Abstract
Emerging evidence suggests inflammation is involved in the development of premenstrual disorders (PMDs). We assessed whether childhood asthma and allergies, as inflammatory conditions that may share etiology with PMDs, are associated with risk of PMDs in adulthood. We conducted a prospective cohort study of 6,524 girls in the Growing Up Today Study between 1996 and 2013. Self- and mother-reported diagnoses of asthma and allergies before age 18 were assessed at baseline and updated multiple times during follow-up. Current premenstrual symptoms and cases of PMDs were evaluated using validated tools in 2013. Log-binomial and linear regressions were employed to assess the associations of asthma/allergies with PMDs and premenstrual symptoms (z score), respectively. At a mean (s.d.) age of 25.7 (3.5) years, 19.3% of participants met the criteria for PMDs. Compared with girls free of asthma, those having asthma had an increased risk of PMDs (adjusted risk ratio (aRR) 1.20 [95% CI 1.07 to 1.34]) and increased symptom score (β = 0.13 [95% CI 0.08 to 0.19]). Allergies were positively associated with PMDs (aRR 1.11 [95% CI 0.99 to 1.24]) and premenstrual symptoms (β = 0.09 [95% CI 0.04 to 0.14]). Specifically, the association with PMDs was statistically significant for food allergy (aRR 1.28 [95% CI 1.06 to 1.54]). The associations between asthma/food allergy and PMDs appeared more pronounced for probable premenstrual dysphoric disorder than for premenstrual syndrome. The findings, which show that individuals with childhood asthma or food allergy are at increased risk of PMDs in adulthood, may provide important evidence for future mechanistic research into inflammation and PMDs.
Similar content being viewed by others
Main
Premenstrual disorders (PMDs) are characterized as recurrence of affective and somatic symptoms in the days before menstruation. Premenstrual syndrome (PMS) is a mild type of PMD, with a prevalence of 20–30%. Premenstrual dysphoric disorder (PMDD) is dominated by psychological manifestations with significantly impaired social functioning, and the prevalence was estimated between 2% and 6% (refs. 1,2). PMDs affect millions of women worldwide. Although most patients are diagnosed in their late twenties3, many in fact have symptoms from adolescence4, indicating that childhood risk factors may be important in the development of PMDs. However, few early-life risk factors of PMDs have been studied, except body size5, pubertal timing4 and childhood abuse6.
Menstruation is featured by the inflammatory response. In the late luteal phase, a cascade of local inflammatory responses results in breakdown and repair of the endometrium7. Cyclic changes in inflammatory marker levels are detectable in blood and urine, indicating system-wide effects8. However, dysregulated systemic inflammatory processes contribute to the pathogenesis of many mental disorders9, including PMDs10,11. Moreover, inflammation may be a potential factor involved in the altered response to hormone changes in premenstrual symptoms12. Furthermore, asthma and allergies are common chronic conditions with the characteristics of allergic inflammation from early childhood13,14. Notably, some inflammatory markers involved in women with PMDs10,11 are also implicated in individuals with asthma or allergies15,16. It is plausible that dysregulated immune function may contribute to asthma/allergies and subsequently to PMDs.
The literature on asthma/allergies and PMDs is, however, limited and inconclusive. Some small retrospective and cross-sectional studies suggested that, compared with women free of PMDs, those having PMDs are more likely to report allergy17,18, yet less likely to have asthma19. However, most of these studies have not addressed confounding, which may have biased the results. In this Article, we examined whether children with asthma or allergies had an increased risk of having PMDs in their adulthood, by leveraging a large, longitudinal population-based cohort study in the United States with detailed information on potential confounders.
Results
Among 6,524 female participants, 1,257 (19.3%) individuals (mean (standard deviation, s.d.) age, 25.7 (3.5) years) were identified as having a PMD. Participants having PMDs were more likely to be overweight/obese and report smoking at baseline compared with those without PMDs (Table 1). In the surveys completed 2–3 years before the assessment of PMDs, participants with PMDs were more likely to report family history of asthma or allergies and no use of hormone contraceptives. Participants with PMDs were more likely to have experienced childhood abuse and have comorbid depression and anxiety.
Asthma and allergies
Across three multivariable models, we found a robust association for childhood asthma/allergies with risk of PMDs. Compared with individuals free of childhood asthma, those having asthma are at an increased risk of PMDs (adjusted relative risk (aRR) 1.20, 95% confidence interval (CI) 1.08–1.34; Table 2, model 2) and higher level of symptom burden (β = 0.13, 95% CI 0.08 to 0.19 per s.d.) in their adulthood. When further adjusting for factors associated with PMDs (model 3), the risk ratio (RR) (1.20, 95% CI 1.07 to 1.34) and β (0.13, 95% CI 0.08 to 0.19) were minimally changed.
