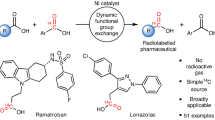
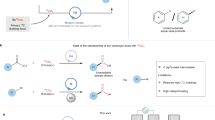
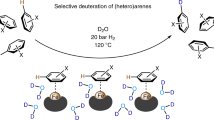
Abstract
Drug metabolism and pharmacokinetic studies play a crucial role in drug discovery and development programmes, assessing a lead drug candidate’s efficacy and safety profile. Quantitative bioanalytical assessment of analytes with mass spectrometry requires the use of stable carbon-13-labelled compounds with a molecular mass difference of ≥3 daltons. The incorporation of three or more carbon isotopes into drug candidates is not trivial, often requiring lengthy and costly syntheses. Here we report a dual catalytic strategy for the synthesis of multi-carbon-labelled isotopologues of active pharmaceutical ingredients. This approach uses isotopically labelled gas surrogates in a three-chamber reactor for sequential release of alkenes, carbon monoxide and hydrogen followed by low-pressure hydroformylation to generate multi-labelled alkyl aldehydes. The method’s utility has been demonstrated through the synthesis of multiple labelled N-alkyl bioactive compounds, site-selective carbon-13 and deuterium introduction and for triple-carbon labelling of small molecules combined with α-functionalization.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data in support and related to this study are available within the paper and the Supplementary Information.
References
Isin, E. M., Elmore, C. S., Nilsson, G. N., Thompson, R. A. & Weidolf, L. Use of radiolabeled compounds in drug metabolism and pharmacokinetic studies. Chem. Res. Toxicol. 25, 532–542 (2012).
Elmore, C. S. & Bragg, R. A. Isotope chemistry; a useful tool in the drug discovery arsenal. Bioorg. Med. Chem. Lett. 25, 167–171 (2015).
Atzrodt, J., Derdau, V., Kerr, W. J. & Reid, M. Deuterium- and tritium-labelled compounds: applications in the life sciences. Angew. Chem. Int. Ed. 57, 1758–1784 (2018).
Wen, B. & Zhu, M. Applications of mass spectrometry in drug metabolism: 50 years of progress. Drug Metab. Rev. 47, 71–87 (2015).
Cuyckens, F. Mass spectrometry in drug metabolism and pharmacokinetics: current trends and future perspectives. Rapid Commun. Mass Spectrom. 33, 90–95 (2019).
Wang, Z. J. et al. Hyperpolarized 13C MRI: state of the art and future directions. Radiology 291, 273–284 (2019).
Liu, R. H. et al. Isotopically labeled analogues for drug quantitation. Anal. Chem. 74, 618–626 (2002).
Atzrodt, J., Derdau, V., Kerr, W. J. & Reid, M. C−H Functionalisation for hydrogen isotope exchange. Angew. Chem. Int. Ed. 57, 3022–3047 (2018).
Loh, Y. Y. et al. Photoredox-catalyzed deuteration and tritiation of pharmaceutical compounds. Science 358, 1182–1187 (2017).
Foster, A. B. Deuterium isotope effects in studies of drug metabolism. Trends Pharmacol. Sci. 5, 524–527 (1984).
Marathe, P., Shyu, W. & Humphreys, W. The use of radiolabeled compounds for ADME studies in discovery and exploratory development. Curr. Pharm. Des. 10, 2991–3008 (2004).
Elmore, C. S. The use of isotopically labeled compounds in drug discovery. Annu. Rep. Med. Chem. 44, 515–534 (2009).
Atzrodt, J., Derdau, V. & Loewe, C. in Drug Discovery and Evaluation: Methods in Clinical Pharmacology (eds Hock, F. J. & Gralinski, M. R.) 1–19 (Springer International, 2017).
Bragg, R. A., Sardana, M., Artelsmair, M. & Elmore, C. S. New trends and applications in carboxylation for isotope chemistry. J. Label. Compd. Radiopharm. 61, 934–948 (2018).
