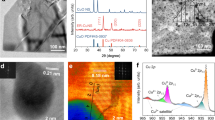
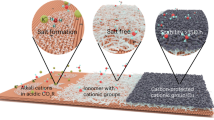
Abstract
Electrosynthesis of multicarbon products from the reduction of CO2 in acidic electrolytes is a promising approach to overcoming CO2 reactant loss in alkaline and neutral electrolytes; however, the proton-rich environment near the catalyst surface favours the hydrogen evolution reaction, leading to low energy efficiency for multicarbon products. Here we report a heterogeneous catalyst adlayer—composed of covalent organic framework nanoparticles and cation-exchange ionomers—that suppresses hydrogen evolution and promotes CO2-to-multicarbon conversion in strong acid. The imine and carbonyl-functionalized covalent organic framework regulates the ionomer structure, creating evenly distributed cation-carrying and hydrophilic–hydrophobic nanochannels that control the catalyst microenvironment. The resulting high local alkalinity and cation-enriched environment enables C–C coupling between 100 and 400 mA cm−2. A multicarbon Faradaic efficiency of 75% is achieved at 200 mA cm−2. The system demonstrates a full-cell multicarbon energy efficiency of 25%, which is a twofold improvement over the literature benchmark acidic system for the reduction of CO2.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All experimental data are available in the main text or the Supplementary Information. Source Data are provided with this paper.
References
Whipple, D. T. & Kenis, P. J. A. Prospects of CO2 utilization via direct heterogeneous electrochemical reduction. J. Phys. Chem. Lett. 1, 3451–3458 (2010).
Luna, P. D. et al. What would it take for renewably powered electrosynthesis to displace petrochemical processes? Science 364, eaav3506 (2019).
Bushuyev, O. S. et al. What should we make with CO2 and how can we make it? Joule 2, 825–832 (2018).
Jouny, M., Luc, W. & Jiao, F. General eechno-economic analysis of CO2 electrolysis systems. Ind. Eng. Chem. Res. 57, 2165–2177 (2018).
Verma, S., Kim, B., Jhong, H.-R. M., Ma, S. & Kenis, P. J. A. A gross-margin model for defining technoeconomic benchmarks in the electroreduction of CO2. ChemSusChem 9, 1972–1979 (2016).
Zhang, X. et al. Selective and high current CO2 electro-reduction to multicarbon products in near-neutral KCl electrolytes. J. Am. Chem. Soc. 143, 3245–3255 (2021).
Fan, L. et al. Proton sponge promotion of electrochemical CO2 reduction to multi-carbon products. Joule 6, 205–220 (2022).
Liu, W. et al. Electrochemical CO2 reduction to ethylene by ultrathin CuO nanoplate arrays. Nat. Commun. 13, 1877 (2022).
Chen, X. et al. Electrochemical CO2-to-ethylene conversion on polyamine-incorporated Cu electrodes. Nat. Catal. 4, 20–27 (2021).
Arquer, F. P. Gd et al. CO2 electrolysis to multicarbon products at activities greater than 1 A cm−2. Science 367, 661–666 (2020).
Endrődi, B. et al. Operando cathode activation with alkali metal cations for high current density operation of water-fed zero-gap carbon dioxide electrolysers. Nat. Energy 6, 439–448 (2021).
Ma, W. et al. Electrocatalytic reduction of CO2 to ethylene and ethanol through hydrogen-assisted C–C coupling over fluorine-modified copper. Nat. Catal. 3, 478–487 (2020).
Yan, Z., Hitt, J. L., Zeng, Z., Hickner, M. A. & Mallouk, T. E. Improving the efficiency of CO2 electrolysis by using a bipolar membrane with a weak-acid cation exchange layer. Nat. Chem. 13, 33–40 (2021).
Xia, C. et al. Continuous production of pure liquid fuel solutions via electrocatalytic CO2 reduction using solid-electrolyte devices. Nat. Energy 4, 776–785 (2019).
Kim, D. et al. Selective CO2 electrocatalysis at the pseudocapacitive nanoparticle/ordered-ligand interlayer. Nat. Energy 5, 1032–1042 (2020).
Dinh, C.-T. et al. CO2 electroreduction to ethylene via hydroxide-mediated copper catalysis at an abrupt interface. Science 360, 783–787 (2018).
Chen, C., Li, Y. & Yang, P. Address the “alkalinity problem” in CO2 electrolysis with catalyst design and translation. Joule 5, 737–742 (2021).
Rabinowitz, J. A. & Kanan, M. W. The future of low-temperature carbon dioxide electrolysis depends on solving one basic problem. Nat. Commun. 11, 5231 (2020).
Huang, J. E. et al. CO2 electrolysis to multicarbon products in strong acid. Science 372, 1074–1078 (2021).
