Abstract
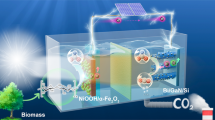
Solar-driven conversion of CO2 and plastics into value-added products provides a potential sustainable route towards a circular economy, but their simultaneous conversion in an integrated process is challenging. Here we introduce a versatile photoelectrochemical platform for CO2 conversion that is coupled to the reforming of plastic. The perovskite-based photocathode enables the integration of different CO2-reduction catalysts such as a molecular cobalt porphyrin, a Cu91In9 alloy and formate dehydrogenase enzyme, which produce CO, syngas and formate, respectively. The Cu27Pd73 alloy anode selectively reforms polyethylene terephthalate plastics into glycolate in alkaline solution. The overall single-light-absorber photoelectrochemical system operates with the help of an internal chemical bias and under zero applied voltage. The system performs similarly to bias-free, dual-light absorber tandems and shows about 10‒100-fold higher production rates than those of photocatalytic suspension processes. This finding demonstrates efficient photoelectrochemical CO2-to-fuel production coupled to plastic-to-chemical conversion as a promising and sustainable technology powered by sunlight.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The raw data supporting the findings of this study are available from the University of Cambridge data repository (https://doi.org/10.17863/CAM.88883). Source data are provided with this paper.
References
Chu, S. & Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 488, 294–303 (2012).
COP26: The Glasgow Climate Pact (UNCC, 2021).
Nitopi, S. et al. Progress and perspectives of electrochemical CO2 reduction on copper in aqueous electrolyte. Chem. Rev. 119, 7610–7672 (2019).
Verma, S., Lu, S. & Kenis, P. J. A. Co-electrolysis of CO2 and glycerol as a pathway to carbon chemicals with improved technoeconomics due to low electricity consumption. Nat. Energy 4, 466–474 (2019).
Morikawa, T., Sato, S., Sekizawa, K., Suzuki, T. M. & Arai, T. Solar-driven CO2 reduction using a semiconductor/molecule hybrid photosystem: from photocatalysts to a monolithic artificial leaf. Acc. Chem. Res. 55, 933–943 (2021).
Andrei, V., Reuillard, B. & Reisner, E. Bias-free solar syngas production by integrating a molecular cobalt catalyst with perovskite–BiVO4 tandems. Nat. Mater. 19, 189–194 (2020).
Sahara, G. et al. Photoelectrochemical reduction of CO2 coupled to water oxidation using a photocathode with a Ru(II)–Re(I) complex photocatalyst and a CoOx/TaON photoanode. J. Am. Chem. Soc. 138, 14152–14158 (2016).
Uekert, T., Pichler, C. M., Schubert, T. & Reisner, E. Solar-driven reforming of solid waste for a sustainable future. Nat. Sustain. 4, 383–391 (2021).
Uekert, T., Kuehnel, M. F., Wakerley, D. W. & Reisner, E. Plastic waste as a feedstock for solar-driven H2 generation. Energy Environ. Sci. 11, 2853–2857 (2018).
Editorial, Plastic upcycling. Nat. Catal. 2, 945–946 (2019).
Lu, S. S. et al. Electrosynthesis of syngas via the Co-reduction of CO2 and H2O. Cell. Rep. Phys. Sci. 1, 100237 (2020).
Syngas Composition (National Energy Technology Laboratory, US Department of Energy, 2002).
Bushuyev, O. S. et al. What should we make with CO2 and how can we make it? Joule 2, 825–832 (2018).
Monteiro, M. C. O., Philips, M. F., Schouten, K. J. P. & Koper, M. T. M. Efficiency and selectivity of CO2 reduction to CO on gold gas diffusion electrodes in acidic media. Nat. Commun. 12, 4943 (2021).
Qiao, J. L., Liu, Y. Y., Hong, F. & Zhang, J. J. A review of catalysts for the electroreduction of carbon dioxide to produce low-carbon fuels. Chem. Soc. Rev. 43, 631–675 (2014).
Khodakov, A. Y., Chu, W. & Fongarland, P. Advances in the development of novel cobalt Fischer–Tropsch catalysts for synthesis of long-chain hydrocarbons and clean fuels. Chem. Rev. 107, 1692–1744 (2007).
Muller, K., Brooks, K. & Autrey, T. Hydrogen storage in formic acid: a comparison of process options. Energy Fuels 31, 12603–12611 (2017).
Moore, E. E. et al. A semi-artificial photoelectrochemical tandem leaf with a CO2-to-formate efficiency approaching 1%. Angew. Chem. Int. Ed. 60, 26303–26307 (2021).
Bharadwaj, S. S. & Schmidt, L. D. Catalytic partial oxidation of natural gas to syngas. Fuel Process. Technol. 42, 109–127 (1995).
