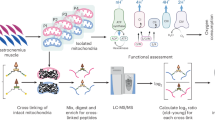
Abstract
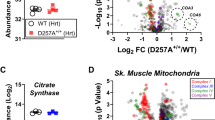
Advancements in cross-linking mass spectrometry bridge the gap between purified systems and native tissue environments, allowing the detection of protein structural interactions in their native state. In this study, we used isobaric quantitative protein interaction reporter (iqPIR) technology to compare the mitochondrial protein interactomes in healthy and failing murine hearts 4 weeks after transverse aortic constriction. The failing heart interactome includes 588 statistically significant cross-linked peptide pairs altered in the disease condition. We observed an increase in the assembly of ketone oxidation oligomers corresponding to an increase in ketone metabolic utilization; remodeling of NDUA4 interaction in Complex IV, likely contributing to impaired mitochondrial respiration; and conformational enrichment of the ADP/ATP carrier ADT1, which is non-functional for ADP/ATP translocation but likely possesses non-selective conductivity. Our application of quantitative cross-linking technology in cardiac tissue provides molecular-level insights into the complex mitochondrial remodeling in heart failure while bringing forth new hypotheses for pathological mechanisms.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Cross-linking data have been deposited at XlinkDB (http://xlinkdb.gs.washington.edu/xlinkdb/index.php) and are publicly available (network name: Caudal_iqPIR_TACsham_Bruce). The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE70 partner repository with the dataset identifiers PXD027757 and PXD035622. The following publicly available files were included: PDB 5Z62, PDB 3DLX, PDB 3OXO, PDB 1OKC, PDB 6GCI, PDB 2LCK and UniProt P48962. Any additional information required to reanalyze the data reported in this paper is available from the lead contacts upon reasonable request. All other data supporting the findings in this study are included in the main article and associated files.
References
Rath, S. et al. MitoCarta3.0: an updated mitochondrial proteome now with sub-organelle localization and pathway annotations. Nucleic Acids Res. 49, D1541–D1547 (2020).
Pagliarini, D. J. et al. A mitochondrial protein compendium elucidates complex I disease biology. Cell 134, 112–123 (2008).
Qi, L. et al. Cryo-EM structure of the human mitochondrial translocase TIM22 complex. Cell Res. 31, 369–372 (2021).
Bridges, H. R. et al. Structure of inhibitor-bound mammalian complex I. Nat. Commun. 11, 5261 (2020).
Gu, J. et al. Cryo-EM structure of the mammalian ATP synthase tetramer bound with inhibitory protein IF1. Science 364, 1068 (2019).
Spikes, T. E., Montgomery, M. G. & Walker, J. E. Structure of the dimeric ATP synthase from bovine mitochondria. Proc. Natl Acad. Sci. USA 117, 23519 (2020).
Tucker, K. & Park, E. Cryo-EM structure of the mitochondrial protein-import channel TOM complex at near-atomic resolution. Nat. Struct. Mol. Biol. 26, 1158–1166 (2019).
Sinz, A. Crosslinking mass spectrometry goes in-tissue. Cell Syst. 6, 10–12 (2018).
Tang, X., Munske, G. R., Siems, W. F. & Bruce, J. E. Mass spectrometry identifiable cross-linking strategy for studying protein−protein interactions. Anal. Chem. 77, 311–318 (2005).
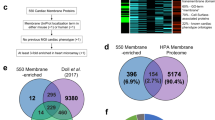
Schweppe, D. K. et al. Mitochondrial protein interactome elucidated by chemical cross-linking mass spectrometry. Proc. Natl Acad. Sci. USA 114, 1732–1737 (2017).
Chavez, J. D. et al. Cross-linking measurements of the Potato leafroll virus reveal protein interaction topologies required for virion stability, aphid transmission, and virus–plant interactions. J. Proteome Res. 11, 2968–2981 (2012).
Weisbrod, C. R. et al. In vivo protein interaction network identified with a novel real-time cross-linked peptide identification strategy. J. Proteome Res. 12, 1569–1579 (2013).
Chavez, J. D., Schweppe, D. K., Eng, J. K. & Bruce, J. E. In vivo conformational dynamics of Hsp90 and its interactors. Cell Chem. Biol. 23, 716–726 (2016).
Chavez, J. D. et al. Chemical crosslinking mass spectrometry analysis of protein conformations and supercomplexes in heart tissue. Cell Syst. 6, 136–141 (2018).
Ong, S.-E. et al. Stable isotope labeling by amino acids in cell culture, silac, as a simple and accurate approach to expression proteomics. Mol. Cell. Proteomics 1, 376–386 (2002).
