Abstract
Atherosclerosis is the major cause of ischemic heart disease and stroke, the leading causes of mortality worldwide. The central pathological features of atherosclerosis include macrophage infiltration and foam cell formation. However, the detailed mechanisms regulating these two processes are unclear. Here we show that oxidative stress-activated Ca2+-permeable transient receptor potential melastatin 2 (TRPM2) plays a critical role in atherogenesis. Both global and macrophage-specific Trpm2 deletions protect Apoe−/− mice against atherosclerosis. Trpm2 deficiency reduces oxidized low-density lipoprotein (oxLDL) uptake by macrophages, thereby minimizing macrophage infiltration, foam cell formation and inflammatory responses. Activation of the oxLDL receptor CD36 induces TRPM2 activity and vice versa. In cultured macrophages, TRPM2 is activated by the CD36 ligands oxLDL and thrombospondin-1 (TSP1); deleting Trpm2 or inhibiting TRPM2 activity suppresses the activation of the CD36 signaling cascade induced by oxLDL and TSP1. Our findings establish the TRPM2–CD36 axis as a molecular mechanism underlying atherogenesis and suggest TRPM2 as a potential therapeutic target for atherosclerosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
All data generated or analyzed are included in the main article and associated files. Source data are provided with this paper.
Change history
23 June 2023
A Correction to this paper has been published: https://doi.org/10.1038/s44161-023-00303-0
08 April 2022
A Correction to this paper has been published: https://doi.org/10.1038/s44161-022-00062-4
References
Libby, P. et al. Atherosclerosis. Nat. Rev. Dis. Primers 5, 56 (2019).
Moore, K. J., Sheedy, F. J. & Fisher, E. A. Macrophages in atherosclerosis: a dynamic balance. Nat. Rev. Immunol. 13, 709–721 (2013).
Moore, K. J. & Tabas, I. Macrophages in the pathogenesis of atherosclerosis. Cell 145, 341–355 (2011).
Silverstein, R. L., Li, W., Park, Y. M. & Rahaman, S. O. Mechanisms of cell signaling by the scavenger receptor CD36: implications in atherosclerosis and thrombosis. Trans. Am. Clin. Climatol. Assoc. 121, 206–220 (2010).
Moore, K. J. & Freeman, M. W. Scavenger receptors in atherosclerosis: beyond lipid uptake. Arterioscler. Thromb. Vasc. Biol. 26, 1702–1711 (2006).
Baldrighi, M., Mallat, Z. & Li, X. NLRP3 inflammasome pathways in atherosclerosis. Atherosclerosis 267, 127–138 (2017).
Cho, S. CD36 as a therapeutic target for endothelial dysfunction in stroke. Curr. Pharm. Des. 18, 3721–3730 (2012).
Hara, Y. et al. LTRPC2 Ca2+-permeable channel activated by changes in redox status confers susceptibility to cell death. Mol. Cell 9, 163–173 (2002).
Perraud, A. L. et al. ADP-ribose gating of the calcium-permeable LTRPC2 channel revealed by Nudix motif homology. Nature 411, 595–599 (2001).
Sano, Y. et al. Immunocyte Ca2+ influx system mediated by LTRPC2. Science 293, 1327–1330 (2001).
Takahashi, N., Kozai, D., Kobayashi, R., Ebert, M. & Mori, Y. Roles of TRPM2 in oxidative stress. Cell Calcium 50, 279–287 (2011).
Desai, B. N. & Leitinger, N. Purinergic and calcium signaling in macrophage function and plasticity. Front. Immunol. 5, 580 (2014).
Syed Mortadza, S. A., Wang, L., Li, D. & Jiang, L.-H. TRPM2 channel-mediated ROS-sensitive Ca2+ signaling mechanisms in immune cells. Front. Immunol. 6, 407 (2015).
Di, A. et al. The redox-sensitive cation channel TRPM2 modulates phagocyte ROS production and inflammation. Nat. Immunol. 13, 29–34 (2011).
Wolf, D. & Ley, K. Immunity and inflammation in atherosclerosis. Circ. Res. 124, 315–327 (2019).
Arida, A., Protogerou, A. D., Kitas, G. D. & Sfikakis, P. P. Systemic inflammatory response and atherosclerosis: the paradigm of chronic inflammatory rheumatic diseases. Int. J. Mol. Sci. 19, 1890 (2018).