Szabolcs, A., Szammer, J. & Noszkó, L. A new method for the preparation of carboxyl-labelled aliphatic carboxylic acids. Tetrahedron 30, 3647–3648 (1974).
Hinsinger, K. & Pieters, G. The emergence of carbon isotope exchange. Angew. Chem. Int. Ed. 58, 9678–9680 (2019).
Gauthier, D. R., Rivera, N. R., Yang, H., Schultz, D. M. & Shultz, C. S. Palladium-catalyzed carbon isotope exchange on aliphatic and benzoic acid chlorides. J. Am. Chem. Soc. 140, 15596–15600 (2018).
Kingston, C. et al. Direct carbon isotope exchange through decarboxylative carboxylation. J. Am. Chem. Soc. 141, 774–779 (2019).
Tortajada, A. et al. Catalytic decarboxylation/carboxylation platform for accessing isotopically labeled carboxylic acids. ACS Catal. 9, 5897–5901 (2019).
Destro, G. et al. Dynamic carbon isotope exchange of pharmaceuticals with labeled CO2. J. Am. Chem. Soc. 141, 780–784 (2019).
Destro, G. et al. Transition‐metal‐free carbon isotope exchange of phenyl acetic acids. Angew. Chem. Int. Ed. 59, 13490–13495 (2020).
Babin, V. et al. Photochemical strategy for carbon isotope exchange with CO2. ACS Catal. 11, 2968–2976 (2021).
Kong, D., Moon, P. J., Lui, E. K. J., Bsharat, O. & Lundgren, R. J. Direct reversible decarboxylation from stable organic acids in dimethylformamide solution. Science 369, 557–561 (2020).
Kong, D. et al. Fast carbon isotope exchange of carboxylic acids enabled by organic photoredox catalysis. J. Am. Chem. Soc. 143, 2200–2206 (2021).
Feng, M. et al. Direct carbon isotope exchange of pharmaceuticals via reversible decyanation. J. Am. Chem. Soc. 143, 5659–5665 (2021).
Reilly, S. W., Lam, Y.-h, Ren, S. & Strotman, N. A. Late-stage carbon isotope exchange of aryl nitriles through Ni-catalyzed C–CN bond activation. J. Am. Chem. Soc. 143, 4817–4823 (2021).
Pipal, R. W. et al. Metallaphotoredox aryl and alkyl radiomethylation for PET ligand discovery. Nature 589, 542–547 (2021).
Carr, R. M., Cable, K. M., Newman, J. J. & Sutherland, D. R. Syntheses of isotopically labelled angiotensin II receptor antagonist GR138950X. J. Label. Compd. Radiopharm. 38, 453–470 (1996).
Pedersen, S. K. et al. Main element chemistry enables gas-cylinder-free hydroformylations. Nat. Catal. 3, 843–850 (2020).
Ricci, A. (ed.) Amino Group Chemistry: From Synthesis to the Life Sciences (Wiley, 2008).
Vitaku, E., Smith, D. T. & Njardarson, J. T. Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among U.S. FDA approved pharmaceuticals. J. Med. Chem. 57, 10257–10274 (2014).
Galan, B. R., Gembicky, M., Dominiak, P. M., Keister, J. B. & Diver, S. T. Carbon monoxide-promoted carbene insertion into the aryl substituent of an N-heterocyclic carbene ligand: Buchner reaction in a ruthenium carbene complex. J. Am. Chem. Soc. 127, 15702–15703 (2005).
Beach, N. J., Camm, K. D. & Fogg, D. E. Hydrogenolysis versus methanolysis of first-and second-generation grubbs catalysts: rates, speciation, and implications for tandem catalysis. Organometallics 29, 5450–5455 (2010).
Jawiczuk, M., Marczyk, A. & Trzaskowski, B. Decomposition of ruthenium olefin metathesis catalyst. Catalysts 10, 887–887 (2020).
Breit, B. & Seiche, W. Self-assembly of bidentate ligands for combinatorial homogeneous catalysis based on an A-T base-pair model. Angew. Chem. Int. Ed. 44, 1640–1643 (2005).
