2019).
Zhao, X. et al. Pollen magnetofection for genetic modification with magnetic nanoparticles as gene carriers. Nature Plants https://doi.org/10.1038/s41477-017-0063-z (2017).
Wang, Z. et al. Efficient and genotype independent maize transformation using pollen transfected by DNA‐coated magnetic nanoparticles. JIPB 64, 1145–1156 (2022).
Law, S. S. Y. et al. Polymer-coated carbon nanotube hybrids with functional peptides for gene delivery into plant mitochondria. Nat. Commun. 13, 2417 (2022).
Wong, M. H. et al. Lipid exchange envelope penetration (LEEP) of nanoparticles for plant engineering: a universal localization mechanism. Nano Lett. 16, 1161–1172 (2016).
Lew, T. T. S. et al. Rational design principles for the transport and subcellular distribution of nanomaterials into plant protoplasts. Small 14, 1802086 (2018).
Su, Y. et al. Delivery, uptake, fate, and transport of engineered nanoparticles in plants: a critical review and data analysis. Environ. Sci. Nano 6, 2311–2331 (2019).
Murali, M. et al. Fate, bioaccumulation and toxicity of engineered nanomaterials in plants: current challenges and future prospects. Sci. Total Environ. 811, 152249 (2022).
Marmiroli, M. et al. Copper oxide nanomaterial fate in plant tissue: nanoscale impacts on reproductive tissues. Environ. Sci. Technol. 55, 10769–10783 (2021).
Milewska-Hendel, A., Zubko, M., Karcz, J., Stróż, D. & Kurczyńska, E. Fate of neutral-charged gold nanoparticles in the roots of the hordeum Vulgare L. Cultivar Karat. Sci. Rep. 7, 3014 (2017).
Santana, I., Wu, H., Hu, P. & Giraldo, J. P. Targeted delivery of nanomaterials with chemical cargoes in plants enabled by a biorecognition motif. Nat. Commun. 11, 2045 (2020).
Yoshizumi, T., Oikawa, K., Chuah, J.-A., Kodama, Y. & Numata, K. Selective gene delivery for integrating exogenous DNA into plastid and mitochondrial genomes using peptide–DNA complexes. Biomacromolecules 19, 1582–1591 (2018).
Liu, W. et al. Lipofection-mediated genome editing using DNA-free delivery of the Cas9/GRNA ribonucleoprotein into plant cells. Plant Cell Rep. 39, 245–257 (2020).
Silva, A. T., Nguyen, A., Ye, C., Verchot, J. & Moon, J. H. Conjugated polymer nanoparticles for effective SiRNA delivery to tobacco BY-2 protoplasts. BMC Plant Biol. 10, 291 (2010).
Liu, Q. et al. Carbon nanotubes as molecular transporters for walled plant cells. Nano Lett. 9, 1007–1010 (2009).
Ali, Z. et al. DNA–carbon nanotube binding mode determines the efficiency of carbon nanotube-mediated DNA delivery to intact plants. ACS Appl. Nano Mater 5, 4663–4676 (2022).
González-Grandío, E. et al. Carbon nanotube biocompatibility in plants is determined by their surface chemistry. J. Nanobiotechnol. 19, 431 (2021).
Lévy, R., Shaheen, U., Cesbron, Y. & Sée, V. Gold nanoparticles delivery in mammalian live cells: a critical review. Nano Rev. 1, 4889 (2010).
Ding, Y. et al. Gold nanoparticles for nucleic acid delivery. Mol. Ther. 22, 1075–1083 (2014).
Vijayakumar, P. S., Abhilash, O. U., Khan, B. M. & Prasad, B. L. V. Nanogold-loaded sharp-edged carbon bullets as plant-gene carriers. Adv. Funct. Mater. 20, 2416–2423 (2010).
Lei, W.-X. et al. Construction of gold-SiRNA NPR1 nanoparticles for effective and quick silencing of NPR1 in Arabidopsis thaliana. RSC Adv. 10, 19300–19308 (2020).
Zhang, H. et al. Gold-nanocluster-mediated delivery of SiRNA to intact plant cells for efficient gene knockdown. Nano Lett. 21, 5859–5866 (2021).
Huang, C., Zhang, Y., Yuan, H., Gao, H. & Zhang, S. Role of nanoparticle geometry in endocytosis: laying down to stand up. Nano Lett. 13, 4546–4550 (2013).