Allergies were overall positively associated with PMDs (aRR 1.11, 95% CI 1.00 to 1.24) and symptom score (β = 0.09, 95% CI 0.04 to 0.14). Similar to asthma, the association with PMDs (aRR 1.11, 95% CI 0.99 to 1.24) and premenstrual symptoms (β = 0.09, 95% CI 0.04 to 0.14) was materially unchanged in model 3. When analysing allergy subtypes, we observed a significant association with PMDs for food allergy (aRR 1.28, 95% CI 1.06 to 1.54), but not for eczema (aRR 1.13, 95% CI 0.96 to 1.32) and hay fever (aRR 1.10, 95% CI 0.98 to 1.25). The association for allergen-specific food allergy with PMDs and premenstrual symptoms was notable for egg allergy and milk allergy (Supplementary Table 1). Furthermore, a stronger association was suggested for asthma/allergies present in adulthood than for asthma/allergies not present in adulthood (RR 1.34 versus 1.18 for asthma, and 1.23 versus 1.09 for allergies; Supplementary Table 2).
As the association with PMDs was most pronounced for childhood asthma and food allergy, we focused on these two exposures in the following analyses.
PMD subtypes
In analyses of PMD subtypes, more pronounced associations of asthma and food allergies with probable PMDD were suggested than with PMS (RR 1.36 versus 1.18 for asthma and RR 1.35 versus 1.23 for food allergies; Table 3), although the associations were statistically significant for PMS only, probably due to greater statistical power for these comparisons. Significant and seemingly stronger associations were noted between asthma and later-onset PMDs (symptom onset after age 20) as well as between food allergies and earlier-onset PMDs.
Age at asthma onset
When analysing by age at onset, a seemingly stronger association was suggested between asthma with a preschool onset (0–5.9 years) and PMDs (Table 4). A higher level of premenstrual symptoms was found for asthma with an onset in preschool and teen ages (12–17.9 years), although the association was significant only for teen-onset asthma (Table 4).
Additional analyses
A greater RR of PMDs was observed for asthma and indicated for food allergies in GUTS II than in GUTS I (Table 5). Statistically comparable associations were found between asthma and PMDs among individuals with and without depression, whereas a stronger association was noted for food allergies among those without depression (P-for-interaction 0.002). However, anxiety and categories of body mass index (BMI) did not clearly modify associations of asthma/food allergies with PMD risk.
Comparable results were observed when further adjusting for childhood abuse, parental smoking or maternal smoking during pregnancy, when adjusting for maternal and paternal history of asthma/food allergy, when mutually adjusted for asthma and food allergy (Supplementary Table 3), when restricting to participants who completed PMD questions and when using premenarcheal exposures (Supplementary Table 4). Furthermore, excluding individuals with milk allergy had no material effect on the result.
Discussion
Childhood asthma and allergies are worldwide public health concerns20. This prospective cohort study showed that girls with asthma or food allergy are at increased risk of PMDs in adulthood. Such associations were independent of most known confounders, for example, BMI category, smoking and childhood abuse, and factors associated with PMDs, for instance, use of hormone contraceptives, and appeared more pronounced for probable PMDD than for PMS.
Asthma and PMDs
Limited evidence supports a link between childhood asthma and depression. One study showed a positive relationship between asthma and depression among girls21, whereas another study showed a non-significant association22. However, studies on the association between asthma and PMDs were scarce and inconclusive. Skrzypulec et al.19 found asthma to be inversely correlated with PMDs, whereas Kljakovic et al.17 reported a null association. However, both small studies did not consider confounders, and the non-prospective designs allowed for reverse causation, as treatment of PMDs (for example, contraceptives) decreases the risk of asthma23. Interestingly, Mirdal et al.24 found eight out of ten women with asthma reported increased irritability, tearfulness and tension during peri-menstruation, indicating a high prevalence of premenstrual symptoms among patients with asthma. With prospectively collected data, and adjustment for most known confounders, our study found that girls with asthma had a 20% higher risk of PMDs in adulthood. Together with robust results in sensitivity analyses (for example, additional adjustment for confounders and restricting to premenarcheal exposures), and a seemingly stronger association with probable PMDD, this study lends some support to the hypothesis that childhood asthma may be related to the development of PMDs.
The mechanisms underlying asthma and PMDs are probably multifactorial. First, genetic variants in oestrogen receptors alpha are observed in patients with PMDD and asthma, indicating shared genetics between these two disorders25,26. However, in our analysis, the association between asthma and PMDs only slightly attenuated after adjusting for family history of asthma or allergies, suggesting our findings cannot be entirely explained by genetic overlap.
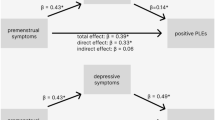
Moreover, association between asthma and PMDs might be driven by psychiatric comorbidities. Indeed, asthma and PMDs are both associated with affective disorders27,28. However, we observed statistically comparable associations between asthma and PMDs across categories of current depression and anxiety, indicating the association between asthma and PMDs is specific and not fully explained by depression or anxiety. Notably, the association was statistically significant only in those with depression. Future studies are warranted to explore whether depression and PMDs share the pathophysiology induced by asthma, or premenstrual alteration of sex hormones amplifies depressive symptoms, so-called premenstrual exacerbation.