Chavez, J. D. Quantitative interactome analysis reveals a chemoresistant edgotype. Nat. Commun. 6, 7928 (2015).
Chavez, J. D., Keller, A., Zhou, B., Tian, R. & Bruce, J. E. Cellular interactome dynamics during paclitaxel treatment. Cell Rep. 29, 2371–2383 (2019).
Chavez, J. D., Keller, A., Mohr, J. P. & Bruce, J. E. Isobaric quantitative protein interaction reporter technology for comparative interactome studies. Anal. Chem. 92, 14094–14102 (2020).
Brown, D. A. et al. Mitochondrial function as a therapeutic target in heart failure. Nat. Rev. Cardiol. 14, 238–250 (2017).
Barth, E., Stämmler, G., Speiser, B. & Schaper, J. Ultrastructural quantitation of mitochondria and myofilaments in cardiac muscle from 10 different animal species including man. J. Mol. Cell. Cardiol. 24, 669–681 (1992).
Schweppe, D. K. et al. XLinkDB 2.0: integrated, large-scale structural analysis of protein crosslinking data. Bioinformatics 32, 2716–2718 (2016).
Broom, B. M. et al. A galaxy implementation of next-generation clustered heatmaps for interactive exploration of molecular profiling data. Cancer Res. 77, e23 (2017).
Zong, S. et al. Structure of the intact 14-subunit human cytochrome c oxidase. Cell Res. 28, 1026–1034 (2018).
Balsa, E. et al. NDUFA4 is a subunit of complex IV of the mammalian electron transport chain. Cell Metab. 16, 378–386 (2012).
Massa, V. et al. Severe infantile encephalomyopathy caused by a mutation in COX6B1, a nucleus-encoded subunit of cytochrome c oxidase. Am. J. Hum. Genet. 82, 1281–1289 (2008).
Abdulhag, U. N. et al. Mitochondrial complex IV deficiency, caused by mutated COX6B1, is associated with encephalomyopathy, hydrocephalus and cardiomyopathy. Eur. J. Hum. Genet. 23, 159–164 (2015).
Rosca, M. G. et al. Cardiac mitochondria in heart failure: decrease in respirasomes and oxidative phosphorylation. Cardiovasc. Res. 80, 30–39 (2008).
Murashige, D. et al. Comprehensive quantification of fuel use by the failing and nonfailing human heart. Science 370, 364–368 (2020).
Aubert, G. et al. The failing heart relies on ketone bodies as a fuel. Circulation 133, 698–705 (2016).
Bedi, K. C. et al. Evidence for intramyocardial disruption of lipid metabolism and increased myocardial ketone utilization in advanced human heart failure. Circulation 133, 706–716 (2016).
Kolwicz, S. C., Airhart, S. & Tian, R. Ketones step to the plate. Circulation 133, 689–691 (2016).
Uchihashi, M. et al. Cardiac-specific Bdh1 overexpression ameliorates oxidative stress and cardiac remodeling in pressure overload-induced heart failure. Circ. Heart. Fail. 10, e004417 (2017).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Tammam, S. D., Rochet, J.-C. & Fraser, M. E. Identification of the cysteine residue exposed by the conformational change in pig heart succinyl-CoA:3-ketoacid coenzyme A transferase on binding coenzyme A. Biochemistry 46, 10852–10863 (2007).
Fraser, M. E., Hayakawa, K. & Brown, W. D. Catalytic role of the conformational change in succinyl-CoA:3-oxoacid CoA transferase on binding CoA. Biochemistry 49, 10319–10328 (2010).
Coker, S.-F. et al. The high-resolution structure of pig heart succinyl-CoA:3-oxoacid coenzyme A transferase. Acta Crystallogr. Biol. D Crystallogr. 66, 797–805 (2010).
Shafqat, N. et al. A structural mapping of mutations causing succinyl-CoA:3-ketoacid CoA transferase (SCOT) deficiency. J. Inherit. Metab. Dis. 36, 983–987 (2013).
Zhang, Y. & Skolnick, J. TM-align: a protein structure alignment algorithm based on the TM-score. Nucleic Acids Res. 33, 2302–2309 (2005).
Pebay-Peyroula, E. et al. Structure of mitochondrial ADP/ATP carrier in complex with carboxyatractyloside. Nature 426, 39–44 (2003).
Ruprecht, J. J. et al. The molecular mechanism of transport by the mitochondrial ADP/ATP carrier. Cell 176, 435–447 (2019).