Yu, M. et al. Rotation-facilitated rapid transport of nanorods in mucosal tissues. Nano Lett 16, 7176–7182 (2016).
Vejlupkova, Z. et al. No evidence for transient transformation via pollen magnetofection in several monocot species. Nat. Plants 6, 1323–1324 (2020).
Vercoulen, P. H. W., Roos, R. A., Marijnissen, J. C. M. & Scarlett, B. Measuring electric charge on pollen. J. Aerosol Sci. 23, 377–380 (1992).
Kneuer, C. et al. A nonviral DNA delivery system based on surface modified silica-nanoparticles can efficiently transfect cells in vitro. Bioconjug. Chem 11, 926–932 (2000).
Radu, D. R. et al. A polyamidoamine dendrimer-capped mesoporous silica nanosphere-based gene transfection reagent. J. Am. Chem. Soc. 126, 13216–13217 (2004).
Bharali, D. J. et al. Organically modified silica nanoparticles: a nonviral vector for in vivo gene delivery and expression in the brain. Proc. Natl Acad. Sci. USA 102, 11539–11544 (2005).
Torney, F., Trewyn, B. G., Lin, V. S.-Y. & Wang, K. Mesoporous silica nanoparticles deliver DNA and chemicals into plants. Nat. Nanotech. 2, 295–300 (2007).
Martin-Ortigosa, S. et al. Mesoporous silica nanoparticle-mediated intracellular Cre protein delivery for maize genome editing via LoxP site excision. Plant Physiol 164, 537–547 (2014).
Mahmoud, L. M., Kaur, P., Stanton, D., Grosser, J. W. & Dutt, M. A cationic lipid mediated CRISPR/Cas9 technique for the production of stable genome edited citrus plants. Plant Methods 18, 33 (2022).
Karny, A., Zinger, A., Kajal, A., Shainsky-Roitman, J. & Schroeder, A. Therapeutic nanoparticles penetrate leaves and deliver nutrients to agricultural crops. Sci. Rep. 8, 7589 (2018).
Cai, Q. et al. Message in a bubble: shuttling small RNAs and proteins between cells and interacting organisms using extracellular vesicles. Annu. Rev. Plant Biol. 72, 497–524 (2021).
Doudna, J. A. & Charpentier, E. The new frontier of genome engineering with CRISPR-Cas9. Science 346, 1258096 (2014).
Shan, S., Soltis, P. S., Soltis, D. E. & Yang, B. Considerations in adapting CRISPR/Cas9 in nongenetic model plant systems. Appl. Plant Sci. https://doi.org/10.1002/aps3.11314 (2020).
Nekrasov, V., Staskawicz, B., Weigel, D., Jones, J. D. G. & Kamoun, S. Targeted mutagenesis in the model plant Nicotiana benthamiana using Cas9 RNA-guided endonuclease. Nat. Biotechnol. 31, 691–693 (2013).
Yuan, M. et al. Mutagenesis of FAD2 genes in peanut with CRISPR/Cas9 based gene editing. BMC Biotechnol. 19, 24 (2019).
Upadhyay, S. K., Kumar, J., Alok, A. & Tuli, R. RNA-guided genome editing for target gene mutations in wheat. G3 3, 2233–2238 (2013).
Kaur, N. et al. CRISPR/Cas9-mediated efficient editing in phytoene desaturase (PDS) demonstrates precise manipulation in banana cv. Rasthali genome. Funct. Integr. Genomics 18, 89–99 (2018).
Peng, A. et al. Engineering canker-resistant plants through CRISPR/Cas9-targeted editing of the susceptibility gene CsLOB1 promoter in citrus. Plant Biotechnol. J. 15, 1509–1519 (2017).
Zhang, Y. et al. Efficient and transgene-free genome editing in wheat through transient expression of CRISPR/Cas9 DNA or RNA. Nat. Commun. 7, 12617 (2016).
Hamada, H. et al. Biolistic-delivery-based transient CRISPR/Cas9 expression enables in planta genome editing in wheat. Sci. Rep. 8, 14422 (2018).
Harrington, L. B. et al. Programmed DNA destruction by miniature CRISPR-Cas14 enzymes. Science 362, 839–842 (2018).
Gangopadhyay, S. A. et al. Precision control of CRISPR-Cas9 using small molecules and light. Biochemistry 58, 234–244 (2019).