Sheedy, F. J. et al. CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nat. Immunol. 14, 812–820 (2013).
Tsiantoulas, D. et al. APRIL limits atherosclerosis by binding to heparan sulfate proteoglycans. Nature 597, 92–96 (2021).
Fonfria, E. et al. TRPM2 is elevated in the tMCAO stroke model, transcriptionally regulated, and functionally expressed in C13 microglia. J. Recept. Signal Transduct. Res. 26, 179–198 (2006).
Belrose, J. C. & Jackson, M. F. TRPM2: a candidate therapeutic target for treating neurological diseases. Acta Pharmacol. Sin. 39, 722–732 (2018).
Deshmane, S. L., Kremlev, S., Amini, S. & Sawaya, B. E. Monocyte chemoattractant protein-1 (MCP-1): an overview. J. Interferon Cytokine Res. 29, 313–326 (2009).
Nowak, W. N., Deng, J., Ruan, X. Z. & Xu, Q. Reactive oxygen species generation and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 37, e41–e52 (2017).
Buttery, L. D. et al. Inducible nitric oxide synthase is present within human atherosclerotic lesions and promotes the formation and activity of peroxynitrite. Lab. Invest. 75, 77–85 (1996).
Zhong, Z. et al. TRPM2 links oxidative stress to NLRP3 inflammasome activation. Nat. Commun. 4, 1611 (2013).
Duewell, P. et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 464, 1357–1361 (2010).
Park, Y. M., Febbraio, M. & Silverstein, R. L. CD36 modulates migration of mouse and human macrophages in response to oxidized LDL and may contribute to macrophage trapping in the arterial intima. J. Clin. Invest. 119, 136–145 (2009).
Barrett, T. J. Macrophages in atherosclerosis regression. Arterioscler. Thromb. Vasc. Biol. 40, 20–33 (2020).
Miller, B. A. et al. TRPM2 channels protect against cardiac ischemia-reperfusion injury: role of mitochondria. J. Biol. Chem. 289, 7615–7629 (2014).
Ferron, M. & Vacher, J. Targeted expression of Cre recombinase in macrophages and osteoclasts in transgenic mice. Genesis 41, 138–145 (2005).
Beckers, C. M. L. et al. Cre/lox studies identify resident macrophages as the major source of circulating coagulation factor XIII-A. Arterioscler. Thromb. Vasc. Biol. 37, 1494–1502 (2017).
Yoshimura, T., Robinson, E. A., Tanaka, S., Appella, E. & Leonard, E. J. Purification and amino acid analysis of two human monocyte chemoattractants produced by phytohemagglutinin-stimulated human blood mononuclear leukocytes. J. Immunol. 142, 1956–1962 (1989).
Harris, J., VanPatten, S., Deen, N. S., Al-Abed, Y. & Morand, E. F. Rediscovering MIF: new tricks for an old cytokine. Trends Immunol. 40, 447–462 (2019).
Zhao, M. et al. Activation of the p38 MAP kinase pathway is required for foam cell formation from macrophages exposed to oxidized LDL. APMIS 110, 458–468 (2002).
Ricci, R. et al. Requirement of JNK2 for scavenger receptor A-mediated foam cell formation in atherogenesis. Science 306, 1558–1561 (2004).
Rahaman, S. O. et al. A CD36-dependent signaling cascade is necessary for macrophage foam cell formation. Cell Metab. 4, 211–221 (2006).
Wang, Y., Wang, G. Z., Rabinovitch, P. S. & Tabas, I. Macrophage mitochondrial oxidative stress promotes atherosclerosis and nuclear factor-κB-mediated inflammation in macrophages. Circ. Res. 114, 421–433 (2014).
Yan, B., Bengtson, C. P., Buchthal, B., Hagenston, A. M. & Bading, H. Coupling of NMDA receptors and TRPM4 guides discovery of unconventional neuroprotectants. Science 370, eaay3302 (2020).
Dugan, L. L. et al. Mitochondrial production of reactive oxygen species in cortical neurons following exposure to N-methyl-d-aspartate. J. Neurosci. 15, 6377–6388 (1995).
