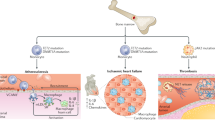
Abstract
Clonal hematopoiesis arises from somatic mutations that provide a fitness advantage to hematopoietic stem cells and the outgrowth of clones of blood cells. Clonal hematopoiesis commonly involves mutations in genes that are involved in epigenetic modifications, signaling and DNA damage repair. Clonal hematopoiesis has emerged as a major independent risk factor in atherosclerotic cardiovascular disease, thrombosis and heart failure. Studies in mouse models of clonal hematopoiesis have shown an increase in atherosclerosis, thrombosis and heart failure, involving increased myeloid cell inflammatory responses and inflammasome activation. Although increased inflammatory responses have emerged as a common underlying principle, some recent studies indicate mutation-specific effects. The discovery of the association of clonal hematopoiesis with cardiovascular disease and the recent demonstration of benefit of anti-inflammatory treatments in human cardiovascular disease converge to suggest that anti-inflammatory treatments should be directed to individuals with clonal hematopoiesis. Such treatments could target specific inflammasomes, common downstream mediators such as IL-1β and IL-6, or mutations linked to clonal hematopoiesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Marnell, C. S., Bick, A. & Natarajan, P. Clonal hematopoiesis of indeterminate potential (CHIP): linking somatic mutations, hematopoiesis, chronic inflammation and cardiovascular disease. J. Mol. Cellular Cardiol. 161, 98–105 (2021).
Steensma, D. P. et al. Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood 126, 9–16 (2015).
Jaiswal, S. et al. Age-related clonal hematopoiesis associated with adverse outcomes. N. Engl. J. Med. 371, 2488–2498 (2014). These authors discovered that people with clonal hematopoiesis were at increased risk of developing both hamatological malignancies and atherosclerotic cardiovascular disease.
Yu, B. et al. Supplemental association of clonal hematopoiesis with incident heart failure. J. Am. Coll. Cardiol. 78, 42–52 (2021). In a meta-analysis of different studies some, but not all, CHIP variants were found to be associated with an increased incidence of heart failure.
Pascual-Figal, D. A. et al. Clonal hematopoiesis and risk of progression of heart failure with reduced left ventricular ejection fraction. J. Am. Coll. Cardiol. 77, 1747–1759 (2021).
Dorsheimer, L. et al. Association of mutations contributing to clonal hematopoiesis with prognosis in chronic ischemic heart failure. JAMA cardiology 4, 25–33 (2019).
Mas-Peiro, S. et al. Clonal haematopoiesis in patients with degenerative aortic valve stenosis undergoing transcatheter aortic valve implantation. Eur. Heart J. 41, 933–939 (2020).
Jaiswal, S. & Ebert, B. L. Clonal hematopoiesis in human aging and disease. Science 366, eaan4673 (2019).
Jaiswal, S. & Libby, P. Clonal haematopoiesis: connecting ageing and inflammation in cardiovascular disease. Nat. Rev. Cardiol. 17, 137–144 (2020).
Libby, P. et al. Clonal hematopoiesis: crossroads of aging, cardiovascular disease, and cancer: JACC review topic of the week. J. Am. Coll. Cardiol. 74, 567–577 (2019).
Yura, Y., Sano, S. & Walsh, K. Clonal hematopoiesis: a new step linking inflammation to heart failure. JACC Basic Transl. Sci. 5, 196–207 (2020).
Heyde, A. et al. Increased stem cell proliferation in atherosclerosis accelerates clonal hematopoiesis. Cell 184, 1348–1361 (2021). This study provides evidence in mice and humans that atherosclerosis can promote the expansion of haematopoietic stem cells containing CH mutations suggesting reverse or bidirectional causation.
Libby, P. The changing landscape of atherosclerosis. Nature 592, 524–533 (2021).
Jaiswal, S. et al. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. New Engl. J. Med. 377, 111–121 (2017).
Zekavat, S. M. et al. TP53-mediated clonal hematopoiesis confers increased risk for incident peripheral artery disease. Preprint at medRxiv https://doi.org/10.1101/2021.08.22.21262430 (2021).
Young, A. L., Challen, G. A., Birmann, B. M. & Druley, T. E. Clonal haematopoiesis harbouring AML-associated mutations is ubiquitous in healthy adults. Nat. Commun. 7, 12484 (2016).