Harrington, L. B. et al. A thermostable Cas9 with increased lifetime in human plasma. Nat. Commun. 8, 1424 (2017).
Wang, J. W., Goh, N., Lien, E., González-Grandío, E. & Landry, M. P. Quantification of cell penetrating peptide mediated delivery of proteins in plant leaves. Preprint at bioRxiv https://doi.org/10.1101/2022.05.03.490515 (2022).
Joung, J. K. & Sander, J. D. TALENs: a widely applicable technology for targeted genome editing. Nat. Rev. Mol. Cell Biol. 14, 49–55 (2013).
Cermak, T. et al. Efficient design and assembly of custom TALEN and other TAL effector-based constructs for DNA targeting. Nucleic Acids Res. 39, e82 (2011).
Clasen, B. M. et al. Improving cold storage and processing traits in potato through targeted gene knockout. Plant Biotechnol. J. 14, 169–176 (2016).
Lor, V. S., Starker, C. G., Voytas, D. F., Weiss, D. & Olszewski, N. E. Targeted mutagenesis of the tomato PROCERA gene using transcription activator-like effector nucleases. Plant Physiol. 166, 1288–1291 (2014).
Li, T., Liu, B., Chen, C. Y. & Yang, B. TALEN-mediated homologous recombination produces site-directed DNA base change and herbicide-resistant rice. J. Genet. Genomics 43, 297–305 (2016).
Wang, Y. et al. Simultaneous editing of three homoeoalleles in hexaploid bread wheat confers heritable resistance to powdery mildew. Nat. Biotechnol. 32, 947–951 (2014).
Luo, S. et al. Non-transgenic plant genome editing using purified sequence-specific nucleases. Mol. Plant 8, 1425–1427 (2015).
Zhao, X. Y., Su, Y. H., Cheng, Z. J. & Zhang, X. S. Cell fate switch during in vitro plant organogenesis. J. Integr. Plant Biol. 50, 816–824 (2008).
An, C. et al. Nanomaterials and nanotechnology for the delivery of agrochemicals: strategies towards sustainable agriculture. J. Nanobiotechnol. 20, 11 (2022).
Pereira, A. E. S., Silva, P. M., Oliveira, J. L., Oliveira, H. C. & Fraceto, L. F. Chitosan nanoparticles as carrier systems for the plant growth hormone Gibberellic acid. Colloids Surf. B Biointerfaces 150, 141–152 (2017).
Li, X. et al. A triple-stimuli responsive hormone delivery system equipped with pillararene magnetic nanovalves. Mater. Chem. Front. 3, 103–110 (2019).
Kokina, I. et al. Target transportation of auxin on mesoporous Au/SiO2 nanoparticles as a method for somaclonal variation increasing in flax (L. Usitatissimum L.). J. Nanomater. 2017, e7143269 (2017).
Hwan Kim, D., Gopal, J. & Sivanesan, I. Nanomaterials in plant tissue culture: the disclosed and undisclosed. RSC Adv. 7, 36492–36505 (2017).
Lowe, K. et al. Rapid genotype “Independent” Zea Mays L. (Maize) transformation via direct somatic embryogenesis. In Vitro Cell. Dev. Biol. Plant 54, 240–252 (2018).
Bouchabké-Coussa, O. et al. Wuschel overexpression promotes somatic embryogenesis and induces organogenesis in cotton (Gossypium hirsutum L.) tissues cultured in vitro. Plant Cell Rep. 32, 675–686 (2013).
Maher, M. F. et al. Plant gene editing through de novo induction of meristems. Nat. Biotechnol. 38, 84–89 (2020).
Debernardi, J. M. et al. A GRF–GIF chimeric protein improves the regeneration efficiency of transgenic plants. Nat. Biotechnol. 38, 1274–1279 (2020).
Liu, Y. et al. Delivery of intact transcription factor by using self-assembled supramolecular nanoparticles. Angew. Chem. Int. Ed. 123, 3114–3118 (2011).
Patel, S. et al. NanoScript: a nanoparticle-based artificial transcription factor for effective gene regulation. ACS Nano 8, 8959–8967 (2014).
Rissanen, T. H. et al. Low intake of fruits, berries and vegetables is associated with excess mortality in men: the Kuopio Ischaemic Heart Disease Risk Factor (KIHD) study. J. Nutr. 133, 199–204 (2003).
