Abstract
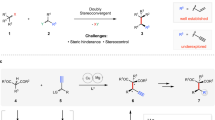
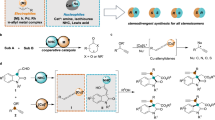
Asymmetric allylic and propargylic substitutions have seen numerous applications in synthetic chemistry, constructing stereogenic centres vicinal to unsaturated C–C bonds. However, the merger of these two substitution processes into alkynylallylic substitution is relatively underdeveloped, probably owing to the challenge of controlling regio- and stereoselectivity. Here we report the development of enantioselective intermolecular and decarboxylative alkynylallylic aminations, alkoxylation and alkylation for the synthesis of a range of enantioenriched 1,4-enynes. Cu(I) and Cu(II) salts are both effective precatalysts for the process, which can also be readily performed on a gram scale in the presence of air and moisture with no erosion in yield or selectivity. The convergent nature of the process was shown through the synthesis of a single product from a mixture of eight regio- and enantiomeric alkynylallylic carbamates and carbonate substrates, in excellent yield and selectivity. X-ray crystallographic and mechanistic analysis reveal that the Cu(I) and Cu(II) processes probably proceed through distinct enantio-determining transition state models, involving multiple ligands.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All relevant data are available within the Article or in the Supplementary Information files. Crystallographic data for the structures reported in this Article have been deposited at the Cambridge Crystallographic Data Centre, under deposition number CCDC 2100528 (rac-3a), 2155927 (C-1), 2143688 (C-2) and 2155930 (C-3).
References
Trost, B. M. & Van Vranken, D. L. Asymmetric transition metal-catalyzed allylic alkylations. Chem. Rev. 96, 395–422 (1996).
Alexakis, A. et al. Transition metal catalyzed enantioselective allylic substitution in organic synthesis in Topics in Organometallic Chemistry Vol. 38 (ed. Kazmaier, U.) (Springer, 2012).
Li, G., Huo, X., Jiang, X. & Zhang, W. Asymmetric synthesis of allylic compounds via hydrofunctionalisation and difunctionalisation of dienes, allenes, and alkynes. Chem. Soc. Rev. 49, 2060–2118 (2020).
Süsse, L. & Stoltz, B. M. Enantioselective formation of quaternary centers by allylic alkylation with first-row transition-metal catalysts. Chem. Rev. 121, 4084–4099 (2021).
Pàmies, O. et al. Recent advances in enantioselective Pd-catalyzed allylic substitution: from design to applications. Chem. Rev. 121, 4373–4505 (2021).
Detz, R. J., Hiemstra, H. & van Maarseveen, J. H. Catalyzed propargylic substitution. Eur. J. Org. Chem. 2009, 6263–6276 (2009).
Miyake, Y., Uemura, S. & Nishibayashi, Y. Catalytic propargylic substitution reactions. ChemCatChem 1, 342–356 (2009).
Ljungdahl, N. & Kann, N. Transition-metal-catalyzed propargylic substitution. Angew. Chem. Int. Ed. 48, 642–644 (2009).
Ding, C.-H. & Hou, X.-L. Catalytic asymmetric propargylation. Chem. Rev. 111, 1914–1937 (2011).
Roy, R. & Saha, S. Scope and advances in the catalytic propargylic substitution reaction. RSC Adv. 8, 31129–31193 (2018).
Shirakura, M. & Suginome, M. Nickel-catalyzed asymmetric addition of alkyne C-H bonds across 1,3-dienes using taddol-based chiral phosphoramidite ligands. Angew. Chem. Int. Ed. 49, 3827–3829 (2010).
Dabrowski, J. A., Gao, F. & Hoveyda, A. H. Enantioselective synthesis of alkyne-substituted quaternary carbon stereogenic centers through NHC-Cu-catalyzed allylic substitution reactions with (i-bu)2(alkynyl)aluminum reagents. J. Am. Chem. Soc. 133, 4778–4781 (2011).
Hamilton, J. Y., Sarlah, D. & Carreira, E. M. Iridium-catalyzed enantioselective allylic alkynylation. Angew. Chem. Int. Ed. 52, 7532–7535 (2013).
Dabrowski, J. A., Haeffner, F. & Hoveyda, A. H. Combining NHC−Cu and Brønsted base catalysis: enantioselective allylic substitution/conjugate additions with alkynylaluminum reagents and stereospecific isomerization of the products to trisubstituted allenes. Angew. Chem. Int. Ed. 52, 7694–7699 (2013).
Harada, A., Makida, Y., Sato, T., Ohmiya, H. & Sawamura, M. Copper-catalyzed enantioselective allylic alkylation of terminal alkyne pronucleophiles. J. Am. Chem. Soc. 136, 13932–13939 (2014).
