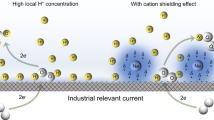
Abstract
Electrocatalytic hydrogenation enables the efficient treatment of oxidized contaminants in water under mild conditions, but its application has been greatly hindered by the need for a supporting electrolyte and the large amounts of unsafe intermediate products. Here we report the development of a rhodium nanoparticle-modified palladium membrane electrochemical reactor (Rh NPs-PdMER) for the hydrogenation of oxidized contaminants in drinking water and industrial effluents. The Rh NPs-PdMER not only enabled the hydrogenation of 12 different oxidized contaminants to safe products with ≥99% conversion and ≥95% yield in water without a supporting electrolyte, but also required a very low operating voltage of only 1.4 V. The performance of the Rh NPs-PdMER can be attributed to the specific reactor configuration and the unique adsorbability of the hydrogenated intermediates of the oxidized contaminants to the Rh nanoparticles.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data supporting the findings in this study are available within the paper and its Supplementary Information. Source data are provided with this paper.
References
Tao, T. & Xin, K. Public health: a sustainable plan for China’s drinking water. Nature 511, 527–528 (2014).
Llorente-Esteban, A. et al. Allosteric regulation of mammalian Na+/I− symporter activity by perchlorate. Nat. Struct. Mol. Biol. 27, 533–539 (2020).
Cai, Y., Long, X., Luo, Y. H., Zhou, C. & Rittmann, B. E. Stable dechlorination of trichloroacetic acid (TCAA) to acetic acid catalyzed by palladium nanoparticles deposited on H2-transfer membranes. Water Res. 192, 116841 (2021).
Liu, J., Olson, C., Qiu, N. & Sayes, C. M. Differential cytotoxicity of haloaromatic disinfection byproducts and lead co-exposures against human intestinal and neuronal cells. Chem. Res. Toxicol. 33, 2401–2407 (2020).
Ford, C. L., Park, Y. J., Matson, E. M., Gordon, Z. & Fout, A. R. A bioinspired iron catalyst for nitrate and perchlorate reduction. Science 354, 741–743 (2016).
Wu, Z. Y. et al. Electrochemical ammonia synthesis via nitrate reduction on Fe single atom catalyst. Nat. Commun. 12, 2870 (2021).
Ye, S. et al. Elucidating the activity, mechanism and application of selective electrosynthesis of ammonia from nitrate on cobalt phosphide. Energy Environ. Sci. 15, 760–770 (2022).
Qin, S., Lei, C., Wang, X., Chen, W. & Huang, B. Electrocatalytic activation of organic chlorides via direct and indirect electron transfer using atomic vacancy control of palladium-based catalyst. Cell Rep. Phys. Sci. 3, 100713 (2022).
Gu, Z., Zhang, Z., Ni, N., Hu, C. & Qu, J. Simultaneous phenol removal and resource recovery from phenolic wastewater by electrocatalytic hydrogenation. Environ. Sci. Technol. 56, 4356–4366 (2022).
Huang, D. et al. Elucidating the role of single-atom Pd for electrocatalytic hydrodechlorination. Environ. Sci. Technol. 55, 13306–13316 (2021).
Gan, G. et al. Nature of intrinsic defects in carbon materials for electrochemical dechlorination of 1,2-dichloroethane to ethylene. ACS Catal. 11, 14284–14292 (2021).
Zhang, J. et al. Electrochemical-driven nanoparticulate catalysis for highly efficient dechlorination of chlorinated environmental pollutant. J. Catal. 395, 362–374 (2021).
Isse, A. A., Huang, B., Durante, C. & Gennaro, A. Electrocatalytic dechlorination of volatile organic compounds at a copper cathode. Part I: polychloromethanes. Appl. Catal. B 126, 347–354 (2012).
Huang, B., Isse, A. A., Durante, C., Wei, C. & Gennaro, A. Electrocatalytic properties of transition metals toward reductive dechlorination of polychloroethanes. Electrochim. Acta 70, 50–61 (2012).
McGuire, C. M. & Peters, D. G. Electrochemical dechlorination of 4,4′-(2,2,2-trichloroethane-1,1-diyl)bis(chlorobenzene) (DDT) at silver cathodes. Electrochim. Acta 137, 423–430 (2014).
