Abstract
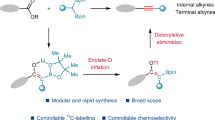
Addition of interelement compounds across alkynes is a straightforward strategy for the synthesis of densely functionalized alkenes, which are versatile units in numerous biologically active compounds. Of these processes the bis-silylation of alkynes is of particular interest, due to the synthetic use of the alkenyl silane reaction products. While cis-bis-silylation of alkynes is well known, trans-bis-silyation of alkynes is relatively underdeveloped. Here, we report the intermolecular trans-bis-silylation of terminal alkynes, using Pd-catalysis and disilane reagent 8-(2-substituted-1,1,2,2-tetramethyldisilanyl)quinoline (TMDQ), to selectively form trans-bis-silylated alkenes. The reaction process was found to be compatible with aryl and alkyl alkynes as well as those bearing electron-withdrawing groups and other alkenyl or alkynyl functionalities. The use of the reaction was displayed through late-stage functionalization of terminal alkynes bearing natural product or drug motifs and onward synthetic transformations of the trans-bis-silylated alkenes reaction products. Experimental and computational mechanistic studies reveal that the reaction probably proceeds through a combined cis-bis-silylation and Z/E isomerization process and that the use of TMDQ as the limiting reagent is key to the desired reactivity.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Full experimental procedures and spectral data for this study are included in the Supplementary Information. The X-ray crystallographic coordinates for structures reported in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition numbers 2217001 (3aa), 2217002 (3am) and 2217003 (6aa). These data can be obtained free of charge from the CCDC via www.ccdc.cam.ac.uk/data_request/cif.
References
Trost, B. M. & Li, C.-J. Modern Alkyne Chemistry: Catalytic and Atom-Economic Transformations (Wiley, 2014).
Stang, P. J. & Diederich, F. Modern Acetylene Chemistry (Wiley, 2008).
Schobert, H. Production of acetylene and acetylene-based chemicals from coal. Chem. Rev. 114, 1743–1760 (2014).
Trotus, I.-T., Zimmermann, T. & Schuth, F. Catalytic reactions of acetylene: a feedstock for the chemical industry revisited. Chem. Rev. 114, 1761–1782 (2014).
Chinchilla, R. & Najera, C. Chemicals from alkynes with palladium catalysts. Chem. Rev. 114, 1783–1826 (2014).
Beletskaya, I. & Moberg, C. Element–element addition to alkynes catalyzed by the group 10 metals. Chem. Rev. 99, 3435–3461 (1999).
Suginome, M. & Ito, Y. Transition-metal-catalyzed additions of silicon–silicon and silicon–heteroatom bonds to unsaturated organic molecules. Chem. Rev. 100, 3221–3256 (2000).
Beletskaya, I. & Moberg, C. Element–element additions to unsaturated carbon–carbon bonds catalyzed by transition metal complexes. Chem. Rev. 106, 2320–2354 (2006).
Feng, J. J., Mao, W., Zhang, L. & Oestreich, M. Activation of the Si-B interelement bond related to catalysis. Chem. Soc. Rev. 50, 2010–2073 (2021).
Ansell, M. B., Navarro, O. & Spencer, J. Transition metal catalyzed element–elementʹ additions to alkynes. Coordin. Chem. Rev. 336, 54–77 (2017).
Suginome, M., Matsuda, T., Ohmura, T., Seki, A. & Murakami, M. in Comprehensive Organometallic Chemistry III Vol. 10 (eds Mingos, D. M. P. & Crabtree, R.) 725–787 (Elsevier, 2007).
Negishi, E., Wang, G., Rao, H. & Xu, Z. Alkyne elementometalation-Pd-catalyzed cross-coupling. Toward synthesis of all conceivable types of acyclic alkenes in high yields, efficiently, selectively, economically, and safely: ‘green’ way. J. Org. Chem. 75, 3151–3182 (2010).
Flynn, A. B. & Ogilvie, W. W. Stereocontrolled synthesis of tetrasubstituted olefins. Chem. Rev. 107, 4698–4745 (2007).
