Abstract
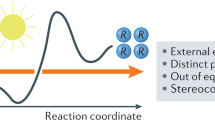
Information gained from in-depth mechanistic investigations can be used to control the selectivity of reactions, leading to expansion of the generality of synthetic processes and discovery of new reactivity. Here, we investigate the mechanism of light-driven [2 + 2] heterocycloadditions (Paternò–Büchi reactions) between indoles and ketones to develop insight into these processes. Using ground-state ultraviolet–visible absorption and transient absorption spectroscopy, together with density functional theory calculations, we found that the reactions can proceed via an exciplex or electron–donor–acceptor complex, which are key intermediates in determining the stereoselectivity of the reactions. We used this discovery to control the diastereoselectivity of the reactions, gaining access to previously inaccessible diastereoisomeric variants. When moving from 370 to 456 nm irradiation, the electron–donor–acceptor complex is increasingly favoured, and the diastereomeric ratio (d.r.) of the product moves from >99:<1 to 47:53. In contrast, switching from methyl to ipropyl substitution favours the exciplex intermediate, reversing the d.r. from 89:11 to 16:84. Our study shows how light and steric parameters can be rationally used to control the diastereoselectivity of photoreactions, creating mechanistic pathways to previously inaccessible stereochemical variants.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
All relevant data supporting the findings of this study, including experimental procedures and compound characterization, NMR spectra and other spectroscopic analysis are available within the article and its Supplementary Information.
References
Yoon, T. P., Ischay, M. A. & Du, J. Visible light photocatalysis as a greener approach to photochemical synthesis. Nat. Chem. 2, 527–532 (2010).
Minisci, F. et al. Additions and corrections – Polar effects in free-radical reactions selectivity and reversibility in the homolytic benzylation of protonated heteroaromatic bases. J. Org. Chem. 51, 4326 (1986).
Shaw, M. H., Twilton, J. & MacMillan, D. W. C. Photoredox catalysis in organic chemistry. J. Org. Chem. 81, 6898–6926 (2016).
Mateos, J. et al. A visible-light Paternò-Büchi dearomatisation process towards the construction of oxeto-indolinic polycycles. Chem. Sci. 11, 6532–6538 (2020).
Franceschi, P., Mateos, J., Vega‐Peñaloza, A. & Dell’Amico, L. Microfluidic visible‐light Paternò–Büchi reaction of oxindole enol ethers. Eur. J. Org. Chem. 2020, 6718–6722 (2020).
Zheng, J., Dong, X. & Yoon, T. P. Divergent photocatalytic reactions of α-ketoesters under triplet sensitization and Photoredox conditions. Org. Lett. 22, 6520–6525 (2020).
Paternò, E. & Chieffi, G. Sintesi in Chimica Organica per Mezzo Della Luce. Nota II. Composti Degli Idrocarburi Non Saturi Con Aldeidi e Chetoni. Gazz. Chim. Ital. 39, 341 (1909).
Büchi, G., Inman, C. G. & Lipinsky, E. S. Light-catalyzed organic reactions. I. The reaction of carbonyl compounds with 2-methyl-2-butene in the presence of ultraviolet light. J. Am. Chem. Soc. 76, 4327–4331 (1954).
Bull, J. A., Croft, R. A., Davis, O. A., Doran, R. & Morgan, K. F. Oxetanes: recent advances in synthesis, reactivity, and medicinal chemistry. Chem. Rev. 116, 12150–12233 (2016).
Turro, N. J. et al. Molecular photochemistry of alkanones in solution: α-cleavage, hydrogen abstraction, cycloaddition, and sensitization reactions. Acc. Chem. Res. 5, 92–101 (1972).
Mattay, J., Gersdorf, J. & Buchkremer, K. Photoreactions of biacetyl with electron‐rich olefins. An extended mechanism. Chem. Ber. 120, 307–318 (1987).
Fréneau, M. & Hoffmann, N. The Paternò-Büchi reaction—mechanisms and application to organic synthesis. J. Photochem. Photobiol. C Photochem. Rev. 33, 83–108 (2017).
Mateos, J., Cuadros, S., Vega-Peñaloza, A. & Dell’Amico, L. Unlocking the synthetic potential of light-excited aryl ketones: applications in direct photochemistry and photoredox catalysis. Synlett 33, 116–128 (2022).
Salem, L. Surface crossings and surface touchings in photochemistry. J. Am. Chem. Soc. 96, 3486–3501 (1974).
Griesbeck, A. G., Abe, M. & Bondock, S. Selectivity control in electron spin inversion processes: regio- and stereochemistry of Paternò-Büchi photocycloadditions as a powerful tool for mapping intersystem crossing processes. Acc. Chem. Res. 37, 919–928 (2004).