Alternatively, chronic inflammation associated with asthma may predispose individuals to a higher risk of PMDs. Inflammatory cytokines affect emotional and behavioural neurocircuitry, neurotransmitter metabolism and hormone response to stress29, all of which are implicated in the aetiology of PMDD30. Indeed, inflammatory cytokines could induce sickness behaviour, including anorexia, disrupted sleep and anhedonia31, which could be part of PMD symptoms. Furthermore, inflammation in early life can influence brain development at critical periods32, thus triggering emotional dysregulation in later life. The chronic inflammation (that is, from childhood to adulthood) hypothesis is further supported by our findings that asthma is more strongly associated with later-onset PMDs and asthma present in adulthood is more strongly related with PMDs. These mechanisms are similar to what has been proposed between inflammation and major depression29. However, there are potential inflammatory pathways specific to PMDs. One of the popular PMD mechanisms is that the premenstrual decline of allopregnanolone (ALLO) levels can provide potent stimulus to re-organize GABAA receptor, change the sensitivity to ALLO and may result in PMDs33. However, as women without PMDs also experience the withdrawal of ALLO in every menstrual cycle, decrease in ALLO alone may not induce those pathological effects. It has been postulated that other factors, for example, inflammation, may be involved in triggering the abnormal response to the withdrawal11. Emerging data suggest that inflammation may reduce the level of ALLO by increasing progesterone–corticosterone metabolism and decreasing progesterone–ALLO metabolism34, which may speed up the decline of ALLO. Future studies, however, are warranted to fully understand the underlying mechanisms linking asthma/inflammation to PMDs.
Importantly, though we did not observe a significant association for PMDs when analysed by age at asthma onset, preschool and adolescence asthma were more strongly associated with premenstrual symptoms, probably due to better statistical power. Compared with children with asthma with onset between age 6 and 11.9, preschool children with asthma have more persistent inflammation35, and adolescents with asthma had higher levels of eosinophils36, further supporting the importance of inflammation in the association between asthma and PMDs.
Allergies and PMDs
A recent study reported a positive association for childhood eczema with depression among girls37. Moreover, one study found that women with PMDs were more likely to have eczema or allergic rhinitis17. This is consistent with our findings that borderline associations were noted between eczema, hay fever and PMDs. More importantly, our study reported that childhood food allergy is linked to an increased risk of PMDs in adulthood. Elimination diet is commonly used to manage food allergy, which could lead to nutritional disorders, especially vitamin D and calcium deficiencies38. Lower intakes of calcium and vitamin D increase risk of PMDs39, which is supported by our findings that milk allergy was more strongly related with PMDs.
We observed statistically comparable associations between food allergies and PMDs across category of anxiety, and a stronger association for food allergies and PMDs in women without depression, suggesting that such association cannot be explained by depression/anxiety. Similar to asthma, the association between food allergy and PMDs may be attributable to shared genetics or underlying inflammation14. However, heterogeneity may exist between the food allergy–PMDs and asthma–PMDs links. For instance, stronger associations were suggested between asthma and late-onset PMDs as well as between food allergy and early-onset PMDs. It is plausible that food allergy has a more immediate effect on the brain via the gut–brain axis. Potential mechanisms may include microbiota composition via the gut–brain axis40,41 and Th2-dominated immunological profiles42. Future studies are, however, warranted to better understand the underlying mechanisms.
Strengths and limitations
Leveraging prospectively collected data on asthma and allergies during follow-up—along with detailed information on confounders—this study presents a comprehensive assessment of associations for asthma, allergies with PMDs during a follow-up up to 18 years. However, this study has potential limitations. First, we used participants’ report to assess asthma and allergies, which may lead to misclassification of some exposures. Nevertheless, the self-reported asthma among children has a high validity43, although food allergy is probably an overestimate44. Moreover, as individuals were healthy or not aware of the status of PMDs when asthma/allergies were measured, this misclassification is possibly non-differential in relation to PMDs and may only dilute associations. Second, we did not use daily symptom diaries to assess PMDs. Although the tool has been validated to identify PMDs in another US study of young females10, it was unable to differentiate PMS/PMDD from premenstrual exacerbation of other mental illness (for example, depression and anxiety with symptom worsening days before menstruation). However, in the stratified analysis we showed comparable associations for asthma among individuals without comorbid mood disorders (that is, the association with ‘pure PMS/PMDD’). Moreover, the questionnaire was not validated to differentiate PMS and PMDD, which may lead to misclassifications of PMDD, although the prevalence in our study (1.9%) is comparable to that found in other studies in the United States1,45,46,47. Third, selection bias could emerge if participation is related to asthma/allergies and PMDs. However, the basic characteristics4 and exposures to asthma and food allergy at first measurement were comparable between included and excluded participants (12.9% versus 13.0% for asthma and 9.3% versus 9.0% for food allergy). Fourth, given the nature of observational study, residual confounding cannot be completely ruled out. However, we have adjusted for most known confounders, including BMI, smoking and childhood abuse, in our analyses. We even controlled for family history of asthma/allergies as a proxy for genetic liability. Finally, our population is homogeneous in terms of socio-economic status and race. Further studies are needed in more ethnically and economically diverse populations.
In conclusion, this study suggests that childhood asthma and food allergy are associated with increased risk of PMDs in adulthood. Clinicians need to be alert of the potential risk of developing PMDs among girls having asthma or food allergy. Our findings may also benefit future mechanistic research in PMDs.