Liu, H. et al. Efficient electrochemical nitrate reduction to ammonia with copper-supported rhodium cluster and single-atom catalysts. Angew. Chem. Int. Ed. https://doi.org/10.1002/anie.202202556 (2022).
Guo, Y. et al. Pd doping-weakened intermediate adsorption to promote electrocatalytic nitrate reduction on TiO2 nanoarrays for ammonia production and energy supply with zinc–nitrate batteries. Energy Environ. Sci. 14, 3938–3944 (2021).
Xu, Y. et al. Single-Ni-atom catalyzes aqueous phase electrochemical reductive dechlorination reaction. Appl. Catal. B 277, 119057 (2020).
Zhou, Y. et al. Enhanced stabilization and effective utilization of atomic hydrogen on Pd−In nanoparticles in a flow-through electrode. Environ. Sci. Technol. 53, 11383–11390 (2019).
Mousset, E. & Doudrick, K. A review of electrochemical reduction processes to treat oxidized contaminants in water. Curr. Opin. Electrochem. 22, 221–227 (2020).
Mao, R. et al. Enhanced indirect atomic H* reduction at a hybrid Pd/graphene cathode for electrochemical dechlorination under low negative potentials. Environ. Sci. Nano 5, 2282–2292 (2018).
Sun, Z., Zhang, J., Wang, X. & Hu, X. Electrochemically reductive dechlorination of 2,4,6-trichlorophenol on palladium loaded titanium cathode modified with graphene/polymeric pyrrole-sodium dodecyl benzene sulfonate. Desalination Water Treat. 88, 128–138 (2017).
Liu, R. et al. Defect sites in ultrathin Pd nanowires facilitate the highly efficient electrochemical hydrodechlorination of pollutants by H. Environ. Sci. Technol. 52, 9992–10002 (2018).
Mao, Z. et al. Atomically dispersed Pd electrocatalyst for efficient aqueous phase dechlorination reaction. Electrochim. Acta 391, 138886 (2021).
Yang, J., Sebastian, P., Duca, M., Hoogenboom, T. & Koper, M. T. pH dependence of the electroreduction of nitrate on Rh and Pt polycrystalline electrodes. Chem. Commun. 50, 2148–2151 (2014).
Xu, Y., Ma, H., Ge, T., Chu, Y. & Ma, C. A. Rhodium-catalyzed electrochemical hydrodefluorination: a mild approach for the degradation of fluoroaromatic pollutants. Electrochem. Commun. 66, 16–20 (2016).
Wang, J., Cui, C., Xin, Y., Zheng, Q. & Zhang, X. High-performance electrocatalytic hydrodechlorination of pentachlorophenol by amorphous Ru-loaded polypyrrole/foam nickel electrode. Electrochim. Acta 296, 874–881 (2019).
Xu, Y. H., Zhang, H., Chu, C. P. & Ma, C. A. J. Dechlorination of chloroacetic acids by electrocatalytic reduction using activated silver electrodes in aqueous solutions of different pH. J. Electroanal. Chem. 664, 39–45 (2012).
Yao, Q. et al. Amorphous nickel phosphide as a noble metal-free cathode for electrochemical dechlorination. Water Res. 165, 114930 (2019).
Mao, R. et al. Dechlorination of trichloroacetic acid using a noble metal-free graphene–Cu foam electrode via direct cathodic reduction and atomic H. Environ. Sci. Technol. 50, 3829–3837 (2016).
Li, J. et al. Kinetics and mechanisms of electrocatalytic hydrodechlorination of diclofenac on Pd-Ni/PPy-rGO/Ni electrodes. Appl. Catal. B 268, 118696 (2020).
Li, A. et al. The electrocatalytic dechlorination of chloroacetic acids at electrodeposited Pd/Fe-modified carbon paper electrode. Appl. Catal. B 111–112, 628–635 (2012).
Xu, Y. et al. Rh-Pd-alloy catalyzed electrochemical hydrodefluorination of 4-fluorophenol in aqueous solutions. Electrochim. Acta 270, 110–119 (2018).
Huang, L. Z. et al. Hierarchical MoS2 nanosheets on flexible carbon felt as an efficient flow-through electrode for dechlorination. Environ. Sci. Nano 4, 2286–2296 (2017).