Hannum, G. et al. Genome-wide methylation profiles reveal quantitative views of human aging rates. Molecular cell 49, 359–367 (2013).
Levine, M. E. et al. An epigenetic biomarker of aging for lifespan and healthspan. Aging 10, 573–591 (2018).
Robertson, N. A. et al. Age-related clonal haemopoiesis is associated with increased epigenetic age. Curr. Biol. 29, R786–R787 (2019).
Nachun, D. et al. Clonal hematopoiesis associated with epigenetic aging and clinical outcomes. Aging Cell 20, e13366 (2021).
Busque, L. et al. Recurrent somatic TET2 mutations in normal elderly individuals with clonal hematopoiesis. Nat. Genet. 44, 1179–1181 (2012).
Ko, M. et al. Ten-eleven-translocation 2 (TET2) negatively regulates homeostasis and differentiation of hematopoietic stem cells in mice. PNAS 108, 14566–14571 (2011).
Moran-Crusio, K. et al. Tet2 loss leads to increased hematopoietic stem cell self-renewal and myeloid transformation. Cancer Cell 20, 11–24 (2011).
Zhang, Q. et al. Tet2 is required to resolve inflammation by recruiting Hdac2 to specifically repress IL-6. Nature 525, 389–393 (2015).
Fuster, J. J. et al. Clonal hematopoiesis associated with Tet2 deficiency accelerates atherosclerosis development in mice. Science 355, 842–847 (2017). This study demonstrated accelerated atherosclerosis in a mouse model of Tet2 deficiency CH and implicated NLRP3 inflammasome activation as a major underlying mechanism.
Liu, D. J. et al. Exome-wide association study of plasma lipids in >300,000 individuals. Nat. Genet. 49, 1758–1766 (2017).
Bick, A. G. et al. Inherited causes of clonal haematopoiesis in 97,691 whole genomes. Nature 586, 763–768 (2020).
Cordua, S. et al. Prevalence and phenotypes of JAK2V617F and calreticulin mutations in a Danish general population. Blood 134, 469–479 (2019).
Yokokawa, T. et al. Crucial role of hematopoietic JAK2V617F in the development of aortic aneurysms. Haematologica 106, 1910–1922 (2021).
Luque Paz, D., Ashcroft, P. & Skoda, R. C. Myeloproliferative neoplasms: the long wait for JAK2-mutant clone expansion. Cell Stem Cell 28, 359–361 (2021).
Wang, W. et al. Macrophage inflammation, erythrophagocytosis, and accelerated atherosclerosis in Jak2 (V617F) mice. Circ. Res. 123, e35–e47 (2018).
Fidler, T. P. et al. The AIM2 inflammasome exacerbates atherosclerosis in clonal haematopoiesis. Nature 592, 296–301 (2021). These authors implicate AIM2 inflammasome activation in the accelerated atherosclerosis of Jak2VF clonal hematopoiesis mice and show beneficial effects of treatment with IL-1β antibodies.
Edelmann, B. et al. JAK2-V617F promotes venous thrombosis through β1/β2 integrin activation. J. Clin. Invest. 128, 4359–4371 (2018).
Shimada, K. et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity 36, 401–414 (2012).
Hu, B. et al. The DNA-sensing AIM2 inflammasome controls radiation-induced cell death and tissue injury. Science 354, 765–768 (2016).
Nilsson, J. & Hansson, G. K. The changing face of atherosclerotic plaque inflammation. J. Intern. Med. 278, 430–432 (2015).
Challen, G. A. & Goodell, M. A. Clonal hematopoiesis: mechanisms driving dominance of stem cell clones. Blood 136, 1590–1598 (2020).
Rauch, P. J. et al. Loss-of-function mutations in Dnmt3a and Tet2 lead to accelerated atherosclerosis and convergent macrophage phenotypes in mice. Blood 132, 745 (2018).
Sano, S. et al. CRISPR-mediated gene editing to assess the roles of Tet2 and Dnmt3a in clonal hematopoiesis and cardiovascular disease. Circ. Res. 123, 335–341 (2018).
Lim, J. Y. et al. DNMT3A haploinsufficiency causes dichotomous DNA methylation defects at enhancers in mature human immune cells. J. Exp. Med. 218, e20202733 (2021)
Kim, P. G. et al. Dnmt3a-mutated clonal hematopoiesis promotes osteoporosis. J. Exp. Med. 218, e20211872 (2021).
