Abstract
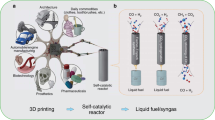
A mass production route to single-atom catalysts (SACs) is crucial for their end use application. To date, the direct fabrication of SACs via a simple and economic manufacturing route remains a challenge, with current approaches relying on convoluted processes using expensive components. Here, a straightforward and cost-effective three-dimensional (3D) printing approach is developed to fabricate a library of SACs. Despite changing synthetic parameters, including centre transition metal atom, metal loading, coordination environment and spatial geometry, the products show similar atomic dispersion nature of single metal sites, demonstrating the generality of the approach. The 3D-printed SACs exhibited excellent activity and stability in the nitrate reduction reaction. It is expected that this 3D-printing technique can be used as a method for large-scale commercial production of SACs, thus enabling the use of these materials in a broad spectrum of industrial applications.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data supporting the findings of this study are available in the article and its Supplementary information. Source data are provided with this paper.
References
Ji, S. et al. Chemical synthesis of single atomic site catalysts. Chem. Rev. 120, 11900–11955 (2020).
Qiao, B. et al. Single-atom catalysis of co oxidation using Pt1/FeOx. Nat. Chem. 3, 634–641 (2011).
Wang, L. et al. Engineering single-atomic iron-catalyst-integrated 3D-printed bioscaffolds for osteosarcoma destruction with antibacterial and bone defect regeneration bioactivity. Adv. Mater. 33, 2100150 (2021).
Chen, B., Zhong, X., Zhou, G., Zhao, N. & Cheng, H. M. Graphene-supported atomically dispersed metals as bifunctional catalysts for next-generation batteries based on conversion reactions. Adv. Mater. 33, 2105812 (2021).
Wang, Y. et al. Advanced electrocatalysts with single-metal-atom active sites. Chem. Rev. 120, 12217–12314 (2020).
Deng, Q. et al. Hydrogen-catalyzed acid transformation for the hydration of alkenes and epoxy alkanes over Co–N frustrated lewis pair surfaces. J. Am. Chem. Soc. 143, 21294–21301 (2021).
Jung, E. et al. Atomic-level tuning of Co–N–C catalyst for high-performance electrochemical H2O2 production. Nat. Mater. 19, 436–442 (2020).
Li, Y. et al. Synthesis of N-doped highly graphitic carbon urchin-like hollow structures loaded with single-Ni atoms towards efficient CO2 electroreduction. Angew. Chem. Int. Ed. Engl. 61, 202201491 (2022).
Zhang, H. B., Lu, X. F., Wu, Z. P. & Lou, X. W. Emerging multifunctional single-atom catalysts/nanozymes. ACS Cent. Sci. 6, 1288–1301 (2020).
Wei, Y. S., Zhang, M., Zou, R. & Xu, Q. Metal–organic framework-based catalysts with single metal sites. Chem. Rev. 120, 12089–12174 (2020).
Hai, X. et al. Scalable two-step annealing method for preparing ultra-high-density single-atom catalyst libraries. Nat. Nanotechnol. 17, 174–181 (2022).
Xia, C. et al. General synthesis of single-atom catalysts with high metal loading using graphene quantum dots. Nat. Chem. 13, 887–894 (2021).
Zhang, X. et al. A general method for transition metal single atoms anchored on honeycomb-like nitrogen-doped carbon nanosheets. Adv. Mater. 32, 1906905 (2020).
Lai, W. H. et al. General π-electron-assisted strategy for Ir, Pt, Ru, Pd, Fe, Ni single-atom electrocatalysts with bifunctional active sites for highly efficient water splitting. Angew. Chem. Int. Ed. Engl. 58, 11868–11873 (2019).
Zhao, L. et al. Cascade anchoring strategy for general mass production of high-loading single-atomic metal-nitrogen catalysts. Nat. Commun. 10, 1278 (2019).
Zhao, Y. et al. Anchoring sites engineering in single-atom catalysts for highly efficient electrochemical energy conversion reactions. Adv. Mater. 33, 2102801 (2021).
Jiao, L. et al. Chemical vapour deposition of Fe–N–C oxygen reduction catalysts with full utilization of dense Fe–N4 sites. Nat. Mater. 20, 1385–1391 (2021).
Liu, P. et al. Photochemical route for synthesizing atomically dispersed palladium catalysts. Science 352, 797–800 (2016).
Zhang, H. et al. Isolated cobalt centers on W18O49 nanowires perform as a reaction switch for efficient CO2 photoreduction. J. Am. Chem. Soc. 143, 2173–2177 (2021).
Wang, Y. et al. Catalysis with two-dimensional materials confining single atoms: concept, design, and applications. Chem. Rev. 119, 1806–1854 (2019).
Peng, P. et al. A pyrolysis-free path toward superiorly catalytic nitrogen-coordinated single atom. Sci. Adv. 5, aaw2322 (2019).