Karch, J. et al. Inhibition of mitochondrial permeability transition by deletion of the ANT family and CypD. Sci. Adv. 5, eaaw4597 (2019).
Bround, M. J., Bers, D. M. & Molkentin, J. D. A 20/20 view of ANT function in mitochondrial biology and necrotic cell death. J. Mol. Cell. Cardiol. 144, A3–A13 (2020).
Bullock, J. M. A., Thalassinos, K. & Topf, M. Jwalk and MNXL web server: model validation using restraints from crosslinking mass spectrometry. Bioinformatics 34, 3584–3585 (2018).
Mirdita, M. et al. ColabFold—making protein folding accessible to all. Nat. Methods 19, 679–682 (2022).
Bertholet, A. M. et al. H+ transport is an integral function of the mitochondrial ADP/ATP carrier. Nature 571, 515–520 (2019).
Ramzan, R., Rhiel, A., Weber, P., Kadenbach, B. & Vogt, S. Reversible dimerization of cytochrome c oxidase regulates mitochondrial respiration. Mitochondrion 49, 149–155 (2019).
Kokoszka, J. E. et al. The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore. Nature 427, 461–465 (2004).
Chavez, J. D. et al. Mitochondrial protein interaction landscape of SS-31. Proc. Natl Acad. Sci. USA 117, 15363 (2020).
Tarnavski, O. et al. Mouse cardiac surgery: comprehensive techniques for the generation of mouse models of human diseases and their application for genomic studies. Physiol. Genomics 16, 349–360 (2004).
Ritterhoff, J. et al. Metabolic remodeling promotes cardiac hypertrophy by directing glucose to aspartate biosynthesis. Circ. Res. 126, 182–196 (2020).
Thompson, A. et al. Tandem mass tags: a novel quantification strategy for comparative analysis of complex protein mixtures by MS/MS. Anal. Chem. 75, 1895–1904 (2003).
Ross, P.ÿL. et al. Multiplexed protein quantitation in Saccharomyces cerevisiae using amine-reactive isobaric tagging reagents. Mol. Cell. Proteomics 3, 1154–1169 (2004).
Chavez, J. D. et al. Systems structural biology measurements by in vivo cross-linking with mass spectrometry. Nat. Protoc. 14, 2318–2343 (2019).
Tyanova, S. et al. The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat. Methods 13, 731–740 (2016).
Rogers, G. W. et al. High throughput microplate respiratory measurements using minimal quantities of isolated mitochondria. PLoS ONE 6, e21746 (2011).
Grinblat, L., Pacheco Bolaños, L. F. & Stoppani, A. O. Decreased rate of ketone-body oxidation and decreased activity of d-3-hydroxybutyrate dehydrogenase and succinyl-CoA:3-oxo-acid CoA-transferase in heart mitochondria of diabetic rats. Biochem. J. 240, 49–56 (1986).
Williamson, D. H., Bates, M. W., Page, M. A. & Krebs, H. A. Activities of enzymes involved in acetoacetate utilization in adult mammalian tissues. Biochem. J. 121, 41–47 (1971).
Brahma, M. K. et al. Increased glucose availability attenuates myocardial ketone body utilization. J. Am. Heart Assoc. 9, e013039 (2020).
Berardi, M. J., Shih, W. M., Harrison, S. C. & Chou, J. J. Mitochondrial uncoupling protein 2 structure determined by NMR molecular fragment searching. Nature 476, 109–113 (2011).
Keller, A., Chavez, J. D., Tang, X. & Bruce, J. E. Leveraging the entirety of the Protein Data Bank to enable improved structure prediction based on cross-link data. J. Proteome Res. 20, 1087–1095 (2021).
Spinazzi, M., Casarin, A., Pertegato, V., Salviati, L. & Angelini, C. Assessment of mitochondrial respiratory chain enzymatic activities on tissues and cultured cells. Nat. Protoc. 7, 1235–1246 (2012).
Chen, Z. et al. Quantitative cross-linking/mass spectrometry reveals subtle protein conformational changes. Wellcome Open Res. 1, 5 (2016).
Chen, Z. A. & Rappsilber, J. Protein dynamics in solution by quantitative crosslinking/mass spectrometry. Trends Biochem. Sci. 43, 908–920 (2018).
Keller, A., Eng, J., Zhang, N., Li, X.-J. & Aebersold, R. A uniform proteomics MS/MS analysis platform utilizing open XML file formats. Mol. Syst. Biol. 1, 2005.0017 (2005).