Falanga, A. & Marchetti, M. Thrombotic disease in the myeloproliferative neoplasms. Hematology/the Education Program of the American Society of Hematology. American Society of Hematology. Education Program 2012, 571–581 (2012).
Wolach, O. et al. Increased neutrophil extracellular trap formation promotes thrombosis in myeloproliferative neoplasms. Sci. Transl. Med. 10, eaan8292 (2018).
Zhao, B. et al. Loss of pleckstrin-2 reverts lethality and vascular occlusions in JAK2V617F-positive myeloproliferative neoplasms. J. Clin. Invest. 128, 125–140 (2018).
Gudbjartsson, D. F. et al. Sequence variants affecting eosinophil numbers associate with asthma and myocardial infarction. Nat. Genet. 41, 342–347 (2009).
Wang, W. et al. LNK/SH2B3 loss of function promotes atherosclerosis and thrombosis. Circ. Res. 119, e91–e103 (2016).
Dou, H. et al. Oxidized phospholipids promote NETosis and arterial thrombosis in LNK(SH2B3) deficiency. Circulation 144, 1940–1954 (2021).
Cremer, S. et al. Multiple somatic mutations for clonal hematopoiesis are associated with increased mortality in patients with chronic heart failure. Circ. Genom. Precis. Med. 13, e003003 (2020).
Palomo, L. et al. Prevalence and characteristics of clonal hematopoiesis in heart failure. Rev. Esp. Cardiol. 74, 996–999 (2021).
Assmus, B. et al. Clonal haematopoiesis in chronic ischaemic heart failure: prognostic role of clone size for DNMT3A- and TET2-driver gene mutations. Eur. Heart J. 42, 257–265 (2021).
Kiefer, K. C. et al. Full spectrum of clonal haematopoiesis-driver mutations in chronic heart failure and their associations with mortality. ESC Heart Fail 8, 1873–1884 (2021).
Adamo, L., Rocha-Resende, C., Prabhu, S. D. & Mann, D. L. Reappraising the role of inflammation in heart failure. Nat. Rev. Cardiol. 17, 269–285 (2020).
Abplanalp, W. T. et al. Clonal hematopoiesis-driver DNMT3A mutations alter immune cells in heart failure. Circ. Res. 128, 216–228 (2021).
Sano, S. et al. Tet2-mediated clonal hematopoiesis accelerates heart failure through a mechanism involving the IL-1β/NLRP3 inflammasome. J. Am. Coll. Cardiol. 71, 875–886 (2018).
Wang, Y. et al. Tet2-mediated clonal hematopoiesis in nonconditioned mice accelerates age-associated cardiac dysfunction. JCI Insight 5, e135204 (2020)
Sano, S. et al. JAK2V617F-mediated clonal hematopoiesis accelerates pathological remodeling in murine heart failure. JACC Basic Transl. Sci. 4, 684–697 (2019).
Bolton, K. L. et al. Cancer therapy shapes the fitness landscape of clonal hematopoiesis. Nat. Genet. 52, 1219–1226 (2020).
Sano, S. et al. TP53-mediated therapy-related clonal hematopoiesis contributes to doxorubicin-induced cardiomyopathy by augmenting a neutrophil-mediated cytotoxic response. JCI Insight 6, e146076 (2021).
Yura, Y. et al. The cancer therapy-related clonal hematopoiesis driver gene Ppm1d promotes inflammation and non-ischemic heart failure in mice. Circ. Res. 129, 684–698 (2021).
Hormaechea-Agulla, D. et al. Chronic infection drives Dnmt3a loss-of-function clonal hematopoiesis via IFNγ signaling. Cell Stem Cell 28, 1428–1442(2021).
Cai, Z. et al. Inhibition of inflammatory signaling in Tet2 mutant preleukemic cells mitigates stress-induced abnormalities and clonal hematopoiesis. Cell Stem Cell 23, 833–849 (2018).
Avagyan, S. et al. Resistance to inflammation underlies enhanced fitness in clonal hematopoiesis. Science 374, 768–772 (2021).
Yvan-Charvet, L. et al. ATP-binding cassette transporters and HDL suppress hematopoietic stem cell proliferation. Science 328, 1689–1693 (2010).
Murphy, A. J. et al. ApoE regulates hematopoietic stem cell proliferation, monocytosis, and monocyte accumulation in atherosclerotic lesions in mice. J. Clin. Invest. 121, 4138–4149 (2011).