Wang, L. et al. A sulfur-tethering synthesis strategy toward high-loading atomically dispersed noble metal catalysts. Sci. Adv. 5, eaax6322 (2019).
Chen, G. et al. Highly accessible and dense surface single metal FeN4 active sites for promoting the oxygen reduction reaction. Energy Environ. Sci. 15, 2619–2628 (2022).
Wan, X. et al. Fe–N–C electrocatalyst with dense active sites and efficient mass transport for high-performance proton exchange membrane fuel cells. Nat. Catal. 2, 259–268 (2019).
Gu, J., Hsu, C. S., Bai, L., Chen, H. M. & Hu, X. Atomically dispersed Fe3+ sites catalyze efficient CO2 electroreduction to CO. Science 364, 1091–1094 (2019).
Li, Z. et al. Iridium single-atom catalyst on nitrogen-doped carbon for formic acid oxidation synthesized using a general host–guest strategy. Nat. Chem. 12, 764–772 (2020).
Chen, W. et al. Deciphering the alternating synergy between interlayer Pt single-atom and NiFe layered double hydroxide for overall water splitting. Energy Environ. Sci. 14, 6428–6440 (2021).
Zheng, T. et al. Copper-catalysed exclusive CO2 to pure formic acid conversion via single-atom alloying. Nat. Nanotechnol. 16, 1386–1393 (2021).
Fei, H. et al. General synthesis and definitive structural identification of MN4C4 single-atom catalysts with tunable electrocatalytic activities. Nat. Catal. 1, 63–72 (2018).
Feng, L., Wang, K. Y., Willman, J. & Zhou, H. C. Hierarchy in metal–organic frameworks. ACS Cent. Sci. 6, 359–367 (2020).
Sorrenti, A. et al. Growing and shaping metal–organic framework single crystals at the millimeter scale. J. Am. Chem. Soc. 142, 9372–9381 (2020).
Venna, S. R., Jasinski, J. B. & Carreon, M. A. Structural evolution of zeolitic imidazolate framework-8. J. Am. Chem. Soc. 132, 18030–18033 (2010).
Shen, K. et al. Ordered macro-microporous metal-organic framework single crystals. Science 359, 206–210 (2018).
Bai, Y. et al. Zr-based metal–organic frameworks: design, synthesis, structure, and applications. Chem. Soc. Rev. 45, 2327–2367 (2016).
Kong, X. J. et al. Constructing new metal–organic frameworks with complicated ligands from ‘one-pot’ in situ reactions. Chem. Sci. 10, 3949–3955 (2019).
Xing, L. et al. Top-down synthetic strategies toward single atoms on the rise. Matter 5, 788–807 (2022).
Han, G. F. et al. Abrading bulk metal into single atoms. Nat. Nanotechnol. 17, 403–407 (2022).
Yao, Y. et al. High temperature shockwave stabilized single atoms. Nat. Nanotechnol. 14, 851–857 (2019).
Peng, Y. et al. Laser solid-phase synthesis of single-atom catalysts. Light Sci. Appl. 10, 168 (2021).
Browne, M. P., Redondo, E. & Pumera, M. 3D printing for electrochemical energy applications. Chem. Rev. 120, 2783–2810 (2020).
Lee, C. Y., Taylor, A. C., Nattestad, A., Beirne, S. & Wallace, G. G. 3D printing for electrocatalytic applications. Joule 3, 1835–1849 (2019).
dos Santos, M. F. et al. 3D-printed low-cost spectroelectrochemical cell for in situ raman measurements. Anal. Chem. 91, 10386–10389 (2019).
Yuk, H. et al. 3D printing of conducting polymers. Nat. Commun. 11, 1604 (2020).
Hartings, M. R. & Ahmed, Z. Chemistry from 3D printed objects. Nat. Rev. Chem. 3, 305–314 (2019).
Zhang, M. et al. Hierarchical-coassembly-enabled 3D-printing of homogeneous and heterogeneous covalent organic frameworks. J. Am. Chem. Soc. 141, 5154–5158 (2019).
Hou, W. et al. Automatic generation of 3D-printed reactionware for chemical synthesis digitization using chemscad. ACS Cent. Sci. 7, 212–218 (2021).
Sullivan, I. et al. 3D printed nickel–molybdenum-based electrocatalysts for hydrogen evolution at low overpotentials in a flow-through configuration. ACS Appl. Mater. Interfaces 13, 20260–20268 (2021).
Wu, Z. Y. et al. Electrochemical ammonia synthesis via nitrate reduction on Fe single atom catalyst. Nat. Commun. 12, 2870 (2021).
Funke, H., Chukalina, M. & Scheinost, A. C. A new FEFF-based wavelet for EXAFS data analysis. J Synchrotron Radiat. 14, 426–432 (2007).
Funke, H., Scheinost, A. C. & Chukalina, M. Wavelet analysis of extended X-ray absorption fine structure data. Phys. Rev. B 71, 094110 (2005).