Hoffmann, A., Kann, O., Ohlemeyer, C., Hanisch, U.-K. & Kettenmann, H. Elevation of basal intracellular calcium as a central element in the activation of brain macrophages (microglia): suppression of receptor-evoked calcium signaling and control of release function. J. Neurosci. 23, 4410–4419 (2003).
Yoshimura, T. The chemokine MCP-1 (CCL2) in the host interaction with cancer: a foe or ally? Cell. Mol. Immunol. 15, 335–345 (2018).
Yamamoto, S. et al. TRPM2-mediated Ca2+ influx induces chemokine production in monocytes that aggravates inflammatory neutrophil infiltration. Nat. Med. 14, 738–747 (2008).
Chu, L.-Y., Ramakrishnan, D. P. & Silverstein, R. L. Thrombospondin-1 modulates VEGF signaling via CD36 by recruiting SHP-1 to VEGFR2 complex in microvascular endothelial cells. Blood 122, 1822–1832 (2013).
Lopez-Dee, Z., Pidcock, K. & Gutierrez, L. S. Thrombospondin-1: multiple paths to inflammation. Mediators Inflamm. 2011, 296069 (2011).
Ganguly, R. et al. TSP-1 (thrombospondin-1) deficiency protects ApoE−/− mice against leptin-induced atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 41, e112–e127 (2021).
Ganguly, R. et al. Oral chromium picolinate impedes hyperglycemia-induced atherosclerosis and inhibits proatherogenic protein TSP-1 expression in STZ-induced type 1 diabetic ApoE-/- mice. Sci. Rep. 7, 45279 (2017).
Du, J., Xie, J. & Yue, L. Intracellular calcium activates TRPM2 and its alternative spliced isoforms. Proc. Natl Acad. Sci. USA 106, 7239–7244 (2009).
Sumoza-Toledo, A. & Penner, R. TRPM2: a multifunctional ion channel for calcium signalling. J. Physiol. 589, 1515–1525 (2011).
Leung, A. W. Y., Chan, R. S. M., Sea, M. M. M. & Woo, J. An overview of factors associated with adherence to lifestyle modification programs for weight management in adults. Int. J. Environ. Res. Public Health 14, 922 (2017).
Jeurissen, M. L. J. et al. Prevention of oxLDL uptake leads to decreased atherosclerosis in hematopoietic NPC1-deficient Ldlr−/− mice. Atherosclerosis 255, 59–65 (2016).
Rahaman, S. O., Zhou, G. & Silverstein, R. L. Vav protein guanine nucleotide exchange factor regulates CD36 protein-mediated macrophage foam cell formation via calcium and dynamin-dependent processes. J. Biol. Chem. 286, 36011–36019 (2011).
Murakami, T. et al. Critical role for calcium mobilization in activation of the NLRP3 inflammasome. Proc. Natl Acad. Sci. USA 109, 11282–11287 (2012).
Fernandez, D. M. & Giannarelli, C. Immune cell profiling in atherosclerosis: role in research and precision medicine. Nat. Rev. Cardiol. 19, 43–58 (2022).
Lin, J.-D. et al. Single-cell analysis of fate-mapped macrophages reveals heterogeneity, including stem-like properties, during atherosclerosis progression and regression. JCI Insight 4, e124574 (2019).
Miller, B. A. et al. The second member of transient receptor potential-melastatin channel family protects hearts from ischemia-reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 304, H1010–H1022 (2013).
Paigen, B., Morrow, A., Holmes, P. A., Mitchell, D. & Williams, R. A. Quantitative assessment of atherosclerotic lesions in mice. Atherosclerosis 68, 231–240 (1987).
Amini-Nik, S. et al. β-Catenin-regulated myeloid cell adhesion and migration determine wound healing. J. Clin. Invest. 124, 2599–2610 (2014).
Du, J. et al. TRPM7-mediated Ca2+ signals confer fibrogenesis in human atrial fibrillation. Circ. Res. 106, 992–1003 (2010).
Sun, Y. et al. A human platelet receptor protein microarray identifies the high affinity immunoglobulin E receptor subunit α (FcεR1α) as an activating platelet endothelium aggregation receptor 1 (PEAR1) ligand. Mol. Cell. Proteomics 14, 1265–1274 (2015).