Reymond, H., Vitas, S., Vernuccio, S. & von Rohr, P. R. Reaction process of resin-catalyzed methyl formate hydrolysis in biphasic continuous flow. Ind. Eng. Chem. Res. 56, 1439–1449 (2017).
Yang, W., Prabhakar, R. R., Tan, J., Tilley, S. D. & Moon, J. Strategies for enhancing the photocurrent, photovoltage, and stability of photoelectrodes for photoelectrochemical water splitting. Chem. Soc. Rev. 48, 4979–5015 (2019).
Jang, Y. J. et al. Unbiased sunlight-driven artificial photosynthesis of carbon monoxide from CO2 using a ZnTe-based photocathode and a perovskite solar cell in tandem. ACS Nano 10, 6980–6987 (2016).
Rahaman, M. et al. Selective CO production from aqueous CO2 using a Cu96In4 catalyst and its integration into a bias-free solar perovskite–BiVO4 tandem device. Energy Environ. Sci. 13, 3536–3543 (2020).
Bhattacharjee, S. et al. Reforming of soluble biomass and plastic derived waste using a bias-free Cu30Pd70|perovskite|Pt photoelectrochemical device. Adv. Funct. Mater. 32, 2109313 (2022).
Antón-García, D. et al. Photoelectrochemical hybrid cell for unbiased CO2 reduction coupled to alcohol oxidation. Nat. Synth. 1, 77–86 (2022).
Lam, E. & Reisner, E. A TiO2-Co(terpyridine)2 photocatalyst for the selective oxidation of cellulose to formate coupled to the reduction of CO2 to syngas. Angew. Chem. Int. Ed. 60, 23306–23312 (2021).
Zhou, X. et al. Glycolic acid production from ethylene glycol via sustainable biomass energy: integrated conceptual process design and comparative techno-economic–society–environment analysis. ACS Sustain. Chem. Eng. 9, 10948–10962 (2021).
Zhang, H. F. et al. Carbon encapsulation of organic–inorganic hybrid perovskite toward efficient and stable photo-electrochemical carbon dioxide reduction. Adv. Energy Mater. 10, 2002105 (2020).
Rodríguez-Jiménez, S. et al. Self-assembled liposomes enhance electron transfer for efficient photocatalytic CO2 reduction. J. Am. Chem. Soc. 144, 9399–9412 (2022).
Rahaman, M., Kiran, K., Montiel, I. Z., Dutta, A. & Broekmann, P. Suppression of the hydrogen evolution reaction is the key: selective electrosynthesis of formate from CO2 over porous In55Cu45 catalysts. ACS Appl. Mater. Interfaces 13, 35677–35688 (2021).
Miller, M. et al. Interfacing formate dehydrogenase with metal oxides for the reversible electrocatalysis and solar-driven reduction of carbon dioxide. Angew. Chem. Int. Ed. 58, 4601–4605 (2019).
Das, S. et al. Bimetallic zero-valent alloy with measured high-valent surface states to reinforce the bifunctional activity in rechargeable zinc–air batteries. ACS Sustain. Chem. Eng. 9, 14868–14880 (2021).
Moore, E. E. et al. Understanding the local chemical environment of bioelectrocatalysis. Proc. Natl Acad. Sci. USA 119, e2114097119 (2022).
Cobb, S. J. et al. Fast CO2 hydration kinetics impair heterogeneous but improve enzymatic CO2 reduction catalysis. Nat. Chem. 14, 417–424 (2022).
Vermaas, D. A., Sassenburg, M. & Smith, W. A. Photo-assisted water splitting with bipolar membrane induced pH gradients for practical solar fuel devices. J. Mater. Chem. A 3, 19556–19562 (2015).
Blommaert, M. A. et al. Insights and challenges for applying bipolar membranes in advanced electrochemical energy systems. ACS Energy Lett. 6, 2539–2548 (2021).
Kim, I. S., Pellin, M. J. & Martinson, A. B. F. Acid-compatible halide perovskite photocathodes utilizing atomic layer deposited TiO2 for solar-driven hydrogen evolution. ACS Energy Lett. 4, 293–298 (2019).
Andrei, V. et al. Scalable triple cation mixed halide perovskite–-BiVO4 tandems for bias-free water splitting. Adv. Energy Mater. 8, 1801403 (2018).
Won, D. H., Choi, C. H., Chung, J. & Woo, S. I. Photoelectrochemical production of formic acid and methanol from carbon dioxide on metal-decorated CuO/Cu2O-layered thin films under visible light irradiation. Appl. Catal. B 158, 217–223 (2014).
Wang, Y. J., Wang, H. J., Woldu, A. R., Zhang, X. H. & He, T. Optimization of charge behavior in nanoporous CuBi2O4 photocathode for photoelectrochemical reduction of CO2. Catal. Today 335, 388–394 (2019).
Leung, J. J. et al. Solar-driven reduction of aqueous CO2 with a cobalt bis(terpyridine)-based photocathode. Nat. Catal. 2, 354–365 (2019).