Mu, Y., Nguyen, T. T., Koh, M. J., Schrock, R. R. & Hoveyda, A. H. E- and Z-, di- and tri-substituted alkenyl nitriles through catalytic cross-metathesis. Nat. Chem. 11, 478–487 (2019).
Prunet, J. Progress in metathesis through natural product synthesis. Eur. J. Org. Chem. 2011, 3634–3647 (2011).
Eissen, M. & Lenoir, D. Mass efficiency of alkene syntheses with tri- and tetrasubstituted double bonds. ACS Sustain. Chem. Eng. 5, 10459–10473 (2017).
Liu, C.-F. et al. Olefin functionalization/isomerization enables stereoselective alkene synthesis. Nat. Catal. 4, 674–683 (2021).
Bottoni, A., Higueruelo, A. P. & Miscione, G. P. A DFT computational study of the bis-silylation reaction of acetylene catalyzed by palladium complexes. J. Am. Chem. Soc. 124, 5506–5513 (2002).
Hada, M. et al. Theoretical study on the reaction mechanism and regioselectivity of silastannation of acetylenes with a palladium catalyst. J. Am. Chem. Soc. 116, 8754–8765 (1994).
Murakami, M., Yoshida, T., Kawanami, S. & Ito, Y. Synthesis, structure, and reaction of the first thermally stable cis-(silyl)(stannyl)palladium(II) complex. J. Am. Chem. Soc. 117, 6408–6409 (1995).
Nagashima, Y., Hirano, K., Takita, R. & Uchiyama, M. Trans-diborylation of alkynes: pseudo-intramolecular strategy utilizing a propargylic alcohol unit. J. Am. Chem. Soc. 136, 8532–8535 (2014).
Suzuki, K., Sugihara, N., Nishimoto, Y. & Yasuda, M. anti-Selective borylstannylation of alkynes with (o-phenylenediaminato)borylstannanes by a radical mechanism. Angew. Chem. Int. Ed. 61, e202201883 (2022).
Romain, E. et al. Trans-selective radical silylzincation of ynamides. Angew. Chem., Int. Ed. 53, 11333–11337 (2014).
Ohmura, T., Oshima, K. & Suginome, M. Palladium-catalysed cis- and trans-silaboration of terminal alkynes: complementary access to stereo-defined trisubstituted alkenes. Chem. Commun. 2008, 1416–1418 (2008).
Nagao, K., Ohmiya, H. & Sawamura, M. Anti-selective vicinal silaboration and diboration of alkynoates through phosphine organocatalysis. Org. Lett. 17, 1304–1307 (2015).
Hiyama, T. & Oestreich, M. Organosilicon Chemistry: Novel Approaches and Reactions (Wiley-VCH, 2019).
Jones, R. G., Ando, W. & Chojnowski, J. Silicon-Containing Polymers (Kluwer Academic Publishers, 2000).
Zelisko, P. M. Bio-Inspired Silicon-Based Materials (Springer, 2014).
Franz, A. K. & Wilson, S. O. Organosilicon molecules with medicinal applications. J. Med. Chem. 56, 388–405 (2013).
Ramesh, R. & Reddy, D. S. Quest for novel chemical entities through incorporation of silicon in drug scaffolds. J. Med. Chem. 61, 3779–3798 (2018).
Dong, J. et al. Manganese-catalysed divergent silylation of alkenes. Nat. Chem. 13, 182–190 (2021).
Toutov, A. A. et al. Silylation of C−H bonds in aromatic heterocycles by an earth-abundant metal catalyst. Nature 518, 80–84 (2015).
Cheng, C. & Hartwig, J. F. Rhodium-catalyzed intermolecular C−H silylation of arenes with high steric regiocontrol. Science 343, 853–857 (2014).
Jia, X. & Huang, Z. Conversion of alkanes to linear alkylsilanes using an iridium–iron-catalysed tandem dehydrogenation–isomerization–hydrosilylation. Nat. Chem. 8, 157–161 (2016).