Harslof, M., Pedersen, K. M., Nordestgaard, B. G. & Afzal, S. Low high-density lipoprotein cholesterol and high white blood cell counts: a mendelian randomization study. Arter. Thromb. Vasc. Biol. 41, 976–987 (2021).
Adamstein, N. H. et al. The neutrophil–lymphocyte ratio and incident atherosclerotic events: analyses from five contemporary randomized trials. Eur. Heart J. 42, 896–903 (2021).
Bonnefond, A. et al. Association between large detectable clonal mosaicism and type 2 diabetes with vascular complications. Nat. Genet. 45, 1040–1043 (2013).
Fuster, J. J. et al. TET2-loss-of-function-driven clonal hematopoiesis exacerbates experimental insulin resistance in aging and obesity. Cell Rep. 33, 108326 (2020).
Honigberg, M. C. et al. Premature menopause, clonal hematopoiesis, and coronary artery disease in postmenopausal women. Circulation 143, 410–423 (2021).
Ruth, K. S. et al. Genetic insights into biological mechanisms governing human ovarian ageing. Nature 596, 393–397 (2021).
Lusis, A. J. A vicious cycle in atherosclerosis. Cell 184, 1139–1141 (2021).
Sanchez-Cabo, F. & Fuster, J. J. Clonal haematopoiesis and atherosclerosis: a chicken or egg question? Nat. Rev. Cardiol. 18, 463–464 (2021).
Ogawa, M. Differentiation and proliferation of hematopoietic stem cells. Blood 81, 2844–2853 (1993).
Bick, A. G. et al. Genetic IL-6 signaling deficiency attenuates cardiovascular risk in clonal hematopoiesis. Circulation https://doi.org/10.1161/CIRCULATIONAHA.119.044362 (2019). In people with TET2 or DNMT3A clonal hematopoiesis the increased risk of atherosclerotic CVD was reversed if they also carried a genetic variant in the IL-6 receptor that reduced its function.
Heron, M. Deaths: leading causes for 2015. Natl. Vital Stat. Rep. 66, 1–76 (2017).
Vallejo-Vaz, A. J. et al. Triglyceride-rich lipoprotein cholesterol and risk of cardiovascular events among patients receiving statin therapy in the TNT trial. Circulation 138, 770–781 (2018).
Cannon, C. P. et al. Ezetimibe added to statin therapy after acute coronary syndromes. N. Engl. J. Med. 372, 2387–2397 (2015).
Sabatine, M. S. et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N. Engl. J. Med. 376, 1713–1722 (2017).
Ridker, P. M. et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 377, 1119–1131 (2017).
Nidorf, S. M. et al. Colchicine in patients with chronic coronary disease. N. Engl. J. Med. 383, 1838–1847 (2020).
Tardif, J. C. et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N. Engl. J. Med. 381, 2497–2505 (2019).
Martinon, F., Petrilli, V., Mayor, A., Tardivel, A. & Tschopp, J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440, 237–241 (2006).
Misawa, T. et al. Microtubule-driven spatial arrangement of mitochondria promotes activation of the NLRP3 inflammasome. Nat. Immunol. 14, 454–460 (2013).
Imazio, M. & Nidorf, M. Colchicine and the heart. Eur. Heart J. 42, 2745–2760 (2021).
Newby, L. K. Inflammation as a treatment target after acute myocardial infarction. N.Engl. J. Med. 381, 2562–2563 (2019).
Ren, G. M. et al. Pharmacological targeting of NLRP3 deubiquitination for treatment of NLRP3-associated inflammatory diseases. Sci. Immunol. 6, eabe2933 (2021).
Mangan, M. S. J. et al. Targeting the NLRP3 inflammasome in inflammatory diseases. Nat. Rev. Drug Discovery 17, 588–606 (2018).
Paulin, N. et al. Double-Strand DNA sensing Aim2 inflammasome regulates atherosclerotic plaque vulnerability. Circulation 138, 321–323 (2018).
Dinarello, C. A., Novick, D., Kim, S. & Kaplanski, G. Interleukin-18 and IL-18 binding protein. Front. Immunol. 4, 289 (2013).
Whitman, S. C., Ravisankar, P. & Daugherty, A. Interleukin-18 enhances atherosclerosis in apolipoprotein E−/− mice through release of interferon-gamma. Circ. Res. 90, E34–E38 (2002).