Crisenza, G. E. M., Mazzarella, D. & Melchiorre, P. Synthetic methods driven by the photoactivity of electron donor-acceptor complexes. J. Am. Chem. Soc. 142, 5461–5476 (2020).
Matsumura, K., Mori, T. & Inoue, Y. Wavelength control of diastereodifferentiating Paternó-Büchi reaction of chiral cyanobenzoates with diphenylethene through direct versus charge-transfer excitation. J. Am. Chem. Soc. 131, 17076–17077 (2009).
Sun, D., Hubig, S. M. & Kochi, J. K. Oxetanes from [2+2] cycloaddition of stilbenes to quinone via photoinduced electron transfer. J. Org. Chem. 64, 2250–2258 (1999).
Zhang, Y., Xue, J., Gao, Y., Fun, H. K. & Xu, J. H. Photoinduced [2+2] cycloadditions (the Paterno–Büchi reaction) of 1-acetylisatin with enol ethers—regioselectivity, diastereoselectivity and acid catalysed transformations of the spirooxetane products. J. Chem. Soc. Perkin Trans. 1 2, 345–353 (2002).
Kandukuri, S. R. et al. X-ray characterization of an electron donor-acceptor complex that drives the photochemical alkylation of indoles. Angew. Chemie Int. Ed. 54, 1485–1489 (2015).
Buzzetti, L., Crisenza, G. E. M. & Melchiorre, P. Mechanistic studies in photocatalysis. Angew. Chemie Int. Ed. 58, 3730–3747 (2019).
Freilich, S. C. & Peters, K. S. Observation of the 1,4-biradical in the Paterno-Buchi reaction. J. Am. Chem. Soc. 103, 6255–6257 (1981).
Rehm, D. & Weller, A. Kinetics of fluorescence quenching by electron and H‐atom transfer. Isr. J. Chem. 8, 259–271 (1970).
Mattay, J., Runsink, J., Rumbach, T., Ly, C. & Gersdorf, J. Selectivity and charge transfer in photoreactions of α, α, α-trifluorotoluene with olefins. J. Am. Chem. Soc. 107, 2557–2558 (1985).
Li, H.-F., Cao, W., Ma, X., Xie, X., Xia, Y. & Ouyang, Z. Visible-Light-Driven [2 + 2] Photocycloadditions between Benzophenone and C═C Bonds in Unsaturated Lipids. J. Am. Chem. Soc. 142, 3499–3505 (2020).
Mieres-Perez, J., Costa, P., Mendez-Vega, E., Crespo-Otero, R. & Sander, W. Switching the spin state of pentafluorophenylnitrene: isolation of a singlet arylnitrene complex. J. Am. Chem. Soc. 140, 17271–17277 (2018).
Gutiérrez-Hernández, A. et al. Deep eutectic solvent choline chloride/ p-toluenesulfonic acid and water favor the enthalpy-driven binding of arylamines to maleimide in Aza-Michael addition. J. Org. Chem. 86, 223–234 (2021).
Mori, T. & Inoue, Y. Charge-transfer excitation: unconventional yet practical means for controlling stereoselectivity in asymmetric photoreactions. Chem. Soc. Rev. 42, 8122–8133 (2013).
Trost, B. M., Dong, G. & Vance, J. A. Cyclic 1,2-diketones as core building blocks: a strategy for the total synthesis of (−)-terpestacin. Chemistry 16, 6265–6277 (2010).
Samanta, S., Roy, D., Khamarui, S. & Maiti, D. K. Ni(ii)-salt catalyzed activation of primary amine-sp3Cα-H and cyclization with 1,2-diketone to tetrasubstituted imidazoles. Chem. Commun. 50, 2477–2480 (2014).
Matsumura, K., Mori, T. & Inoue, Y. Solvent and temperature effects on diastereodifferentiating Paternó-Büchi reaction of chiral alkyl cyanobenzoates with diphenylethene upon direct versus charge-transfer excitation. J. Org. Chem. 75, 5461–5469 (2010).
Saito, H., Mori, T., Wada, T. & Inoue, Y. Diastereoselective [2 + 2] photocycloaddition of stilbene to chiral fumarate. Direct versus charge-transfer excitation. J. Am. Chem. Soc. 126, 1900–1906 (2004).
Aoki, Y., Matsuki, N., Mori, T., Ikeda, H. & Inoue, Y. Exciplex ensemble modulated by excitation mode in intramolecular charge-transfer dyad: effects of temperature, solvent polarity, and wavelength on photochemistry and photophysics of tethered naphthalene-dicyanoethene system. Org. Lett. 16, 4888–4891 (2014).
Nagasaki, K., Inoue, Y. & Mori, T. Entropy-driven diastereoselectivity improvement in the Paternò–Büchi reaction of 1-naphthyl aryl ethenes with a chiral cyanobenzoate through remote alkylation. Angew. Chemie Int. Ed. 57, 4880–4885 (2018).