Okusu, S., Okazaki, H., Tokunaga, E., Soloshonok, V. A. & Shibata, N. Organocatalytic enantioselective nucleophilic alkynylation of allyl fluorides affording chiral skipped ene-ynes. Angew. Chem. Int. Ed. 55, 6744–6748 (2016).
Cui, X.-Y. et al. Guanidine–copper complex-catalyzed enantioselective dynamic kinetic allylic alkynylation under biphasic condition. J. Am. Chem. Soc. 140, 8448–8455 (2018).
Huang, W.-Y., Lu, C.-H., Ghorai, S., Li, B. & Li, C. Regio- and enantioselective allylic alkylation of terminal alkynes by synergistic Rh/Cu catalysis. J. Am. Chem. Soc. 142, 15276–15281 (2020).
Huang, Y. et al. Ligand coordination- and dissociation-induced divergent allylic alkylations using alkynes. Chem 7, 812–826 (2021).
Liu, L. et al. Copper-catalyzed intermolecular enantioselective radical oxidative C(sp3)-H/C(sp)-H cross-coupling with rationally designed oxazoline-derived N,N,P(O)-ligands. Angew. Chem. Int. Ed. 60, 26710–26717 (2021).
Bai, J.-F., Yasumoto, K., Kano, T. & Maruoka, K. Asymmetric synthesis of chiral 1,4-enynes through organocatalytic alkenylation of propargyl alcohols with trialkenylboroxines. Angew. Chem. Int. Ed. 58, 8898–8901 (2019).
Zhang, K. et al. Enantioconvergent copper catalysis: in situ generation of the chiral phosphorus ylide and its Wittig reactions. J. Am. Chem. Soc. 139, 12847–12854 (2017).
Trost, B. M., Hildbrand, S. & Dogra, K. Regio- and enantioselective molybdenum-catalyzed alkylations of polyenyl esters. J. Am. Chem. Soc. 121, 10416–10417 (1999).
Kacprzynski, M. A. & Hoveyda, A. H. Cu-catalyzed asymmetric allylic alkylation of aromatic and aliphatic phosphates with alkylzinc reagents. An effective method for enantioselective synthesis of tertiary and quaternary carbons. J. Am. Chem. Soc. 126, 10676–10681 (2004).
Li, H. & Alexakis, A. Enyne chlorides: substrates for copper-catalyzed asymmetric allylic alkylation. Angew. Chem. Int. Ed. 51, 1055–1058 (2012).
Detz, R. J., Delville, M. M. E., Hiemstra, H. & van Maarseveen, J. H. Enantioselective copper-catalyzed propargylic amination. Angew. Chem. Int. Ed. 47, 3777–3780 (2008).
Hattori, G., Matsuzawa, H., Miyake, Y. & Nishibayashi, Y. Copper-catalyzed asymmetric propargylic substitution reactions of propargylic acetates with amines. Angew. Chem. Int. Ed. 47, 3781–3783 (2008).
Zhang, D. Y. & Hu, X. P. Recent advances in copper-catalyzed propargylic substitution. Tetrahedron Lett. 56, 283–295 (2015).
Hattori, G. et al. Copper-catalyzed enantioselective propargylic amination of propargylic esters with amines: Copper-allenylidene complexes as key intermediates. J. Am. Chem. Soc. 132, 10592–10608 (2010).
Nakajima, K., Shibata, M. & Nishibayashi, Y. Copper-catalyzed enantioselective propargylic etherification of propargylic esters with alcohols. J. Am. Chem. Soc. 137, 2472–2475 (2015).
Li, R.-Z. et al. Enantioselective propargylation of polyols and desymmetrization of meso 1,2-diols by copper/borinic acid dual catalysis. Angew. Chem. Int. Ed. 56, 7213–7217 (2017).
Cheng, L.-J., Brown, A. P. N. & Cordier, C. J. Enantioselective propargylic [1,3]-rearrangements: copper-catalyzed O-to-N migrations toward C−N bond formation. Chem. Sci. 8, 4299–4305 (2017).
Gómez, J. E., Cristòfol, À. & Kleij, A. W. Copper-catalyzed enantioselective construction of tertiary propargylic sulfones. Angew. Chem. Int. Ed. 58, 3903–3907 (2019).
Li, R.-Z., Liu, D.-Q. & Niu, D. Asymmetric O-propargylation of secondary aliphatic alcohols. Nat. Catal. 3, 672–680 (2020).