Keith, D. W., Holmes, G., St. Angelo, D. & Heidel, K. A process for capturing CO2 from the atmosphere. Joule 2, 1573–1594 (2018).
Sisler, J. et al. Ethylene electrosynthesis: a comparative techno-economic analysis of alkaline vs membrane electrode assembly vs CO2–CO–C2H4 tandems. ACS Energy Lett. 6, 997–1002 (2021).
Greenblatt, J. B., Miller, D. J., Ager, J. W., Houle, F. A. & Sharp, I. D. The technical and energetic challenges of separating (photo)electrochemical carbon dioxide reduction products. Joule 2, 381–420 (2018).
Aaron, D. & Tsouris, C. Separation of CO2 from flue gas: a review. Sep. Sci. Technol. 40, 321–348 (2005).
Bondue, C. J., Graf, M., Goyal, A. & Koper, M. T. M. Suppression of hydrogen evolution in acidic electrolytes by electrochemical CO2 reduction. J. Am. Chem. Soc. 143, 279–285 (2021).
Kortlever, R., Balemans, C., Kwon, Y. & Koper, M. T. M. Electrochemical CO2 reduction to formic acid on a Pd-based formic acid oxidation catalyst. Catal. Today 244, 58–62 (2015).
Ooka, H., Figueiredo, M. C. & Koper, M. T. M. Competition between hydrogen evolution and carbon dioxide reduction on copper electrodes in mildly acidic media. Langmuir 33, 9307–9313 (2017).
Wu, Y. et al. Electrochemical CO2 reduction using gas diffusion electrode loading Ni-doped covalent triazine frameworks in acidic electrolytes. Electrochemistry 88, 359–364 (2020).
Liu, Z. et al. Acidic electrocatalytic CO2 reduction using space-confined nanoreactors. ACS Appl. Mater. Interfaces 14, 7900–7908 (2022).
Dickinson, E. J. F. & Wain, A. J. The Butler-Volmer equation in electrochemical theory: origins, value, and practical application. J. Electroanal. Chem. 872, 114145 (2020).
Noren, D. A. & Hoffman, M. A. Clarifying the Butler–Volmer equation and related approximations for calculating activation losses in solid oxide fuel cell models. J. Power Sources 152, 175–181 (2005).
Salvatore, D. & Berlinguette, C. P. Voltage matters when reducing CO2 in an electrochemical flow cell. ACS Energy Lett. 5, 215–220 (2020).
Tao, Z., Pearce, A. J., Mayer, J. M. & Wang, H. Bridge sites of Au surfaces are active for electrocatalytic CO2 reduction. J. Am. Chem. Soc. 144, 8641–8648 (2022).
Gu, J. et al. Modulating electric field distribution by alkali cations for CO2 electroreduction in strongly acidic medium. Nat. Catal. 5, 268–276 (2022).
Monteiro, M. C. O. et al. Absence of CO2 electroreduction on copper, gold and silver electrodes without metal cations in solution. Nat. Catal. 4, 654–662 (2021).
Vennekoetter, J.-B., Sengpiel, R. & Wessling, M. Beyond the catalyst: how electrode and reactor design determine the product spectrum during electrochemical CO2 reduction. Chem. Eng. J. 364, 89–101 (2019).
Kusoglu, A. & Weber, A. Z. New insights into perfluorinated sulfonic-acid ionomers. Chem. Rev. 117, 987–1104 (2017).
Yang, F. et al. Investigation of the interaction between Nafion ionomer and surface functionalized carbon black using both ultrasmall angle X-ray scattering and cryo-TEM. ACS Appl. Mater. Interfaces 9, 6530–6538 (2017).
Ott, S. et al. Ionomer distribution control in porous carbon-supported catalyst layers for high-power and low Pt-loaded proton exchange membrane fuel cells. Nat. Mater. 19, 77–85 (2020).
Lopez-Haro, M. et al. Three-dimensional analysis of Nafion layers in fuel cell electrodes. Nat. Commun. 5, 5229 (2014).
Orfanidi, A. et al. The key to high performance low Pt loaded electrodes. J. Electrochem. Soc. 164, F418–F426 (2017).
Xu, H., Tao, S. & Jiang, D. Proton conduction in crystalline and porous covalent organic frameworks. Nat. Mater. 15, 722–726 (2016).
Lin, S. et al. Covalent organic frameworks comprising cobalt porphyrins for catalytic CO2 reduction in water. Science 349, 1208–1213 (2015).
Wang, H. et al. Organic molecular sieve membranes for chemical separations. Chem. Soc. Rev. 50, 5468–5516 (2021).
Resasco, J. et al. Promoter effects of alkali metal cations on the electrochemical reduction of carbon dioxide. J. Am. Chem. Soc. 139, 11277–11287 (2017).