D’Auria, M. The Paternò-Büchi reaction – a comprehensive review. Photochem. Photobiolog. Sci. 18, 2297–2362 (2019).
Griesbeck, A. G., Buhr, S., Fiege, M., Schmickler, H. & Lex, J. Stereoselectivity of triplet photocycloadditions: 1 diene-carbonyl reactions and solvent effects. J. Org. Chem. 63, 3847–3854 (1998).
Harris, C. B., Ippen, E. P., Mourou, G. & Zewail A. H. Ultrafast Phenomena VIII (Springer, 1993).
Ikeda, N., Koshioka, M., Masuhara, H. & Yoshihara, K. Picosecond dynamics of excited singlet states in organic microcrystals: diffuse reflectance laser photolysis study. Chem. Phys. Lett. 150, 452–456 (1988).
Singh, A. K., Palit, D. K. & Mittal, J. P. Conformational relaxation dynamics in the excited electronic states of benzil in solution. Chem. Phys. Lett. 360, 443–452 (2002).
Fang, T. S. & Singer, L. A. Variable temperature studies on the luminescence from benzil in a polymethylmethacrylate glass. An example of matrix controlled photorotamerism. Chem. Phys. Lett. 60, 117–121 (1978).
Charton, M. Steric effects. I. Esterification and acid-catalyzed hydrolysis of esters. J. Am. Chem. Soc. 97, 1552–1556 (1975).
Harper, K. C., Bess, E. N. & Sigman, M. S. Multidimensional steric parameters in the analysis of asymmetric catalytic reactions. Nat. Chem. 4, 366–374 (2012).
Sigman, M. S. & Miller, J. J. Examination of the role of taft-type steric parameters in asymmetric catalysis. J. Org. Chem. 74, 7633–7643 (2009).
Cambié, D., Bottecchia, C., Straathof, N. J. W., Hessel, V. & Noël, T. Applications of continuous-flow photochemistry in organic synthesis, material science, and water treatment. Chem. Rev. 116, 10276–10341 (2016).
Mateos, J. et al. A microfluidic photoreactor enables 2-methylbenzophenone light-driven reactions with superior performance. Chem. Commun. 54, 6820–6823 (2018).
Chapman, S. J. et al. Cooperative stereoinduction in asymmetric photocatalysis. J. Am. Chem. Soc. 144, 4206–4213 (2022).
Poplata, S., Tröster, A., Zou, Y.-Q. & Bach, T. Recent advances in the synthesis of cyclobutanes by olefin [2+2] photocycloaddition reactions. Chem. Rev. 116, 9748–9815 (2016).
Acknowledgements
We acknowledge P. Franceschi, P. Andreetta and G. Simionato (Department of Chemical Sciences, University of Padova) for preliminary experiments, and S. Bonacchi (Department of Chemical Sciences, University of Padova) for insightful discussions. This project was supported by Ministero dell’Università (MUR) (grant No. PRIN 2020927WY3_002 to L.D.), the European Union, European Research Concilium (ERC) (starting grant No. SYNPHOCAT 101040025 to L.D.), Cariparo, project Synergy within the call Ricerca Scientifica d’Eccellenza 2018 (to L.D.), University of Padova (to P.C.), Seal of Excellence@UNIPD (to P.C.) and QuantaCOF (to P.C.).
Author information
Authors and Affiliations
Contributions
J.M. and L.D. wrote the manuscript with contributions from all the authors. J.M. and L.D. conceived the project and devised the experiments. J.M. and A.V.-P. carried out the reactions and isolated and characterized the products. J.M., L.D., M.B. and A.S. rationalized the experimental results. F.R., M.N., E.C. and E.F. performed the spectroscopic investigations using TAS. P.C. and A.S. performed the DFT calculations.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks Axel Griesbeck, Norbert Hoffmann and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Peter Seavill, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Experimental details and DFT calculations, Supplementary sections A–M, and Supplementary Figs. S.A.1–S.K.17.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mateos, J., Rigodanza, F., Costa, P. et al. Unveiling the impact of the light source and steric factors on [2 + 2] heterocycloaddition reactions. Nat. Synth 2, 26–36 (2023). https://doi.org/10.1038/s44160-022-00191-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44160-022-00191-5
This article is cited by
-
The impact of UV light on synthetic photochemistry and photocatalysis
Nature Chemistry (2024)
-
Accessing ladder-shape azetidine-fused indoline pentacycles through intermolecular regiodivergent aza-Paternò–Büchi reactions
Nature Communications (2024)
-
Two photons are better than one: continuous flow synthesis of ꞵ-lactones through a doubly photochemically-activated Paternò-Büchi reaction
Journal of Flow Chemistry (2024)
-
Paternò–Büchi pathways
Nature Synthesis (2022)