He, J., Ela, W. P., Betterton, E. A., Arnold, R. G. & Saez, A. E. Reductive dehalogenation of aqueous-phase chlorinated hydrocarbons in an electrochemical reactor. Ind. Eng. Chem. Res. 43, 7965–7974 (2004).
Bhat, H. K., Kanz, M. F., Campbell, G. A. & Ansari, G. A. S. Ninety day toxicity study of chloroacetic acids in rats. Fundam. Appl. Toxicol. 17, 240–253 (1991).
Sherbo, R. S., Delima, R. S., Chiykowski, V. A., MacLeod, B. P. & Berlinguette, C. P. Complete electron economy by pairing electrolysis with hydrogenation. Nat. Catal. 1, 501–507 (2018).
Kurimoto, A., Sherbo, R. S., Cao, Y., Loo, N. W. X. & Berlinguette, C. P. Electrolytic deuteration of unsaturated bonds without using D2. Nat. Catal. 3, 719–726 (2020).
Inoue, H., Abe, T. & Iwakura, C. Successive hydrogenation of styrene at a palladium sheet electrode combined with electrochemical supply of hydrogen. Chem. Commun.55–56 (1996).
Niwa, S.-I. et al. A one-step conversion of benzene to phenol with a palladium membrane. Science 295, 105–107 (2002).
Liu, Y. et al. Reductive dechlorination of trichloroacetic acid (TCAA) by electrochemical process over Pd-In/Al2O3 catalyst. Electrochim. Acta 232, 13–21 (2017).
Zhang, J. et al. Synchronous reduction–oxidation process for efficient removal of trichloroacetic acid: H* initiates dechlorination and ·OH Is responsible for removal efficiency. Environ. Sci. Technol. 53, 14586–14594 (2019).
Guilian, W. & Naibin, B. Structure–activity relationships for rat and mouse LD50 of miscellaneous alcohols. Chemosphere 36, 1475–1483 (1998).
VandeVondele, J. et al. Quickstep: fast and accurate density functional calculations using a mixed Gaussian and plane waves approach. Comput. Phys. Commun. 167, 103–128 (2005).
Hutter, J., Iannuzzi, M., Schiffmann, F. & VandeVondele, J. CP2K: atomistic simulations of condensed matter systems. Wiley Interdiscip. Res. Comput. Mol. Sci. 4, 15–25 (2014).
Goedecker, S., Teter, M. & Hutter, J. Separable dual-space Gaussian pseudopotentials. Phys. Rev. B 54, 1703–1710 (1996).
Seifitokaldani, A. et al. Hydronium-induced switching between CO2 electroreduction pathways. J. Am. Chem. Soc. 140, 3833–3837 (2018).
Acknowledgements
This work was supported by funds from the National Natural Science Foundation of China (21576238 and 21106133, to Y.X.), the Natural Science Foundation of Zhejiang Province, China (LY16B060012, to Y.X.), Zhejiang Provincial Key Research and Development Program (2020C03085, to J.Y.) and the Singapore Ministry of Education Academic Research Fund (AcRF) Tier 1 (RG4/20 and RG2/21) and Tier 2 (MOE-MOET2EP10120-0002, to B.L.).
Author information
Authors and Affiliations
Contributions
Y.X. and B.L. conceived and designed the project and prepared the manuscript. Z.M. prepared the catalytic Pd membranes and carried out the hydrogenation experiments. R.Q. and J.W. performed the UPD and related experiments. X.L. and J.D. carried out the DFT calculations. J.Y., M.S. and X.M. contributed to the materials characterizations and analysed the experimental data.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Water thanks Emmanuel Mousset, Rui Liu and Binbin Huang for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–19, Table 1 and references 49–53.
Source data
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, Y., Mao, Z., Qu, R. et al. Electrochemical hydrogenation of oxidized contaminants for water purification without supporting electrolyte. Nat Water 1, 95–103 (2023). https://doi.org/10.1038/s44221-022-00002-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44221-022-00002-3
This article is cited by
-
Electrochemical hydrogenation and oxidation of organic species involving water
Nature Reviews Chemistry (2024)
-
Decoupled oxidation process enabled by atomically dispersed copper electrodes for in-situ chemical water treatment
Nature Communications (2024)
-
Separate electro from the chemistry
Nature Catalysis (2023)
-
Electrochemical hydrogenation for water purification made easy
Nature Water (2023)