Acknowledgements
We thank A. M. Scharenberg (University of Washington) for providing the TRPM2 plasmid. CD36-bio-His was a gift from G. Wright (Addgene plasmid no. 52025; http://n2t.net/addgene:52025; Research Resource Identifier: Addgene_52025)58. We thank Q. Lin (University of Connecticut) for preparing the artistic graphic works for our study. This work was partially supported by the National Institutes of Health (no. R01-HL143750) and American Heart Association (no. 19TPA34890022) to L.Y. and Canadian Institutes of Health Research (no. MOP 86655) to J.V.
Author information
Authors and Affiliations
Contributions
L.Y. conceived the research. P.Z. designed and performed the in vitro experiments. Z.Y. and J.F. performed most of the in vivo experiments. A.S.Y. conducted some of the in vitro experiments. J. V., B. M. and Y.M. generated the transgenic mice. E.R.J. and P.Z. conducted the flow cytometry experiments. P.Z. and L.Y. wrote the manuscript with contributions from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Cardiovascular Research thanks Lin-Hua Jiang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated supplementary information
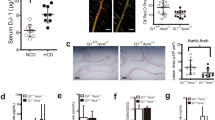
Extended Data Fig. 1 Knockout of Trpm2 in Apoe-/- mice.
(a) Representative PCR genotyping results showing a 514 bp and 740 bp products for WT and M2KO mice. (b) Representative WB analysis of TRPM2 expression in macrophages isolated from Apoe single knockout (WT (n = 3)) and Apoe / Trpm2 double knockout (M2KO (n = 3)) mice (c-e) Representative recording (c, I-V curve; d, time-current trace) and quantification of TRPM2 current in macrophages isolated from Apoe single knockout (WT) and Apoe / Trpm2 double knockout (M2KO) mice. ACA is a TRPM2 blocker. (***: p < 0.001; unpaired t test; mean ± SEM) (f) Graphic illustration showing the atherosclerotic area chosen for taking images of F4/80&CD80 staining in Fig. 1i and Fig. 3h. (g) Representative WB analysis of TRPM2 expression in macrophages isolated from Trpm2fl/flCd11b-cre- (n = 3) and Trpm2fl/flCd11b-cre+ mice (n = 3) with Apoe knockout. (h, i) Representative recording and quantification of TRPM2 current in macrophages isolated from Trpm2fl/flCd11b-cre- and Trpm2fl/flCd11b-cre+ mice with Apoe knockout.
Extended Data Fig. 2 Expression of TRPM2 is increased during atherogenesis.
(a, b) Representative WB analysis of TRPM2 expression in aorta from wild-type mice (WT) with or without the treatment of high-fat diet (HFD) for 4 months (n = 6/group). (c, d) Representative WB analysis of TRPM2 expression in macrophages isolated from WT mice with or without the treatment of oxLDL (50 µg/ml) for 24 h (n = 6/group). (***: p < 0.001; unpaired t test, two-tailed; mean ± SEM)
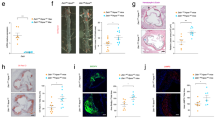
Extended Data Fig. 3 In vitro macrophage migration and emigration assay.
(a) Graphic illustration of in vitro examination of macrophage infiltration across endothelial cells induced by MCP1. Aorta-derived endothelial cells were plated on the transwell inserts (pore size: 12 µm) for 3-5 days. Bone marrow derived macrophages were added into the upper chamber after endothelial cells completely covered the upper surface of transwells. After 4 h, F4/80 and CD80 staining of macrophages in the lower chamber was performed as in Fig. 2l. (b) Graphic illustration of in vitro examination of macrophage emigration across endothelial cells induced by MCP1. Aorta-derived endothelial cells were plated on the transwell inserts (pore size: 12 µm) for 3-5 days. Bone marrow derived macrophages preloaded with oxLDL for 24 h were added into the upper chamber after endothelial cells completely covered the upper surface of transwells. After 24 h, F4/80 and CD80 staining of macrophages in lower chamber was performed as in Fig. 2n. (c) In vitro macrophage migration assay induced by MIF instead of MCP1 as shown in Extended Data Fig. 3a. l, F4/80 and CD80 staining of macrophages in the lower chamber (Red: F4/80; Blue: DAPI; Green: CD80). (d), Quantification of the number of infiltrated macrophages within a x 10 field. 6 dishes from each group were chosen for quantification. (***: p < 0.001; two-way ANOVA, Bonferroni’s test; mean ± SEM).