Methods
Study design
Data were from the Growing Up Today Study (GUTS). GUTS enroled offspring of participants in the Nurses’ Health Study II (NHS II), which is composed of registered female nurses in the United States48. Details of the study, including recruitment and response rates, are described elsewhere4,5. Briefly, in 1996, 16,882 children from age 9 to 14 years were enroled, forming the original GUTS I cohort. In 2004, an additional 10,923 adolescents from age 10 to 17 years were enroled and formed GUTS II. At enrolment, mothers provided informed consent, because the recruitment of GUTS participants was initiated by sending invitations to NHS II participants. GUTS participants assented by returning baseline questionnaires. The GUTS cohort was not designed to examine the relation of interest, and the current study is a secondary analysis. This study is approved by the Institutional Review Board of the Brigham and Women’s Hospital and the Swedish Ethical Review Authority.
Participants were sent questionnaires every 1–3 years. Mothers of these participants received questionnaires regarding their children’s health during 1997–2011. In total, 99% mothers of GUTS I participants49 and 87% of mothers of GUTS II participants completed at least one questionnaire. In 2013, GUTS I and II were combined into the GUTS cohort and have been followed in parallel since then. The 2013 questionnaire, which assessed premenstrual symptoms, was returned by 8,266 (55%) women. Participants were excluded from the analysis if they reported amenorrhoea (n = 8) or lacked information on menstruation (n = 362) or missed every question on the PMD assessment (n = 1,372), yielding a final sample of 6,524 women. Our previous work showed that demographic and lifestyle characteristics were comparable between these women and those who did not respond to the 2013 questionnaire or missed PMD assessment4.
Asthma and allergies
Both asthma and allergies were assessed at baseline and then were prospectively surveyed throughout follow-up. Asthma was defined as either self-report of a clinical diagnosis by a GUTS participant, or the report by the participant’s mother (an NHS II participant) that their daughter had been clinically diagnosed with asthma. GUTS participants were asked if they had asthma on every questionnaire (the timeline of data collection is summarized in Fig. 1). In addition, in 2010, 2011 and 2013, participants reported the year of first diagnosis of asthma. In 1997, 1999, 2004 and 2009, mothers were asked if their children had been diagnosed with asthma, and the year of first diagnosis (1999 and 2004 only).
Exposures were assessed from 1996 to 2011 in GUTS I and GUTS II (with circles beside the years), separately. Asthma/food allergies/hay fever/eczema was assessed by asking ‘Has a doctor ever said you have asthma/food allergies/hay fever/eczema’. Food allergies also included allergic reactions to peanuts or tree nuts. GUTS I and GUTS II were combined in 2013, when PMDs were evaluated. dr-dx, doctor diagnosed.
Allergies included eczema, food allergy and hay fever, and were defined from self- and mother reports. Physician-diagnosed eczema and hay fever was reported in 2006–2008 by daughters and in 1997, 1999 and 2009 by mothers. In 1999, mothers recalled the age at eczema and hay fever onset. In addition, in 2006, 2007, 2008 and 2010, girls were asked if they had a clinician-diagnosed food allergy, and in 2010, to estimate the year of first diagnosis. In 2009, mothers were asked whether their daughters had clinician-diagnosed food allergies. We also classified individuals reporting allergic reactions to peanuts or tree nuts as having a food allergy, using reports in 2006–2008 by daughters and 2006 by mothers.
Participants were ascertained as having asthma or allergies if reported by either themselves or the mother. Childhood asthma/allergies were defined as either reporting asthma/allergies or reporting age at onset before age 18. In 2013, use of asthma medications (for example, albuterol and Flovent) and allergy medications (for example, Allegra, Claritin and Zyrtec) in the past year was reported by participants. This information was used to classify whether childhood asthma/allergy was present in adulthood.
PMDs
In 2013, PMDs were assessed using a scale adapted from the Calendar of Premenstrual Experiences50. A similar tool was validated in NHS II10,51 Participants were asked if they experienced 19 physical/behavioural and 8 affective symptoms for some days before the start of menstruation in most cycles. Participants evaluated the severity of every symptom as none, mild, moderate or severe. We scored the severity between 0 and 3, and then converted the summed score (range 0–81) to a z-score. Individual’s mean symptom score was used to impute missing symptoms, though only 1% of participants missed more than five symptoms. Participants also recalled the age when their symptoms began, with four response options: since the first period, in teens, when aged 20+ and when aged 30+.
The severity of overall symptoms and social impairment were further rated. Consistent with previous studies4,5, women were identified as having PMDs if they reported all these items: (1) ≥1 affective and ≥1 physical symptoms; (2) impact on social functioning or overall symptom evaluated as moderate or severe or ≥1 severe emotional symptoms; (3) symptoms beginning ≤2 weeks before menstruation onset and ending ≤1 week of menstruation onset; and (4) symptoms completely withdrew in 1 week after menstruation. The validity of the tool has been demonstrated, with a positive predictive value of 80%10.