Suginome, M. & Ito, Y. Activation of silicon–silicon σ bonds by transition-metal complexes: synthesis and catalysis of new organosilyl transition-metal complexes. J. Chem. Soc., Dalton Trans. 1998, 1925–1934 (1998).
Sakurai, H., Kamiyama, Y. & Nakadaira, Y. Novel [σ+π] reactions of hexaorganodisilanes with acetylenes catalyzed by palladium complexes. J. Am. Chem. Soc. 97, 931–932 (1975).
Ito, Y., Suginome, M. & Murakami, M. Palladium(II) acetate-tert-alkyl isocyanide as a highly efficient catalyst for the inter- and intramolecular bis-silylation of carbon-carbon triple bonds. J. Org. Chem. 56, 1948–1951 (1991).
Ozawa, F., Sugawara, M. & Hayashi, T. A new reactive system for catalytic bis-silylation of acetylenes and olefins. Organometallics 13, 3237–3243 (1994).
Braunschweig, H. & Kupfer, T. Transition-metal-catalyzed bis-silylation of propyne by [2]chromoarenophanes. Organometallics 26, 4634–4638 (2007).
Ansell, M. B., Roberts, D. E., Cloke, F. G. N., Navarro, O. & Spencer, J. Synthesis of an [(NHC)2Pd(SiMe3)2] complex and catalytic cis-bis(silyl)ations of alkynes with unactivated disilanes. Angew. Chem. Int. Ed. 54, 5578–5582 (2015).
Ahmad, M., Gaumont, A. C., Durandetti, M. & Maddaluno, J. Direct syn addition of two silicon atoms to a C≡C triple bond by Si−Si bond activation: access to reactive disilylated olefins. Angew. Chem. Int. Ed. 56, 2464–2468 (2017).
Xiao, P., Cao, Y., Gui, Y., Gao, L. & Song, Z. Me3Si–SiMe2[oCON(iPr)2–C6H4]: an unsymmetrical disilane reagent for regio- and stereoselective bis-silylation of alkynes. Angew. Chem. Int. Ed. 57, 4769–4773 (2018).
Matsuda, T. & Ichioka, Y. Rhodium-catalysed intramolecular trans-bis-silylation of alkynes to synthesise 3-silyl-1-benzosiloles. Org. Biomol. Chem. 10, 3175–3177 (2012).
Naka, A., Shimomura, N. & Kobayashi, H. Synthesis of pyridine-fused siloles by palladium-catalyzed intramolecular bis-silylation. ACS Omega 7, 30369–30375 (2022).
He, T. et al. Rhodium catalyzed intermolecular trans-disilylation of alkynones with unactivated disilanes. Angew. Chem. Int. Ed. 57, 10868–10872 (2018).
Zhang, Y., Wang, X.-C., Ju, C.-W. & Zhao, D. Bis-silylation of internal alkynes enabled by Ni(0) catalysis. Nat. Commun. 12, 68 (2021).
Mei, J., Leung, N. L. C., Kwok, R. T. K., Lam, J. W. Y. & Tang, B. Z. Aggregation-induced emission: together we shine, united we soar! Chem. Rev. 115, 11718–11940 (2015).
Zafrani, Y. et al. Difluoromethyl bioisostere: examining the ‘lipophilic hydrogen bond donor’ concept. J. Med. Chem. 60, 797–804 (2017).
Zhang, L.-H. et al. The synthetic compound CC-5079 is a potent inhibitor of tubulin polymerization and tumor necrosis factor-α production with antitumor activity. Cancer Res. 66, 951–959 (2006).
Ruchelman, A. L. et al. 1,1-Diarylalkenes as anticancer agents: dual inhibitors of tubulin polymerization and phosphodiesterase 4. Bioorg. Med. Chem. 19, 6356–6374 (2011).
Kupfer, D. & Bulger, W. H. Inactivation of the uterine estrogen receptor binding of estradiol during P-450 catalyzed metabolism of chlorotrianisene (TACE). Speculation that TACE antiestrogenic activity involves covalent binding to the estrogen receptor. FEBS Lett. 261, 59–62 (1990).