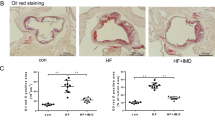
Extended Data Fig. 4 TRPM2 is required for CD36 activation in macrophages induced by oxLDL.
(a) Quantification of Fig. 4a by counting percentage of Oil red O staining macrophages (n = 8/group). (b, c) 30-min oxLDL treatment (50 µg/ml) induce the activation of CD36 signaling without upregulating CD36 expression. Representative WB analysis of CD36, pFyn, pJNK and pp38 expression in macrophages after oxLDL treatment for 30 min (n = 6/group). (d, e) NADPH oxidase inhibitor apocynin does not inhibit CD36 activation induced by 24-h oxLDL treatment (50 µg/ml) in macrophages isolated from wild-type (WT) mice. Representative WB analysis of CD36, pFyn, pJNK and pp38 expression in macrophages after oxLDL treatment for 24 h (n = 6/group). (f) Quantification of Fig. 4k by counting percentage of Oil red O staining macrophages (n = 6/group). (g) Quantification of Fig. 4o by counting percentage of Oil red O staining macrophages (n = 8/group). (h) A set of original Fura-2 real time recording traces without normalization during oxLDL treatment as in Fig. 4o. (i, j) Trpm2 deletion does not influence the production of MCP1/MIF in endothelial cells isolated from aorta in response to 24-h oxLDL treatment (50 µg/ml). Representative WB analysis of MCP1 and MIF expression in endothelial cells after oxLDL treatment for 24 h (n = 6/group). (ns: no statistical significance; *: p < 0.05; **: p < 0.01; ***: p < 0.001; ANOVA, two-way, Bonferroni’s test; mean ± SEM).
Extended Data Fig. 5 TRPM2 is required for CD36 activation in macrophages induced by TSP1.
(a, b) Representative WB analysis of TRPM2 expression in macrophages isolated from WT mice with or without the treatment of TSP1 (10 µg/ml) for 24 h (n = 6/group). (c, d) 30-min TSP1 (10 µg/ml) treatment induce the activation of CD36 signaling without upregulating CD36 expression. Representative WB analysis of CD36, pFyn, pJNK and pp38 expression in macrophages after TSP1 treatment for 30 min (n = 6/group). (e, f) NADPH oxidase inhibitor apocynin does not inhibit CD36 activation induced by 24-h TSP1 (10 µg/ml) treatment in macrophages isolated from wild-type (WT) mice. Representative WB analysis of CD36, pFyn, pJNK and pp38 expression in macrophages after TSP1 treatment for 24 h. (g) A set of original Fura-2 real time recording traces without normalization during TSP1 treatment as in Fig. 5g. (h, i) Trpm2 deletion does not influence the production of MCP1/MIF in endothelial cells isolated from aorta in response to 24-h TSP1 (10 µg/ml) treatment. Representative WB analysis of MCP1 and MIF expression in endothelial cells after TSP1 treatment for 24 h (n = 6/group). (ns: no statistical significance; *: p < 0.05; **: p < 0.01; ***: p < 0.001; ANOVA, two-way, Bonferroni’s test; mean ± SEM).
Extended Data Fig. 6 Different inhibitors suppressed the activation of TRPM2 by oxLDL or TSP1 treatment.
(a, b) Representative recording of TRPM2 current in HEK293T cells transfected with CD36 and TRPM2 during oxLDL treatment (50 µg/ml) as in a, and during TSP1 treatment (10 µg/ml) as in b. Transfected cells were treated with different inhibitors as indicated before current recording.
Extended Data Fig. 7 Inhibition of CD36 or TRPM2 did not produce additional inhibitory effect on M2KO macrophages.