Mohr, J. P., Perumalla, P., Chavez, J. D., Eng, J. K. & Bruce, J. E. Mango: a general tool for collision induced dissociation-cleavable cross-linked peptide identification. Anal. Chem. 90, 6028–6034 (2018).
Chavez, J. D., Weisbrod, C. R., Zheng, C., Eng, J. K. & Bruce, J. E. Protein interactions, post-translational modifications and topologies in human cells. Mol. Cell. Proteomics 12, 1451–1467 (2013).
Calvo, S. E., Clauser, K. R. & Mootha, V. K. MitoCarta2.0: an updated inventory of mammalian mitochondrial proteins. Nucleic Acids Res. 44, D1251–D1257 (2015).
Keller, A., Chavez, J. D. & Bruce, J. E. Increased sensitivity with automated validation of XL-MS cleavable peptide crosslinks. Bioinformatics 35, 895–897 (2018).
Keller, A., Nesvizhskii, A. I., Kolker, E. & Aebersold, R. Empirical statistical model to estimate the accuracy of peptide identifications Made by MS/MS and database search. Anal. Chem. 74, 5383–5392 (2002).
Perez-Riverol, Y. et al. The PRIDE database and related tools and resources in 2019: improving support for quantification data. Nucleic Acids Res. 47, D442–D450 (2018).
Acknowledgements
We thank all Tian and Bruce laboratory members for their thoughtful discussions and support. We thank the University of Washington Proteomics Resource for advice and helpful discussions. We thank Y.-W. A. Hsu for assistance with the animal models. We thank J. Ritterhoff and F. Drees for their technical support and guidance. This work was supported, in part, by National Institutes of Health (NIH) grants HL110349, HL129510 and HL142628 (to R.T.); HL144778, GM097112, GM086688 and R35GM136255 (to J.E.B); American Heart Association (AHA) Predoctoral Fellowship 20PRE35120126 (to A.C.); AHA Postdoctoral Fellowship 18POST33990352 (to B.Z.); China Scholarship Council Fellowship 202006320416 (to H.C.); and NIH 2T32DK007247-41 and AHA Career Development Award 930223 (to M.A.W).
Author information
Authors and Affiliations
Contributions
A.C., X.T., J.D.C., R.T. and J.E.B. designed the experiments. A.C., X.T., J.D.C., A.K., M.A.W., R.T. and J.E.B. wrote the manuscript. A.C., X.T., R.T. and J.E.B. edited the manuscript. A.C., X.T., J.D.C. and A.K. performed formal analysis. A.C., B.Z. and M.A.W. performed animal experiments. A.C. and J.D.C. performed cross-linking experiments. J.D.C. performed protein preparation. J.D.C., X.T. and A.K. performed mass spectrometry raw data acquisition and processing. A.K. developed computational tools to support structural protein analysis and cross-linking quantitation. J.P.M. and A.A.B. performed cross-linking analysis and structural modeling. O.V. and H.C. performed animal surgeries. R.T. and J.E.B. supervised the project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Cardiovascular Research thanks Claudio Iacobucci, Martin Steinegger, Daniel Kelly and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 (a-b) Left ventricle internal dimension and wall thickness in TAC and Sham groups determined by echocardiography four weeks post-surgery.
(c-d) Lung and liver edema (wet weight/dry weight in mg) measured at tissue harvest. (e) Quantitation of mean cross-link (XL) ratio vs mean dead-end (DE) ratio for each cross-linked peptide pair statistically changed in TAC in at least 4/6 biological pairs (Log2 TAC/Sham). The sum of the Mean DE ratio for Protein A and Protein B is shown to account for cross-links between two different proteins. (f) Interaction network of lysine residues (black nodes) connected by observed cross-links (edges). Edges are colored according to increasing (red) and decreasing (blue) quantitation of statistically significant subset of cross-links (Log2 TAC/Sham) shown by color scale. For (a-d), all data are n=12 animals, AVG+/-SEM, *denotes p<0.05 by unpaired, two-tailed Student’s t-test.
Extended Data Fig. 2 (a) Structural insight into CX6B1 R20 forming salt-bridges at NDUA4-CX6B1 interface.