Zhang, C. et al. Highly diastereo- and enantioselective Cu-catalyzed [3 + 3] cycloaddition of propargyl esters with cyclic enamines toward chiral bicyclo[n.3.1] frameworks. J. Am. Chem. Soc. 134, 9585–9588 (2012).
Zhu, F.-L., Wang, Y.-H., Zhang, D.-Y., Xu, J. & Hu, X.-P. Enantioselective synthesis of highly functionalized dihydrofurans through copper-catalyzed asymmetric formal [3+2] cycloaddition of β-ketoesters with propargylic esters. Angew. Chem. Int. Ed. 53, 10223–10227 (2014).
Shao, W., Li, H., Liu, C., Liu, C. J. & You, S. L. Copper-catalyzed intermolecular asymmetric propargylic dearomatization of indoles. Angew. Chem. Int. Ed. 54, 7684–7687 (2015).
Wang, Q. et al. Catalytic asymmetric [4 + 1] annulation of sulfur ylides with copper-allenylidene intermediates. J. Am. Chem. Soc. 138, 8360–8363 (2016).
Song, J., Zhang, Z.-J. & Gong, L.-Z. Asymmetric [4 + 2] annulation of C1 ammonium enolates with copper-allenylidenes. Angew. Chem. Int. Ed. 56, 5212–5216 (2017).
Gao, X., Cheng, R., Xiao, Y.-L., Wan, X.-L. & Zhang, X. Copper-catalyzed highly enantioselective difluoroalkylation of secondary propargyl sulfonates with difluoroenoxysilanes. Chem. 5, 2987–2999 (2019).
Zhang, Z.-J. et al. N-heterocyclic carbene/copper cooperative catalysis for the asymmetric synthesis of spirooxindoles. Angew. Chem. Int. Ed. 58, 12190–12194 (2019).
Simlandy, A. K. & Brown, M. K. Allenylidene induced 1,2-metalate rearrangement of indole-boronates: diastereoselective access to highly substituted indolines. Angew. Chem. Int. Ed. 60, 12366–12370 (2021).
Niu, S. et al. Copper-catalyzed yne-allylic substitutions using stabilized nucleophiles. ACS Catal. 12, 6840–6850 (2022).
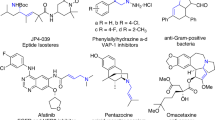
Rej, R. K., Das, T., Hazra, S. & Nanda, S. Chemoenzymatic asymmetric synthesis of fluoxetine, atomoxetine, nisoxetine, and duloxetine. Tetrahedron: Asymmetry 24, 913–918 (2013).
Smilovic, I. G. et al. Synthesis of enantiopure antiobesity drug lorcaserin. Bioorg. Med. Chem. 26, 2686–2690 (2018).
Tewari, N., Maheshwari, N., Medhane, R., Nizar, H. & Prasad, M. A novel method for the large scale synthesis of cinacalcet hydrochloride using iron catalyzed C−C coupling. Org. Process Res. Dev. 16, 1566–1568 (2012).
Lee, S. H. et al. Stereoselective amination of chiral benzylic ethers using chlorosulfonyl isocyanate: total synthesis of (+)-sertraline. J. Org. Chem. 76, 10011–10019 (2011).
Ma, G. et al. A novel synthesis of rasagiline via a chemoenzymatic dynamic kinetic resolution. Org. Process Res. Dev. 18, 1169–1174 (2014).
Otani, T. et al. Construction of dibenzo-fused seven- to nine-membered carbocycles via Brønsted acid-promoted intramolecular Friedel−Crafts-type alkenylation. Chem. Commun. 51, 7895–7898 (2015).
Bymaster, F. P. et al. Duloxetine (cymbaltaTM), a dual inhibitor of serotonin and norepinephrine reuptake. Bioorg. Med. Chem. Lett. 13, 4477–4480 (2003).
Liu, Z. & Xiang, J. A high yield and pilot-scale process for the preparation of adapalene. Org. Process Res. Dev. 10, 285–288 (2006).
Rouden, J., Lasne, M.-C., Blanchet, J. & Baudoux, J. (−)-Cytisine and derivatives: synthesis, reactivity, and applications. Chem. Rev. 114, 712–778 (2014).
Hughes, G., Kimura, M. & Buchwald, S. L. Catalytic enantioselective conjugate reduction of lactones and lactams. J. Am. Chem. Soc. 125, 11253–11258 (2003).
Wang, H. et al. Selective inhibition of the Kir2 family of inward rectifier potassium channels by a small molecule probe: the discovery, SAR, and pharmacological characterization of ML133. ACS Chem. Biol. 6, 845–856 (2011).