Ringe, S. et al. Understanding cation effects in electrochemical CO2 reduction. Energy Environ. Sci. 12, 3001–3014 (2019).
Poojary, S., Islam, M. N., Shrivastava, U. N., Roberts, E. P. L. & Karan, K. Transport and electrochemical interface properties of ionomers in low-Pt loading catalyst layers: effect of ionomer equivalent weight and relative humidity. Molecules 25, 3387 (2020).
Sun, R. et al. Periodic evolution of the ionomer/catalyst interfacial structures towards proton conductance and oxygen transport in polymer electrolyte membrane fuel cells. Nano Energy 75, 104919 (2020).
Chen, C. et al. Boosting the productivity of electrochemical CO2 reduction to multi-carbon products by enhancing CO2 diffusion through a porous organic cage. Angew. Chem. Int. Ed. 61, e202202607 (2022).
Tan, Y. C., Lee, K. B., Song, H. & Oh, J. Modulating local CO2 concentration as a general strategy for enhancing C–C coupling in CO2 electroreduction. Joule 4, 1104–1120 (2020).
O’Brien, C. P. et al. Single pass CO2 conversion exceeding 85% in the electrosynthesis of multicarbon products via local CO2 regeneration. ACS Energy Lett. 6, 2952–2959 (2021).
Ozden, A. et al. High-rate and efficient ethylene electrosynthesis using a catalyst/promoter/transport layer. ACS Energy Lett. 5, 2811–2818 (2020).
Wang, R., Kong, W., Zhou, T., Wang, C. & Guo, J. Organobase modulated synthesis of high-quality β-ketoenamine-linked covalent organic frameworks. Chem. Commun. 57, 331–334 (2021).
Bai, L., Gao, Q. & Zhao, Y. Two fully conjugated covalent organic frameworks as anode materials for lithium ion batteries. J. Mater. Chem. A 4, 14106–14110 (2016).
Ma, W. et al. Size-controllable synthesis of uniform spherical covalent organic frameworks at room temperature for highly efficient and selective enrichment of hydrophobic peptides. J. Am. Chem. Soc. 141, 18271–18277 (2019).
Acknowledgements
This work was financially supported by the Ontario Research Fund—Research Excellence Program (D.S.), the Natural Sciences and Engineering Research Council (NSERC) of Canada (D.S.), the Australian Research Council through a Discovery Early Career Researcher Award (grant no. DE200100477 to F.L.) and the National Natural Science Foundation of China (grant no. 51603114 to L.H.).
Author information
Authors and Affiliations
Contributions
D.S. and F.L. supervised the project. Y.Z. designed and performed all of the electrochemical experiments. L.H. and J.N. synthesized and characterized the COF materials. A.O. prepared PTFE–Cu substrates and fabricated the slim flow-cell. R.K.M. and K.X. designed the permeation flow-cell. R.K.M. and S.Z. performed NMR and ICP analyses. Y.L. performed TEM, zeta potential and contact angle measurements. S.L. and T.A. performed COMSOL modelling. P.O. performed DFT calculations. Y.X., M.F., Y.C., J.E.H. and J.Z. assisted in electrochemical measurements and contributed to data analysis. Y.Z. wrote the manuscript. A.O., C.P.O'.B., F.L., E.H.S. and D.S. contributed to manuscript editing. All of the authors discussed the results and assisted during the manuscript preparation.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks Peng Kang, Anna Klinkova and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary handling editor: Alexandra Groves, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Figs. 1–42 and Tables 1–6.
Source data
Source Data Fig. 1
Statistical Source Data.
Source Data Fig. 2
Statistical Source Data.
Source Data Fig. 3
Statistical Source Data.
Source Data Fig. 4
Statistical Source Data.
Rights and permissions
About this article
Cite this article
Zhao, Y., Hao, L., Ozden, A. et al. Conversion of CO2 to multicarbon products in strong acid by controlling the catalyst microenvironment. Nat. Synth 2, 403–412 (2023). https://doi.org/10.1038/s44160-022-00234-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44160-022-00234-x
This article is cited by
-
Gas diffusion enhanced electrode with ultrathin superhydrophobic macropore structure for acidic CO2 electroreduction
Nature Communications (2024)
-
Acidic media enables oxygen-tolerant electrosynthesis of multicarbon products from simulated flue gas
Nature Communications (2024)
-
Acidic electroreduction CO2 to formic acid via interfacial modification of Bi nanoparticles at industrial-level current
Nano Research (2024)
-
Electrochemical Carbon Dioxide Reduction in Acidic Media
Electrochemical Energy Reviews (2024)
-
Engineering the catalyst microenvironment
Nature Synthesis (2023)