Women with PMDs were classified as having PMS or probable PMDD, on the basis of the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, (DSM-5) criteria52: (1) ≥1 of 4 emotional manifestations (depression, anxiety, irritability/anger and mood swings/tearful) classified as severe; (2) ≥5 of 11 symptoms including the 4 emotional symptoms and insomnia, food cravings, hypersensitized state, desire for loneliness, tiredness, poor concentrating and/or other physical manifestations; and (3) social impairment classified as moderate or severe.
Covariates
Age, race and use of multi-vitamins was measured at baseline from GUTS questionnaires. Maternal marital status was ascertained from 1997 and 2005 NHS II questionnaires, and paternal educational level was ascertained from 1999 NHS II questionnaires.
Factors associated with PMDs and asthma/allergies were potential confounders. Ever drinking alcohol and ever smoking were assessed at baseline. Baseline BMI (as kg m−2) was generated using height and weight reported by participants. On the basis of the extended International Obesity Task Force53, participants were further grouped into four BMI categories: underweight, normal, overweight and obese. Physical activity at baseline was calculated by multiplying the weekly hours spent in moderate to vigorous activities by the activities’ metabolic equivalent of task (MET) score54, with the products then summed to derive weekly MET hours. Experiences of abuse before age 11, including physical, emotional and sexual abuse, were collected from GUTS I in 2007 (ref. 55). Furthermore, as evidence showed that women with PMDs are more likely to report family history of allergies17,56, we collected information on family history of asthma or allergies from mothers’ questionnaires in 2006 and 2009. Parental smoking was reported by GUTS I participants in 1999. Maternal smoking during pregnancy was reported by mothers of GUTS II participants in 2009.
Number of pregnancy and use of birth control pills were evaluated in 2010 in GUTS I and 2011 in GUTS II. Age at menarche was reported from serial questionnaires from cohort entry until 2003 in GUTS I and until 2008 in GUTS II.
Anxiety and depression are commonly comorbid with asthma57 and PMDs27. Anxiety was evaluated in 2013, on the basis of self-reported physician-diagnosed anxiety disorders or medication (minor tranquilizers) use. Depression was evaluated on the basis of clinician-diagnosed depression, antidepressant use or high depressive symptom (scoring above 11 based on the Center for Epidemiologic Studies Depression Scale-10 (ref. 55) (positive predictive values 73–95%)), all surveyed in self-administrated questionnaire in 2013.
Statistical analysis
Background variables between women with and without PMDs were compared using t-test or χ2 test. Using log-binomial regression modelling, we then estimated RRs of PMDs by comparing individuals with childhood asthma or allergies with those without. Furthermore, we estimated β for the associations with premenstrual symptom score, the secondary outcome, using linear regression. To provide insights into asthma/allergy subtypes, we analysed the associations by: (1) eczema, food allergies (further divided by common allergens) and hay fever; and (2) whether conditions present in adulthood.
We built three models. Model 1 was adjusted for demographics, cohort membership, and paternal educational attainment, use of multi-vitamin and maternal civil status. Model 2 was further adjusted for potential confounders, including baseline lifestyles (physical activity58,59, ever smoking60,61 and consuming alcohol62,63), baseline BMI5,64,65 and family history of asthma or allergies25,26. Model 3 was further adjusted for factors associated with PMDs, including parity, use of contraceptives and age at menarche.
To determine the potentially different associations between asthma/allergies with PMD subtypes, we estimated separately the association for (1) PMS and probable PMDD; and (2) PMDs with symptom onset before and after age 20. The latter analysis was to alleviate the concern of reverse causation, namely PMDs with an onset after age 20 should have occurred after childhood asthma/allergies. In addition, presuming that age recalled by mothers (nurses) is more accurate, we classified individuals with asthma according to mother-reported age at onset (<6, 6–11.9 and 12–17.9; only available in GUTS I), and compared them with individuals without childhood asthma.
We conducted several additional analyses. First, inflammation is involved in depression, anxiety66 and obesity67; to test potential risk modifications, we stratified analyses by these factors. We also stratified analyses by cohort membership. Second, to address residual confounding, we further adjusted for childhood abuse, parental smoking in GUTS I, and maternal smoking during pregnancy in GUTS II. Third, maternal and paternal atopy have differential impacts on offspring’s immunoglobulin E production68; instead of adjusting for family history of asthma and allergies as a whole, we adjusted for maternal and paternal history of asthma/allergies, respectively. Fourth, to evaluate the independent role of asthma/allergies, we mutually adjusted them. Fifth, to examine the impact of missing information, we limited analyses to participants who completed all PMD symptom items. Sixth, we restricted analyses to premenarcheal exposures to rule out reverse causality, as PMDs cannot emerge until menarche. Finally, as people frequently conflate milk allergy with lactose intolerance69, we performed analyses of food allergies by excluding individuals with milk allergy.
All analyses were performed in SAS, version 9.4 (SAS Institute) and R, version 3.6.3 (R Foundation for Statistical Computing). A two-tailed P < 0.05 indicates statistical significance.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The datasets used in the current study are not publicly available due to paticipant confidentiality. External investigators who plan to use data from the GUTS are supposed to fill out a request form (https://docs.google.com/forms/d/e/1FAIpQLScAPV23ZIBpkk9CyEJ1OcFJjMol9elKEpLYnPu7g3PgBL57XA/viewform) and describe the study proposal. Most of the requests will be approved. Investigators can contact guts@channing.harvard.edu for more details.