Kuehnel, M. F., Orchard, K. L., Dalle, K. E. & Reisner, E. Selective photocatalytic CO2 reduction in water through anchoring of a molecular Ni catalyst on CdS nanocrystals. J. Am. Chem. Soc. 139, 7217–7223 (2017).
Wang, Q. et al. Molecularly engineered photocatalyst sheet for scalable solar formate production from carbon dioxide and water. Nat. Energy 5, 703–710 (2020).
Wang, Q., Pornrungroj, C., Linley, S. & Reisner, E. Strategies to improve light utilization in solar fuel synthesis. Nat. Energy 7, 13–24 (2022).
Boutin, E. et al. Photo‐electrochemical conversion of CO2 under concentrated sunlight enables combination of high reaction rate and efficiency. Adv. Energy Mater. 12, 2200585 (2022).
Oliveira, A. R. et al. Toward the mechanistic understanding of enzymatic CO2 reduction. ACS Catal. 10, 3844–3856 (2020).
Pornrungroj, C. et al. Bifunctional perovskite–BiVO4 tandem devices for uninterrupted solar and electrocatalytic water splitting cycles. Adv. Funct. Mater. 31, 2008182 (2021).
Sun, K. et al. A stabilized, intrinsically safe, 10% efficient, solar-driven water-splitting cell incorporating earth-abundant electrocatalysts with steady-state pH gradients and product separation enabled by a bipolar membrane. Adv. Energy Mater. 6, 1600379 (2016).
Acknowledgements
This work was supported by the Cambridge Trust (HRH The Prince of Wales Commonwealth Scholarship to S.B.), the European Commission for Horizon 2020 Marie Skłodowska-Curie Individual European Fellowships (GAN 839763 to M.R. and GAN 891338 to S.R.-J.), the Cambridge Trusts (Vice-Chancellor’s Award to V.A. and Cambridge Thai Foundation Award to C.P.), the Winton Programme for the Physics of Sustainability and St John’s College (Title A Research Fellowship to V.A.), a European Research Council (ERC) Consolidator Grant “MatEnSAP” (682833, to M.M. and E.R.), an EPSRC Impact Acceleration Account Award (to E.L. and E.R.), the UKRI Cambridge Circular Plastics Centre (CirPlas, EP/S025308/1 to E.R.), the Hermann and Marianne Straniak Stiftung (to E.R.) and the Swiss National Science Foundation (Early Postdoc Fellowship P2EZP2_191791 to E.L.). We also acknowledge use of the Cambridge XPS system, part of the Sir Henry Royce Institute (EPSRC grant EP/P024947/1). We are thankful to H. Greer (University of Cambridge) for assistance with the electron microscopy, C. M. Fernández-Posada (University of Cambridge) for assistance with the XPS, A. R. Oliveira and I. A. C. Pereira (ITQB, Universidade Nova de Lisboa) for a sample of FDH as well as S. Linley and S. Kar (University of Cambridge) for useful feedback on the manuscript.
Author information
Authors and Affiliations
Contributions
S.B., M.R. and E.R. conceived the idea and designed the project. S.B. and M.R. synthesized the bimetallic catalysts, fabricated the (photo)electrodes and carried out all the electrochemical and PEC experiments. V.A. prepared the PVK devices and carried out the EQE experiments. M.M. prepared the IO-TiO2 electrodes and drop-casted the FDH catalyst. S.R.-J. synthesized the CoPL molecular catalyst. S.B. and E.L. performed the density functional theory calculations. C.P. assisted with the catalyst characterization, artwork and schematic diagrams. S.B., M.R. and E.R. co-wrote the manuscript with input from all the co-authors. E.R. supervised the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks Wooseok Yang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Alexandra Groves, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–16 and Tables 1–6.
Supplementary Video 1
The evolution of CO from a PVK|CoPL photocathode during PEC operation of a Cu27Pd73||PVK|CoPL device with pre-treated PET substrate at the anode.
Source data
Source Data Fig. 2
Raw Data for Fig. 2.
Source Data Fig. 3
Raw Data for Fig. 3.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bhattacharjee, S., Rahaman, M., Andrei, V. et al. Photoelectrochemical CO2-to-fuel conversion with simultaneous plastic reforming. Nat. Synth 2, 182–192 (2023). https://doi.org/10.1038/s44160-022-00196-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44160-022-00196-0
This article is cited by
-
Solar reforming as an emerging technology for circular chemical industries
Nature Reviews Chemistry (2024)
-
Photocatalytic CO2 reduction
Nature Reviews Methods Primers (2023)
-
Scalable electrosynthesis of commodity chemicals from biomass by suppressing non-Faradaic transformations
Nature Communications (2023)
-
Solar-driven liquid multi-carbon fuel production using a standalone perovskite–BiVO4 artificial leaf
Nature Energy (2023)