Malik, K. A. & Maqbool, A. Transgenic crops for biofortification. Front. Sustain. Food Syst. 4, 571402 (2020).
Blando, F. et al. Nutraceutical characterization of anthocyanin-rich fruits produced by “Sun black” tomato line. Front. Nutr. 6, 133 (2019).
Butelli, E. et al. Enrichment of tomato fruit with health-promoting anthocyanins by expression of select transcription factors. Nat. Biotechnol. 26, 1301–1308 (2008).
Nonaka, S., Arai, C., Takayama, M., Matsukura, C. & Ezura, H. Efficient increase of γ-aminobutyric acid (GABA) content in tomato fruits by targeted mutagenesis. Sci. Rep. 7, 7057 (2017).
Ye, X. et al. Engineering the provitamin A (β-carotene) biosynthetic pathway into (carotenoid-free) rice endosperm. Science 287, 303–305 (2000).
Paine, J. A. et al. Improving the nutritional value of golden rice through increased pro-vitamin A content. Nat. Biotechnol. 23, 482–487 (2005).
Tian, Y.-S. et al. Enhancing carotenoid biosynthesis in rice endosperm by metabolic engineering. Plant Biotechnol. J. 17, 849–851 (2019).
Qu, Y. et al. Solution of the multistep pathway for assembly of corynanthean, strychnos, iboga, and aspidosperma monoterpenoid indole alkaloids from 19E-geissoschizine. Proc. Natl Acad. Sci. 115, 3180–3185 (2018).
Caputi, L. et al. Missing enzymes in the biosynthesis of the anticancer drug vinblastine in Madagascar Periwinkle. Science 360, 1235–1239 (2018).
Onda, Y. & Mochida, K. Exploring genetic diversity in plants using high-throughput sequencing techniques. Curr. Genomics 17, 358–367 (2016).
Santos, R. B., Abranches, R., Fischer, R., Sack, M. & Holland, T. Putting the spotlight back on plant suspension cultures. Front. Plant Sci. 7, 297 (2016).
Schillberg, S., Raven, N., Fischer, R., Twyman, R. M. & Schiermeyer, A. Molecular farming of pharmaceutical proteins using plant suspension cell and tissue cultures. Curr. Pharm. Des. 19, 5531–5542 (2013).
Lawson, C. E. et al. Machine learning for metabolic engineering: a review. Metab. Eng. 63, 34–60 (2021).
Moore, B. M. et al. Robust predictions of specialized metabolism genes through machine learning. Proc. Natl Acad. Sci. USA https://doi.org/10.1073/pnas.1817074116 (2019).
Colantonio, V. et al. Metabolomic selection for enhanced fruit flavor. Proc. Natl Acad. Sci. USA 119, e2115865119 (2022).
Demirer, G. S. et al. Carbon nanocarriers deliver siRNA to intact plant cells for efficient gene knockdown. Sci. Adv. 6, eaaz0495 (2020).
Derossi, D., Joliot, A. H., Chassaing, G. & Prochiantz, A. The third helix of the antennapedia homeodomain translocates through biological membranes. J. Biol. Chem. 269, 10444–10450 (1994).
Jönsson, H., Heisler, M. G., Shapiro, B. E., Meyerowitz, E. M. & Mjolsness, E. An auxin-driven polarized transport model for phyllotaxis. Proc. Natl Acad. Sci. USA 103, 1633–1638 (2006).
Zhang, T. et al. Phyllotactic patterning of gerbera flower heads. Proc. Natl Acad. Sci. USA 118, e2016304118 (2021).
Kuzma, J. Nanotechnology oversight and regulation — just do it. Environ. Law Rev. 36, 10913–10923 (2006).
Subramanian, K. S. & Rajkishore, S. K. in Nanomaterials: Ecotoxicity, Safety, and Public Perception (eds. Rai, M. & Biswas, J. K.) 319–342 (Springer International Publishing, 2018).
Landry, M. P. & Mitter, N. How nanocarriers delivering cargos in plants can change the GMO landscape. Nat. Nanotechnol. 14, 512–514 (2019).
Santana, I. et al. Targeted carbon nanostructures for chemical and gene delivery to plant chloroplasts. ACS Nano 16, 12156–12173 (2022).