Code availability
The codes used to generate all the results are available from the corresponding authors upon request.
References
Cohen, L. S. et al. Prevalence and predictors of premenstrual dysphoric disorder (PMDD) in older premenopausal women. The Harvard Study of Moods and Cycles. J. Affect. Disord. 70, 125–132 (2002).
Qiao, M. et al. Prevalence of premenstrual syndrome and premenstrual dysphoric disorder in a population-based sample in China. Eur. J. Obstet. Gynecol. Reprod. Biol. 162, 83–86 (2012).
Robinson, R. L. & Swindle, R. W. Premenstrual symptom severity: impact on social functioning and treatment-seeking behaviors. J. Womens Health Gend. Based Med. 9, 757–768 (2000).
Lu, D. et al. Pubertal development and risk of premenstrual disorders in young adulthood. Hum. Reprod. 36, 455–464 (2021).
Lu, D. et al. Association between childhood body size and premenstrual disorders in young adulthood. JAMA Netw. Open 5, e221256 (2022).
Bertone-Johnson, E. R. et al. Early life emotional, physical, and sexual abuse and the development of premenstrual syndrome: a longitudinal study. J. Womens Health 23, 729–739 (2014).
Evans, J. & Salamonsen, L. A. Inflammation, leukocytes and menstruation. Rev. Endocr. Metab. Disord. 13, 277–288 (2012).
Blum, C. A. et al. Low-grade inflammation and estimates of insulin resistance during the menstrual cycle in lean and overweight women. J. Clin. Endocrinol. Metab. 90, 3230–3235 (2005).
Bauer, M. E. & Teixeira, A. L. Inflammation in psychiatric disorders: what comes first? Ann. NY Acad. Sci. 1437, 57–67 (2019).
Bertone-Johnson, E. R. et al. Association of inflammation markers with menstrual symptom severity and premenstrual syndrome in young women. Hum. Reprod. 29, 1987–1994 (2014).
Granda, D., Szmidt, M. K. & Kaluza, J. Is premenstrual syndrome associated with inflammation, oxidative stress and antioxidant status? A systematic review of case–control and cross-sectional studies. Antioxidants 10, 604 (2021).
Tiranini, L. & Nappi, R. E. Recent advances in understanding/management of premenstrual dysphoric disorder/premenstrual syndrome. Fac. Rev. 11, 11 (2022).
Mims, J. W. Asthma: definitions and pathophysiology. Int. Forum Allergy Rhinol. 5, S2–S6 (2015).
Yu, W., Freeland, D. M. H. & Nadeau, K. C. Food allergy: immune mechanisms, diagnosis and immunotherapy. Nat. Rev. Immunol. 16, 751–765 (2016).
Gour, N. & Wills-Karp, M. IL-4 and IL-13 signaling in allergic airway disease. Cytokine 75, 68–78 (2015).
Turcanu, V., Maleki, S. J. & Lack, G. Characterization of lymphocyte responses to peanuts in normal children, peanut-allergic children, and allergic children who acquired tolerance to peanuts. J. Clin. Invest. 111, 1065–1072 (2003).
Kljakovic, M. & Pullon, S. Allergy and the premenstrual syndrome (PMS). Allergy 52, 681–683 (1997).
Boyle, C. A., Berkowitz, G. S. & Kelsey, J. L. Epidemiology of premenstrual symptoms. Am. J. Public Health 77, 349–350 (1987).
Skrzypulec, V., Doniec, Z., Drosdzol, A., Nowosielski, K. & Pawlińska-Chmara, R. The influence of bronchial asthma on premenstrual syndrome prevalence among girls. J. Physiol. Pharmacol. 58, 639–646 (2007).
Eder, W., Ege, M. J. & von Mutius, E. The asthma epidemic. N. Engl. J. Med. 355, 2226–2235 (2006).
Ahmadiafshar, A. et al. The high prevalence of depression among adolescents with asthma in Iran. Psychosom. Med. 78, 113–114 (2016).
Bahreinian, S., Ball, G. D. C., Colman, I., Becker, A. B. & Kozyrskyj, A. L. Depression is more common in girls with nonatopic asthma. Chest 140, 1138–1145 (2011).
Wei, J. et al. Hormonal factors and incident asthma and allergic rhinitis during puberty in girls. Ann. Allergy Asthma Immunol. 115, 21–27.e2 (2015).
Mirdal, G. M., Petersson, B., Weeke, B. & Vibits, A. Asthma and menstruation: the relationship between psychological and bronchial hyperreactivity. Br. J. Med. Psychol. 71, 47–55 (1998).
Miller, A. et al. Estrogen receptor alpha (ESR-1) associations with psychological traits in women with PMDD and controls. J. Psychiatr. Res. 44, 788–794 (2010).