(a) Representative WB analysis of the expression of CD36, pFyn, Fyn, pJNK, JNK, pp38 and p38 in isolated macrophages from M2KO mice after oxLDL treatment (50 µg/ml). Macrophages were treated with different inhibitors as indicated before protein extraction. (b) Quantification of WB bands (n = 3/group). (c) Representative WB analysis of the expression of CD36, pFyn, Fyn, pJNK, JNK, pp38 and p38 in isolated macrophages from M2KO mice after TSP1 treatment (10 µg/ml). Macrophages were treated with different inhibitors as indicated before protein extraction. (d) Quantification of WB bands (n = 3/group). (e) Representative WB analysis of the expression of iNOS in isolated macrophages from M2KO mice after oxLDL treatment (50 µg/ml). Macrophages were treated with different inhibitors as indicated before protein extraction. (f) Representative WB analysis of the expression of iNOS in isolated macrophages from M2KO mice after TSP1 treatment (10 µg/ml). Macrophages were treated with different inhibitors as indicated before protein extraction. (g) Quantification of WB bands (n = 3/group). (h) Quantification of WB bands (n = 3/group). (i) Representative WB analysis of the expression of MCP1 and MIF in isolated macrophages from M2KO mice after oxLDL treatment (50 μg/ml). Macrophages were treated with different inhibitors as indicated before protein extraction. (j) Representative WB analysis of the expression of MCP1 and MIF in isolated macrophages from M2KO mice after TSP1 treatment (10 μg/ml). Macrophages were treated with different inhibitors as indicated before protein extraction. (k) Quantification of WB bands (n = 3/group). (l) Quantification of WB bands (n = 3/group). (ns: no statistical significance; ANOVA, two-way, Bonferroni’s test; mean ± SEM).
Extended Data Fig. 8 Graphic abstract, the activation of CD36 and TRPM2 form a positive feedback loop in atherogenesis.
(a) Global Trpm2 deletion and macrophage-specific Trpm2 deletion protect against atherosclerosis in Apoe-/- mice fed with a high-fat diet (HFD). (b) Trpm2 deficiency in macrophages inhibits atherogenesis by inhibiting macrophage infiltration and minimizing foam cell formation. (c) TRPM2 activation is required for CD36-induced oxLDL uptake and subsequent inflammatory responses in macrophages. (d) The ligands of CD36, oxLDL and TSP1, activate TRPM2, thereby perpetuating TRPM2-CD36 inflammatory cycle in atherogenesis cascade. (e) Our data establish TRPM2-CD36 axis in macrophages as an important atherogenesis mechanism and TRPM2 as a promising therapeutic target for atherosclerosis.
Supplementary information
Supplementary Information
Flow cytometry gating strategy for Figs. 1 and 3 and all uncropped original scanned gel images in the Extended Data figures.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Current traces and statistical source data.
Source Data Fig. 2
Uncropped gels.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 3
Uncropped gels.
Source Data Fig. 4
Fura2 imaging traces and statistical source data.
Source Data Fig. 4
Uncropped gels.
Source Data Fig. 5
Fura2 imaging traces and statistical source data.
Source Data Fig. 5
Uncropped gels.
Source Data Fig. 6
Current traces and statistical source data.
Source Data Fig. 6
Uncropped gels.
Source Data Fig. 7
Fura2 imaging traces and statistical source data.
Source Data Fig. 7
Uncropped gels.
Source Data Fig. 8
Statistical source data.
Source Data Fig. 8
Uncropped gels.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zong, P., Feng, J., Yue, Z. et al. TRPM2 deficiency in mice protects against atherosclerosis by inhibiting TRPM2–CD36 inflammatory axis in macrophages. Nat Cardiovasc Res 1, 344–360 (2022). https://doi.org/10.1038/s44161-022-00027-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44161-022-00027-7
This article is cited by
-
Downregulation of HMGB1 carried by macrophage-derived extracellular vesicles delays atherosclerotic plaque formation through Caspase-11-dependent macrophage pyroptosis
Molecular Medicine (2024)
-
TRP Channels in Stroke
Neuroscience Bulletin (2023)
-
PIAS1 impedes vascular endothelial injury and atherosclerotic plaque formation in diabetes by blocking the RUNX3/TSP-1 axis
Human Cell (2023)
-
ROS-Activated TRPM2 Channel: Calcium Homeostasis in Cardiovascular/renal System and Speculation in Cardiorenal Syndrome
Cardiovascular Drugs and Therapy (2023)