Bollag, W. & Ott, F. Inhibition of rat mammary carcinogenesis by an arotinoid without a polar end group. Eur. J. Cancer Clin. Oncol. 23, 131–135 (1987).
Guerrero, P. G. Jr. et al. Synthesis of arotinoid acid and temarotene using mixed (Z)-1,2-bis(organylchalcogene)-1-alkene as precursor. Tetrahedron Lett. 53, 5302–5305 (2012).
Chen, H.-C., Wu, Y., Yu, Y. & Wang, P. Pd-catalyzed isomerization of alkenes. Chin. J. Org. Chem. 42, 742–757 (2022).
Fiorito, D., Scaringi, S. & Mazet, C. Transition metal-catalyzed alkene isomerization as an enabling technology in tandem, sequential and domino processes. Chem. Soc. Rev. 50, 1391–1406 (2021).
Molloy, J. J., Morack, T. & Gilmour, R. Positional and geometrical isomerisation of alkenes: the pinnacle of atom economy. Angew. Chem. Int. Ed. 58, 13654–13664 (2019).
Albéniz, A. C., Espinet, P., López-Fernández, R. & Sen, A. A warning on the use of radical traps as a test for radical mechanisms: they react with palladium hydrido complexes. J. Am. Chem. Soc. 124, 11278–11279 (2002).
Ozawa, F. & Kamite, J. Mechanistic study on the insertion of phenylacetylene into cis-bis(silyl)platinum(II) complexes. Organometallics 17, 5630–5639 (1998).
Acknowledgements
D.Z. is grateful for the financial support from the National Natural Science Foundation of China (grant nos. 22022103, 21871146, 22188101 and 22221002, D.Z.), the National Key Research and Development Program of China (grant nos. 2019YFA0210500 and 2020YFA0711504, D.Z.), the ‘Frontiers Science Center for New Organic Matter’, Nankai University (grant no. 63181206, D.Z.) and the Fundamental Research Funds for the Central Universities and Nankai University (D.Z.). X.H. is grateful for the financial support from National Natural Science Foundation of China (grant nos. 21873081 and 22122109, X.H.), the Starry Night Science Fund of Zhejiang University Shanghai Institute for Advanced Study (grant no. SN-ZJU-SIAS-006, X.H.), Beijing National Laboratory for Molecular Sciences (grant no. BNLMS202102, X.H.), CAS Youth Interdisciplinary Team (grant no. JCTD-2021-11, X.H.), Fundamental Research Funds for the Central Universities (grant nos. 226-2022-00140 and 226-2022-00224, X.H.), the Center of Chemistry for Frontier Technologies and Key Laboratory of Precise Synthesis of Functional Molecules of Zhejiang Province (grant no. PSFM 2021-01, X.H.), and the State Key Laboratory of Clean Energy Utilization (grant no. ZJUCEU2020007, X.H.). Calculations were performed on the high-performance computing system at Department of Chemistry, Zhejiang University.
Author information
Authors and Affiliations
Contributions
S.Z., Y.Z. and K.Y. performed the experiments. X.H. and R.W. designed and carried out the DFT calculations. D.Z. conceived the concept, directed the project and wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks Boris Maryasin, Akinobu Naka, John Spencer and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Thomas West, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Tables 1–5, Figs. 1–4, Methods, Text, Experiments, NMR spectra, HRMS spectra and References.
Supplementary Data 1
Crystallographic data for 3aa, CCDC 2217001.
Supplementary Data 2
Crystallographic data for 3am, CCDC 2217002.
Supplementary Data 3
Crystallographic data for 6aa, CCDC 2217003.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, S., Zhang, Y., Wu, R. et al. Intermolecular trans-bis-silylation of terminal alkynes. Nat. Synth 2, 937–948 (2023). https://doi.org/10.1038/s44160-023-00325-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44160-023-00325-3
This article is cited by
-
A change of direction through trans-bis-silylation of alkynes
Nature Synthesis (2023)