Bloodworth, M. H., Rusznak, M., Bastarache, L., Wang, J. & Newcomb, D. C. Association of estrogen receptor α polymorphism rs1999805 with asthma. Ann. Allergy Asthma Immunol. 122, 208–210 (2019).
Kim, D. R. et al. Premenstrual dysphoric disorder and psychiatric co-morbidity. Arch. Womens Ment. Health. 7, 37–47 (2004).
Choi, H. G. et al. Association between asthma and depression: a national cohort study. J. Allergy Clin. Immunol. Pract. 7, 1239–1245.e1 (2019).
Raison, C. L., Capuron, L. & Miller, A. H. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 27, 24–31 (2006).
Raffi, E. The etiology of premenstrual dysphoric disorder: 5 interwoven pieces. Curr. Psychiatry 16, 20–28 (2017).
Dantzer, R. Cytokine-induced sickness behaviour: a neuroimmune response to activation of innate immunity. Eur. J. Pharmacol. 500, 399–411 (2004).
Hagberg, H., Gressens, P. & Mallard, C. Inflammation during fetal and neonatal life: implications for neurologic and neuropsychiatric disease in children and adults. Ann. Neurol. 71, 444–457 (2012).
Lovick, T. A. Plasticity of GABAA receptor subunit expression during the oestrous cycle of the rat: implications for premenstrual syndrome in women. Exp. Physiol. 91, 655–660 (2006).
Parks, E. E. et al. Interleukin 6 reduces allopregnanolone synthesis in the brain and contributes to age-related cognitive decline in mice. J. Lipid Res. 61, 1308–1319 (2020).
Saglani, S. et al. Early detection of airway wall remodeling and eosinophilic inflammation in preschool wheezers. Am. J. Respir. Crit. Care Med. 176, 858–864 (2007).
Miranda, C., Busacker, A., Balzar, S., Trudeau, J. & Wenzel, S. E. Distinguishing severe asthma phenotypes: role of age at onset and eosinophilic inflammation. J. Allergy Clin. Immunol. 113, 101–108 (2004).
Teichgräber, F. et al. Association between skin disorders and depression in children and adolescents: a retrospective case–control study. J. Affect. Disord. 282, 939–944 (2021).
Meyer, R. Nutritional disorders resulting from food allergy in children. Pediatr. Allergy Immunol. 29, 689–704 (2018).
Bertone-Johnson, E. R. et al. Calcium and vitamin D intake and risk of incident premenstrual syndrome. Arch. Intern. Med. 165, 1246–1252 (2005).
Bunyavanich, S. & Berin, M. C. Food allergy and the microbiome: current understandings and future directions. J. Allergy Clin. Immunol. 144, 1468–1477 (2019).
Liang, L., Zhou, H., Zhang, S., Yuan, J. & Wu, H. Effects of gut microbiota disturbance induced in early life on the expression of extrasynaptic GABA-A receptor α5 and δ subunits in the hippocampus of adult rats. Brain Res Bull. 135, 113–119 (2017).
Ellenbogen, Y. et al. The initiation of Th2 immunity towards food allergens. Int. J. Mol. Sci. 19, 1447 (2018).
Remes, S., Korppi, M., Remes, K. & Pekkanen, J. Prevalence of asthma at school age: a clinical population-based study in eastern Finland. Acta Paediatr. 85, 59–63 (1996).
Rona, R. J. et al. The prevalence of food allergy: a meta-analysis. J. Allergy Clin. Immunol. 120, 638–646 (2007).
Rivera-Tovar, A. D. & Frank, E. Late luteal phase dysphoric disorder in young women. Am. J. Psychiatry 147, 1634–1636 (1990).
Gehlert, S., Song, I. H., Chang, C. H. & Hartlage, S. A. The prevalence of premenstrual dysphoric disorder in a randomly selected group of urban and rural women. Psychol. Med. 39, 129–136 (2009).
Gehlert, S. & Hartlage, S. A design for studying the DSM-IV research criteria of premenstrual dysphoric disorder. J. Psychosom. Obstet. Gynaecol. 18, 36–44 (1997).
Rockett, H. R., Berkey, C. S., Field, A. E. & Colditz, G. A. Cross-sectional measurement of nutrient intake among adolescents in 1996. Prev. Med. 33, 27–37 (2001).
Dumas, O., Varraso, R., Gillman, M. W., Field, A. E. & Camargo, C. A. Longitudinal study of maternal body mass index, gestational weight gain, and offspring asthma. Allergy 71, 1295–1304 (2016).
Mortola, J. F., Girton, L., Beck, L. & Yen, S. S. Diagnosis of premenstrual syndrome by a simple, prospective, and reliable instrument: the calendar of premenstrual experiences. Obstet. Gynecol. 76, 302–307 (1990).
Bertone-Johnson, E. R., Hankinson, S. E., Johnson, S. R. & Manson, J. E. A simple method of assessing premenstrual syndrome in large prospective studies. J. Reprod. Med. 52, 779–786 (2007).
Bertone-Johnson, E. R., Hankinson, S. E., Johnson, S. R. & Manson, J. E. Timing of alcohol use and the incidence of premenstrual syndrome and probable premenstrual dysphoric disorder. J. Womens Health 18, 1945–1953 (2009).