R20 side chain (green) forms a salt bridge with CX6B1 D16 (green) and NDUA4 D60 (black), which pinpoints an interface necessary for the stability of CIV. Side-chains of R20, D16, and NDUA4 D60 (partially resolved) are depicted in stick representation. Cross-linked lysine sidechains are shown as yellow (CX6B1) or orange (NDUA4) spheres. (b) Cytochrome C oxidase enzymatic activity assay in tissue homogenates from TAC and Sham hearts, normalized to Citrate Synthase activity. N=4 animals, AVG+/-SEM, *denotes p<0.05 by unpaired, two-tailed Student’s t-test. (c) Table summarizing the mean cross-linking ratio and DE ratio for cross-linked peptide pairs, including values obtained across biological replicates for cross-links in ADT isoforms. (d) Representative Blue Native-PAGE (BN-PAGE) analysis of mitochondria isolated from Sham and TAC groups. Coomassie stain (left) for total protein loading, In-gel CIV activity stain (middle), with CI activity stain overlay (right). Gels were run in duplicates. (e) Representative BN-PAGE immunoblot of NDUA4 containing SCs from digitonin solubilized isolated mitochondria. Blots were run in duplicates. (f) Representative BN-PAGE immunoblot of NDUA4 containing SCs from DDM solubilized isolated mitochondria. Blots were run in duplicates. (g) Cross-linked peptide pairs (yellow lysine side chains) mapped onto the M-state conformation of ADT1 (PDB: 6GCI). Salt bridges between K96-D196 and K199-D292 contribute to the gating mechanism, which closes the M-state to the IMS and would make lysines unavailable for cross-linking. Aspartic acid sidechains are shown in magenta. (h) Cross-linked peptide pairs (yellow lysine side chains) mapped onto the C-state conformation of bovine ADT1 (PDB: 1OKC). K33 and D231 are known to form a salt bridge that stabilizes the C-state and would make K33 unavailable for cross-linking. Aspartic acid sidechains are shown in magenta.
Extended Data Fig. 3 (a) AlphaFold-predicted BDH1_MOUSE structure.
Crosslinked residues were indicated in yellow spheres, and crosslinks shown in red lines mean they were increased in TAC samples. (b) The pLDDT plot of the predicted BDH_mouse structure. (c) Alignment of apo (grey, PDB: 3OXO chain A) and substrate-bound (yellow, PDB: 3OXO chain E) monomers of porcine SCOT1. CoA is colored in magenta and bound to the active site. Lysine sidechains are shown in stick representation. Alignment depicts the structural differences between the dynamic C-terminal domain and the static N-terminal domain during substrate-binding. A close-up view specifies cross-linked lysines (K418 and K421). (d) Ketone-driven oxygen consumption rate (OCR) of mitochondria isolated from TAC and Sham hearts. Baseline OCR (State 1) was measured, followed by sequential injections of β-hydroxybutyrate/malate (State 2), ADP (State 3), Oligomycin (State 4μ), and FCCP (FCCPmax). N=6 animals, AVG+/-SEM, *denotes p<0.05 by unpaired, two-tailed Student’s t-test.
Supplementary information
Supplementary Video 1
Cross-linking determines ADT1 conformational states
Supplementary Table
Supplementary Data 1: log2 ratios of non-redundant peptide pairs across six biological replicates and their mean log2 ratios showed significant changes between TAC and Sham samples and DE mean log2 ratios of the corresponding proteins. Supplementary Data 2: LFQ of mitochondrial proteins from six biological replicates of TAC and Sham samples and combined log2 ratios of TAC/Sham was reported. Supplementary Data 3: R2 values of pairwise linear regression of six pairs of biological replicates and mean R2 values of FF and FR regression. Supplementary Data 6: log2 ratios of residue pairs across six biological replicates and their mean log2 ratios for each residue pair with P value and 95% confidence level and several distinct peptide pairs reported. Supplementary Data 7: ANOVA test of the quantitation of the same residue pair generated from fully-cleaved peptide pairs and its corresponding missed-cleaved peptide pairs
Supplementary Data 4
SCOT1 octamer structure file generated by superimposition of AlphaFold monomer structures onto 3OXO to provide structural data on missing regions containing K296
Supplementary Data 5
AlphaFold model structure of ADT1 open channel consistent with increased cross-link levels quantified in TAC hearts
Source data
Source Data Extended Data Fig. 2
Uncropped gels for Extended Data Fig. 2c–e
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Caudal, A., Tang, X., Chavez, J.D. et al. Mitochondrial interactome quantitation reveals structural changes in metabolic machinery in the failing murine heart. Nat Cardiovasc Res 1, 855–866 (2022). https://doi.org/10.1038/s44161-022-00127-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44161-022-00127-4
This article is cited by
-
Skeletal muscle mitochondrial interactome remodeling is linked to functional decline in aged female mice
Nature Aging (2023)
-
Metabolic mechanisms in physiological and pathological cardiac hypertrophy: new paradigms and challenges
Nature Reviews Cardiology (2023)