Seiche, W., Schuschkowski, A. & Breit, B. Bidentate ligands by self-assembly through hydrogen bonding: a general room temperature/ambient pressure regioselective hydroformylation of terminal alkenes. Adv. Synth. Catal. 347, 1488–1494 (2005).
Ulman, M. & Grubbs, R. H. Relative reaction rates of olefin substrates with ruthenium(II) carbene metathesis initiators. Organometallics 17, 2484–2489 (1998).
Won, E.-J., Yun, H.-Y., Lee, D.-H. & Shin, K.-H. Application of compound-specific isotope analysis in environmental forensic and strategic management avenue for pesticide residues. Molecules 26, 4412–4412 (2021).
Friis, S. D., Skrydstrup, T. & Buchwald, S. L. Mild Pd-catalyzed aminocarbonylation of (hetero)aryl bromides with a palladacycle precatalyst. Org. Lett. 16, 4296–4299 (2014).
Collin, H. P. et al. COtab: expedient and safe setup for Pd-catalyzed carbonylation chemistry. Org. Lett. 21, 5775–5778 (2019).
Friis, S. D., Lindhardt, A. T. & Skrydstrup, T. The development and application of two-chamber reactors and carbon monoxide precursors for safe carbonylation reactions. Acc. Chem. Res. 49, 594–605 (2016).
Nielsen, D. U., Neumann, K. T., Lindhardt, A. T. & Skrydstrup, T. Recent developments in carbonylation chemistry using [13C]CO, [11C]CO, and [14C]CO. J. Label. Compd. Radiopharm. 61, 949–987 (2018).
List, B. Direct catalytic asymmetric α-amination of aldehydes. J. Am. Chem. Soc. 124, 5656–5657 (2002).
Reed-Berendt, B. G., Latham, D. E., Dambatta, M. B. & Morrill, L. C. Borrowing hydrogen for organic synthesis. ACS Cent. Sci. 7, 570–585 (2021).
Fleischer, I. et al. From olefins to alcohols: efficient and regioselective ruthenium-catalyzed domino hydroformylation/reduction sequence. Angew. Chem. 125, 3021–3025 (2013).
Hollmann, D., Bähn, S., Tillack, A. & Beller, M. N-Dealkylation of aliphatic amines and selective synthesis of monoalkylated aryl amines. Chem. Commun. 27, 3199–3201 (2008).
Flinker, M. et al. Efficient water reduction with sp3-sp3 diboron(4) compounds: application to hydrogenations, H–D exchange reactions, and carbonyl reductions. Angew. Chem. Int. Ed. 56, 15910–15915 (2017).
Acknowledgements
We thank the following funding agencies for supporting this research: The Danish National Research Foundation (grant no. DNRF118), NordForsk (grant no. 85378), European Union’s Horizon 2020 research and innovation programme under grant agreement 862179 and Marie Skłodowska-Curie grant agreement 859910. Furthermore, we thank K. Baldvinsson for his generous donation of Citanest. This publication reflects the views only of the authors, and the European Commission cannot be held responsible for any use which may be made of the information contained therein.
Author information
Authors and Affiliations
Contributions
H.G.G. and T.S. devised the original idea. H.C.D.H., S.K., H.G.G. and T.S. conceptualized the strategy. H.C.D.H., S.K., H.G.G. and T.S. designed the experiments. H.C.D.H., S.K., H.G.G., J.B. and J.K. performed the experiments. H.G.G. and T.S. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
T.S. is co-owner of SyTracks A/S, which commercializes silacarboxylic acid 2. All remaining authors have no competing interests.
Peer review
Peer review information
Nature Synthesis thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: Thomas West, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–5, general experimental details and procedures, Discussion, Tables 1 and 2, NMR spectra and references.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hammershøj, H.C.D., Gudmundsson, H.G., Kjærsgaard, S. et al. Multi-carbon labelling of active pharmaceutical ingredients enabled by a three-gas surrogate hydroformylation. Nat. Synth 2, 243–250 (2023). https://doi.org/10.1038/s44160-022-00223-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44160-022-00223-0