Cole, T. J. & Lobstein, T. Extended international (IOTF) body mass index cut-offs for thinness, overweight and obesity. Pediatr. Obes. 7, 284–294 (2012).
Ainsworth, B. E. et al. 2011 Compendium of Physical Activities: a second update of codes and MET values. Med. Sci. Sports Exerc. 43, 1575–1581 (2011).
Opoliner, A., Carwile, J. L., Blacker, D., Fitzmaurice, G. M. & Austin, S. B. Early and late menarche and risk of depressive symptoms in young adulthood. Arch. Womens Ment. Health 17, 511–518 (2014).
Atton-Chamla, A. et al. Premenstrual syndrome and atopy: a double-blind clinical evaluation of treatment with a gamma-globulin/histamine complex. Pharmatherapeutica 2, 481–486 (1980).
Jiang, M., Qin, P. & Yang, X. Comorbidity between depression and asthma via immune-inflammatory pathways: a meta-analysis. J. Affect. Disord. 166, 22–29 (2014).
Byberg, K. K., Eide, G. E., Forman, M. R., Júlíusson, P. B. & Øymar, K. Body mass index and physical activity in early childhood are associated with atopic sensitization, atopic dermatitis and asthma in later childhood. Clin. Transl. Allergy 6, 33 (2016).
Vaghela, N., Mishra, D., Sheth, M. & Dani, V. B. To compare the effects of aerobic exercise and yoga on premenstrual syndrome. J. Educ. Health Promot. 8, 199 (2019).
Accordini, S., Janson, C., Svanes, C. & Jarvis, D. The role of smoking in allergy and asthma: lessons from the ECRHS. Curr. Allergy Asthma Rep. 12, 185–191 (2012).
Bertone-Johnson, E. R., Hankinson, S. E., Johnson, S. R. & Manson, J. E. Cigarette smoking and the development of premenstrual syndrome. Am. J. Epidemiol. 168, 938–945 (2008).
Skaaby, T. et al. Association of alcohol consumption with allergic disease and asthma: a multi-centre Mendelian randomization analysis. Addiction 114, 216–225 (2019).
Fernández, M. D. M., Saulyte, J., Inskip, H. M. & Takkouche, B. Premenstrual syndrome and alcohol consumption: a systematic review and meta-analysis. BMJ Open 8, e019490 (2018).
Black, M. H., Zhou, H., Takayanagi, M., Jacobsen, S. J. & Koebnick, C. Increased asthma risk and asthma-related health care complications associated with childhood obesity. Am. J. Epidemiol. 178, 1120–1128 (2013).
Chang, C. L. et al. Associations between body mass index trajectories in the first two years of life and allergic rhinitis, eczema and food allergy outcomes up to early adulthood. Pediatr. Allergy Immunol. 33, e13765 (2022).
Peirce, J. M. & Alviña, K. The role of inflammation and the gut microbiome in depression and anxiety. J. Neurosci. Res. 97, 1223–1241 (2019).
Welsh, P. et al. Unraveling the directional link between adiposity and inflammation: a bidirectional Mendelian randomization approach. J. Clin. Endocrinol. Metab. 95, 93–99 (2010).
Wu, C. C., Chen, R. F. & Kuo, H. C. Different implications of paternal and maternal atopy for perinatal IgE production and asthma development. Clin. Dev. Immunol. 2012, 132142 (2012).
Di Costanzo, M. & Berni Canani, R. Lactose intolerance: common misunderstandings. Ann. Nutr. Metab. 73, 30–37 (2018).
Acknowledgements
We thank the Channing Division of Network Medicine, Brigham and Women’s hospital for data access and administrative support, as well as the participants of the Growing Up Today Study. The work is supported by the scholarship from the China Scholarship Council (No. 202106100010 to Y.Y.), grant 2020-01003 from the Swedish Research Council (Vetenskapsrådet) (to D.L.), and grant 2020-00971 from the Swedish Research Council for Health, Working Life, and Welfare (FORTE) (to D.L.). The Growing Up Today Study is supported by grants R03 CA106238 and U01 HL145386 from the National Institutes of Health. The funders did not participate in the study design, data collection, manuscript approval and submission decision. Part of the methods is from our previous work 4,5.
Funding
Open access funding provided by Karolinska Institute.
Author information
Authors and Affiliations
Contributions
Y.Y., E.B.-J. and D.L. conceived and designed the study. Y.Y. analysed the data. Y.Y., T.G., C.A.C., U.A.V., E.B.-J. and D.L. interpreted the results. Y.Y. drafted the paper, and T.G., C.A.C., U.A.V., E.B.-J. and D.L. contributed to revision and perfection of the draft. E.B.-J. and D.L. provided supervision and administrative support. D.L. obtained funds. All authors have approved the final submission version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Mental Health thanks Chighaf Bakour and the other, anonymous, reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Tables 1–4.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yang, Y., Gong, T., Camargo, C.A. et al. Childhood asthma, allergies and risk of premenstrual disorders in young adulthood. Nat. Mental Health 1, 410–419 (2023). https://doi.org/10.1038/s44220-023-00066-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44220-023-00066-4