Wang, J. et al. Interleukin 18 function in atherosclerosis is mediated by the interleukin 18 receptor and the Na-Cl co-transporter. Nat. Med. 21, 820–826 (2015).
Gupta, S. et al. IFN-γ potentiates atherosclerosis in ApoE knock-out mice. J. Clin. Invest. 99, 2752–2761 (1997).
Ridker, P. M. & Rane, M. Interleukin-6 signaling and anti-interleukin-6 therapeutics in cardiovascular Disease. Circ. Res. 128, 1728–1746 (2021).
Galicia, J. C. et al. Polymorphisms in the IL-6 receptor (IL-6R) gene: strong evidence that serum levels of soluble IL-6R are genetically influenced. Genes Immun. 5, 513–516 (2004).
Huber, S. A., Sakkinen, P., Conze, D., Hardin, N. & Tracy, R. Interleukin-6 exacerbates early atherosclerosis in mice. Arter. Thromb. Vasc. Biol. 19, 2364–2367 (1999).
Schuett, H. et al. Transsignaling of interleukin-6 crucially contributes to atherosclerosis in mice. Arter. Thromb. Vasc. Biol. 32, 281–290 (2012).
Schieffer, B. et al. Impact of interleukin-6 on plaque development and morphology in experimental atherosclerosis. Circulation 110, 3493–3500 (2004).
Pierini, F. S. et al. Effect of tocilizumab on LDL and HDL characteristics in patients with rheumatoid arthritis. An observational study. Rheumatol. Ther. 8, 803–815 (2021).
Ridker, P. M. et al. IL-6 inhibition with ziltivekimab in patients at high atherosclerotic risk (RESCUE): a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet 397, 2060–2069 (2021).
Blaschke, K. et al. Vitamin C induces Tet-dependent DNA demethylation and a blastocyst-like state in ES cells. Nature 500, 222–226 (2013).
Cimmino, L. et al. Restoration of TET2 function blocks aberrant self-renewal and leukemia progression. Cell 170, 1079–1095(2017).
Tang, Y. et al. Inhibition of JAK2 suppresses myelopoiesis and atherosclerosis in Apoe−/− mice. Cardiovasc. Drugs Ther. 34, 145–152 (2020).
Rao, T. N. et al. JAK2-mutant hematopoietic cells display metabolic alterations that can be targeted to treat myeloproliferative neoplasms. Blood 134, 1832–1846 (2019).
Rocca, B. et al. A randomized double-blind trial of 3 aspirin regimens to optimize antiplatelet therapy in essential thrombocythemia. Blood 136, 171–182 (2020).
Bersenev, A. et al. Lnk constrains myeloproliferative diseases in mice. J. Clin. Invest. 120, 2058–2069 (2010).
Lv, K. et al. CBL family E3 ubiquitin ligases control JAK2 ubiquitination and stability in hematopoietic stem cells and myeloid malignancies. Genes Dev. 31, 1007–1023 (2017).
Acknowledgements
A.T. and J.J.F. are supported by a grant from the Leducq Foundation (TNE-18CVD04). A.T. is supported by NIH grant 155431. We thank M. A. Zuriaga for assistance with figure design.
Author information
Authors and Affiliations
Contributions
A.T. and J.J.F. researched the data for the article, provided substantial contributions to discussions of its content, wrote the article and undertook review and/or editing of the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
A.T. is a consultant for Amgen and CSL and is on the scientific advisory board of Staten Biotech and 1016 Biotech. J.J.F. declares no potential conflicts of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tall, A.R., Fuster, J.J. Clonal hematopoiesis in cardiovascular disease and therapeutic implications. Nat Cardiovasc Res 1, 116–124 (2022). https://doi.org/10.1038/s44161-021-00015-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44161-021-00015-3
This article is cited by
-
Uncovering atherosclerotic cardiovascular disease by PET imaging
Nature Reviews Cardiology (2024)
-
Inflammatory recruitment of healthy hematopoietic stem and progenitor cells in the acute myeloid leukemia niche
Leukemia (2024)
-
The immune hunger games: the effects of fasting on monocytes
Cellular & Molecular Immunology (2023)
-
Blockade of IL-6 signaling alleviates atherosclerosis in Tet2-deficient clonal hematopoiesis
Nature Cardiovascular Research (2023)
-
TP53 clonal hematopoiesis promotes atherosclerosis
Nature Cardiovascular Research (2023)