Martin-Ortigosa, S. et al. Parameters affecting the efficient delivery of mesoporous silica nanoparticle materials and gold nanorods into plant tissues by the biolistic method. Small 8, 413–422 (2012).
Hahn, S., Giritch, A., Bartels, D., Bortesi, L. & Gleba, Y. A novel and fully scalable Agrobacterium spray-based process for manufacturing cellulases and other cost-sensitive proteins in plants. Plant Biotechnol. J. 13, 708–716 (2015).
Stadler, R. et al. Expression of GFP-fusions in arabidopsis companion cells reveals non-specific protein trafficking into sieve elements and identifies a novel post-phloem domain in roots: protein trafficking in the phloem. Plant J. 41, 319–331 (2004).
Zhang, Y., Klepsch, M. & Jansen, S. Bordered pits in xylem of vesselless angiosperms and their possible misinterpretation as perforation plates: bordered pits in xylem of vesselless angiosperms. Plant Cell Environ. 40, 2133–2146 (2017).
Zhu, Z.-J. et al. Effect of surface charge on the uptake and distribution of gold nanoparticles in four plant species. Environ. Sci. Technol. 46, 12391–12398 (2012).
Contento, A. L. & Bassham, D. C. Structure and function of endosomes in plant cells. J. Cell Sci. 125, 3511–3518 (2012).
Block, M. A., Douce, R., Joyard, J. & Rolland, N. Chloroplast envelope membranes: a dynamic interface between plastids and the cytosol. Photosynth. Res. 92, 225–244 (2007).
Murcha, M. W. et al. Protein import into plant mitochondria: signals, machinery, processing, and regulation. J. Exp. Bot. 65, 6301–6335 (2014).
Li, S., Chang, L. & Zhang, J. Advancing organelle genome transformation and editing for crop improvement. Plant Commun. 2, 100141 (2021).
Oey, M., Lohse, M., Kreikemeyer, B. & Bock, R. Exhaustion of the chloroplast protein synthesis capacity by massive expression of a highly stable protein antibiotic. Plant J. 57, 436–445 (2009).
Remacle, C., Cardol, P., Coosemans, N., Gaisne, M. & Bonnefoy, N. High-efficiency biolistic transformation of Chlamydomonas mitochondria can be used to insert mutations in complex I genes. Proc. Natl Acad. Sci. USA 103, 4771–4776 (2006).
Turnbull, C., Lillemo, M. & Hvoslef-Eide, T. A. K. Global regulation of genetically modified crops amid the gene edited crop boom — a review. Front. Plant Sci. 12, 630396 (2021).
ISAAA Inc. ISAAA Brief 55-2019; https://www.isaaa.org/resources/publications/briefs/55/executivesummary/default.asp (2020).
Potrykus, I. Regulation must be revolutionized. Nature 466, 561–561 (2010).
Wu, F. et al. Allow golden rice to save lives. Proc. Natl Acad. Sci. USA 118, e2120901118 (2021).
Paarlberg, R. A dubious success: the NGO campaign against GMOs. GM Crops Food 5, 223–228 (2014).
Hudson, M. et al. Indigenous perspectives and gene editing in Aotearoa New Zealand. Front. Bioeng. Biotechnol. 7, 70 (2019).
Rock, J. & Schurman, R. The complex choreography of agricultural biotechnology in Africa. Afr. Aff. 119, 499–525 (2020).
Acknowledgements
Co-lead authors contributed equally to this work and may revise the order of authorship when presenting this work. M.P.L. is a Chan-Zuckerberg Biohub investigator, a Hellen Wills Neuroscience Institute Investigator, and an IGI Investigator. H.J.S. and E.V. were supported by the Department of Defense (DoD) through the National Defense Science & Engineering Graduate (NDSEG) Fellowship Program.
Author information
Authors and Affiliations
Contributions
H.J.S. and S.T. contributed equally in leading the writing of the manuscript and drafting figures. E.V. and E.G.G. contributed to the writing of the manuscript. M.P.L. provided funding and direction for the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Bioengineering thanks Johannes Buyel, Keiji Numata and Edward Rybicki for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Squire, H.J., Tomatz, S., Voke, E. et al. The emerging role of nanotechnology in plant genetic engineering. Nat Rev Bioeng 1, 314–328 (2023). https://doi.org/10.1038/s44222-023-00037-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44222-023-00037-5