Wang, Y. et al. Phthalocyanine precursors to construct atomically dispersed iron electrocatalysts. ACS Catal. 9, 6252–6261 (2019).
Zhang, S. et al. Electrocatalytically active Fe-(O-C2)4 single-atom sites for efficient reduction of nitrogen to ammonia. Angew. Chem. Int. Ed. Engl. 59, 13423–13429 (2020).
Wang, Y. et al. High-efficiency oxygen reduction to hydrogen peroxide catalyzed by nickel single-atom catalysts with tetradentate N2O2 coordination in a three-phase flow cell. Angew. Chem. Int. Ed. Engl. 59, 13057–13062 (2020).
Li, Z. et al. Axial chlorine coordinated iron-nitrogen-carbon single-atom catalysts for efficient electrochemical CO2 reduction. Chem. Eng. J. 430, 132882 (2022).
Han, Y. et al. Electronic structure engineering to boost oxygen reduction activity by controlling the coordination of the central metal. Energy Environ. Sci. 11, 2348–2352 (2018).
Liu, M. et al. Atomically-dispersed NiN4–Cl active sites with axial Ni–Cl coordination for accelerating electrocatalytic hydrogen evolution. J. Mater. Chem. A 10, 6007–6015 (2022).
Li, P., Jin, Z., Fang, Z. & Yu, G. A single-site iron catalyst with preoccupied active centers that achieves selective ammonia electrosynthesis from nitrate. Energy Environ. Sci. 14, 3522–3531 (2021).
Singh, B. et al. Single-atom (iron-based) catalysts: synthesis and applications. Chem. Rev. 121, 13620–13697 (2021).
Tang, J. et al. Injection-free delivery of MSC-derived extracellular vesicles for myocardial infarction therapeutics. Adv. Healthcare Mater. 11, 2100312 (2022).
Ravel, B. & Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 12, 537–541 (2005).
Li, L. et al. Efficient nitrogen fixation to ammonia through integration of plasma oxidation with electrocatalytic reduction. Angew. Chem. Int. Ed. Engl. 60, 14131–14137 (2021).
Kresse, G. & Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999).
Nørskov, J. K. et al. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B 108, 17886–17892 (2004).
Peterson, A. A., Abild-Pedersen, F., Studt, F., Rossmeisl, J. & Nørskov, J. K. How copper catalyzes the electroreduction of carbon dioxide into hydrocarbon fuels. Energy Environ. Sci. 3, 1311–1315 (2010).
Wan, H., Bagger, A. & Rossmeisl, J. Electrochemical nitric oxide reduction on metal surfaces. Angew. Chem. Int. Ed. Engl. 60, 21966–21972 (2021).
Calle-Vallejo, F., Huang, M., Henry, J. B., Koper, M. T. M. & Bandarenka, A. S. Theoretical design and experimental implementation of Ag/Au electrodes for the electrochemical reduction of nitrate. Phys. Chem. Chem. Phys. 15, 3196–3202 (2013).
Acknowledgements
DFT computations within this work were supported by computational resources provided by the Australian Government through NCI under the National Computational Merit Allocation Scheme and the Phoenix High Performance Compute (HPC) Service at The University of Adelaide. This research was undertaken on the XAS and Soft X-ray beamlines at the Australian Synchrotron, part of ANSTO. F.X. thanks H. Jin, C. Tang, Y. Jiao, Y. Zheng, P. Wang and J. Shan from the school of CEAM, A. Slattery and S. Gilbert from Adelaide Microscopy at the University of Adelaide, and H. Yu from the University of South Australia for constructive suggestions and help. This study was supported by the Australian Research Council (grant Nos. FL170100154-S.Z.Q and DP220102596-S.Z.Q), the New Zealand Health Research Council (grant No. 19/779 to C.X.), the New Zealand Ministry for Business, Innovation and Employment (grant No. MBIE Science Whitinga Fellowship, MWF-UOO2103 to C.X.) and the National Heart Foundation of New Zealand (grant Nos. 1891, 1896 to C.X.).
Author information
Authors and Affiliations
Contributions
F.X. and C.X. conceived the project. S.-Z.Q. supervised the project and whole studies. F.X. and C.X. performed the materials preparations with the assistance of J.T. and T.W. F.X. and D.Y. conducted the electrochemical measurements. X.Z. conducted the DFT calculations. F.X., B.J., T.L. and G.L. performed the physicochemical characterizations. F.X., C.X., X.Z., D.Y. and S.-Z.Q. wrote the manuscript. S.-Z.Q. revised the manuscript for submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks Yu Chen, Xiong Wen (David) Lou and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Alexandra Groves, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–44 and Tables 1 and 2.
Supplementary Video 1
Typical printing process.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xie, F., Cui, X., Zhi, X. et al. A general approach to 3D-printed single-atom catalysts. Nat. Synth 2, 129–139 (2023). https://doi.org/10.1038/s44160-022-00193-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44160-022-00193-3