Mohr, J. T. & Stoltz, B. M. Enantioselective Tsuji allylations. Chem. - Asian J. 2, 1476–1491 (2007).
Weaver, J. D., Recio, A. III, Grenning, A. J. & Tunge, J. A. Transition metal-catalyzed decarboxylative allylation and benzylation reactions. Chem. Rev. 111, 1846–1913 (2011).
James, J., Jackson, M. & Guiry, P. J. Palladium-catalyzed decarboxylative asymmetric allylic alkylation: development, mechanistic understanding and recent advances. Adv. Synth. Catal. 361, 3016–3049 (2019).
Zhu, F.-L. et al. Enantioselective copper-catalyzed decarboxylative propargylic alkylation of propargyl β-ketoesters with a chiral ketimine P,N,N-ligand. Angew. Chem. Int. Ed. 53, 1410–1414 (2014).
Zou, Y. et al. Enantioselective Cu-catalyzed decarboxylative propargylic amination of propargyl carbamates. Tetrahedron Lett. 55, 2033–2036 (2014).
Lu, W.-Y. et al. Copper-catalyzed decarboxylative [3 + 2] annulation of ethynylethylene carbonates with azlactones: access to γ-butyrolactones bearing two vicinal quaternary carbon centers. J. Org. Chem. 86, 1779–1788 (2021).
Guo, W., Zuo, L., Cui, M., Yan, B. & Ni, S. Propargylic amination enabled the access to enantioenriched acyclic α-quaternary α-amino ketones. J. Am. Chem. Soc. 143, 7629–7634 (2021).
Xia, J.-T., Li, L. & Hu, X.-P. Copper-catalyzed decarboxylative propargylic alkylation of enol carbonates: stereoselective synthesis of quaternary α‑amino acids. ACS Catal. 11, 11843–11848 (2021).
Ma, J. et al. Enantioselective synthesis of pyroglutamic acid esters from glycinate via carbonyl catalysis. Angew. Chem. Int. Ed. 60, 10588–10592 (2021).
Chai, W. et al. Lewis-acid-promoted ligand-controlled regiodivergent cycloaddition of Pd-oxyallyl with 1,3-dienes: reaction development and origins of selectivities. J. Am. Chem. Soc. 143, 3595–3603 (2021).
Larock, R. C., Yum, E. K. & Refvik, M. D. Synthesis of 2,3-disubstituted indoles via palladium-catalyzed annulation of internal alkynes. J. Org. Chem. 63, 7652–7662 (1998).
Gommermann, N. & Knochel, P. Practical highly enantioselective synthesis of terminal propargylamines. An expeditious synthesis of (S)-(1)-coniine. Chem. Commun. 2004, 2324–2325 (2004).
Acknowledgements
We acknowledge the National Natural Science Foundation of China (grant nos. NSFC 22071262 to Z.-T.H., and 22101296, 21871284 and 91956113 to G.-Q.L.), the Shanghai Rising-Star programme (grant no. 20QA1411300 to Z.-T.H.), the Shanghai Municipal Committee of Science and Technology (grant no. 22ZR1475200 to Z.-T.H.), the CAS Key Laboratory of Synthetic Chemistry of Natural Substances and the Shanghai Institute of Organic Chemistry for financial support. H. Luo and X.-S. Xue are thanked for help with preliminary trials and discussions on Density Functional Theory calculations.
Author information
Authors and Affiliations
Contributions
Z.-T.H. conceived the project. J.-S.M., H.-Y.L., Y.-W.C., Y.-Z.S., W.-C.Z., R.-P.L. and H.-X.W. performed the experiments. G.-Q.L. and Z.-T.H. supervised the project. Z.-T.H. wrote the manuscript with feedback from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks Jan van Maarseveen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Thomas West, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–176 and Tables 1–14.
Supplementary Data 1
X-ray crystallographic data for C-1, CCDC 2155927.
Supplementary Data 2
X-ray crystallographic data for C-2, CCDC 2143688.
Supplementary Data 3
X-ray crystallographic data for C-3, CCDC 2155930.
Supplementary Data 4
X-ray crystallographic data for rac-3a, CCDC 2100528.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ma, JS., Lu, HY., Chen, YW. et al. Copper-catalysed convergent regio- and enantioselective alkynylallylic substitution. Nat. Synth 2, 37–48 (2023). https://doi.org/10.1038/s44160-022-00176-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44160-022-00176-4
This article is cited by
-
Catalytic enantioselective reductive alkynylation of amides enables one-pot syntheses of pyrrolidine, piperidine and indolizidine alkaloids
Nature Communications (2023)
-
Copper for alkynylallylic substitution
Nature Synthesis (2022)