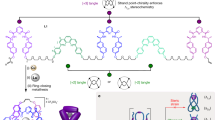
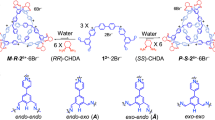
Abstract
Molecular knots attract attention on account of their topological intricacies and potential application. Tying molecular knots with different topologies, on larger length scales, remains challenging, not to mention the difficulties with harnessing their topological characteristics in order to modulate their properties. Here, we report a general approach to construct torus knots from two coaxially nested multistranded contra-helices. As a proof of concept, a series of two iron(II)-templated contra-helical trefoil knots have been synthesized near-quantitatively in one step. Among these, one features a long trefoil knot—a 111-atom closed loop that is ~11 nm long. The thermally induced spin crossover of the two iron(II) centres in each knot can be modulated in opposing directions by changing the intramolecular mechanical strain. The synthesis of molecular knots with mechanically tuneable properties enables the unleashing of their stimuli-responsive multifunctionalities. One of these molecular knots exhibits, during crystallization, narcissistic self-sorting, which allows the manual separation of enantiomers. A purely organic trefoil knot, obtained by reductive demetallation of its precursor, is also characterized in the solid state by X-ray crystallography.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Crystallographic data for the structures reported in this Article are available from the Cambridge Crystallographic Data Centre with the following codes: TK3-100K (CCDC 2143936), TK3-273K (CCDC 2143937), TK4-100K (CCDC 2143938), TK4-273K (CCDC 2143939), Λ-(+)-TK6-100K (CCDC 2143940), Λ-(+)-TK6-273K (CCDC 2143941), Δ-(−)-TK6-100K (CCDC 2143942), Δ-(−)-TK6-273K (CCDC 2143943), TK4D-100K (CCDC 2143944) and TK7-100K (CCDC 2151636). Other data that support the findings of this study are available in the paper and Supplementary Information.
References
Fielden, S. D. P., Leigh, D. A. & Woltering, S. L. Molecular knots. Angew. Chem. Int. Ed. 56, 11166–11194 (2017).
Stoddart, J. F. Dawning of the age of molecular nanotopology. Nano Lett. 20, 5597–5600 (2020).
Guo, Q.-H., Jiao, Y., Feng, Y. & Stoddart, J. F. The rise and promise of molecular nanotopology. CCS Chem. 3, 1542–1572 (2021).
Forgan, R. S., Sauvage, J.-P. & Stoddart, J. F. Chemical topology: complex molecular knots, links, and entanglements. Chem. Rev. 111, 5434–5464 (2011).
Dietrich-Buchecker, C. O. & Sauvage, J.-P. A synthetic molecular trefoil knot. Angew. Chem. Int. Ed. Engl. 28, 189–192 (1989).
Sauvage, J.-P. Interlacing molecular threads on transition metals: catenands, catenates, and knots. Acc. Chem. Res. 23, 319–327 (1990).
Guo, J., Mayers, P. C., Breault, G. A. & Hunter, C. A. Synthesis of a molecular trefoil knot by folding and closing on an octahedral coordination template. Nat. Chem. 2, 218–222 (2010).
Barran, P. E. et al. Active-metal template synthesis of a molecular trefoil knot. Angew. Chem. Int. Ed. 50, 12488–12492 (2011).
Prakasam, T. et al. Simultaneous self-assembly of a [2]catenane, a trefoil knot, and a Solomon link from a simple pair of ligands. Angew. Chem. Int. Ed. 52, 9956–9960 (2013).
Zhang, G. et al. Lanthanide template synthesis of trefoil knots of single handedness. J. Am. Chem. Soc. 137, 10437–10442 (2015).
Inomata, Y., Sawada, T. & Fujita, M. Metal-peptide torus knots from flexible short peptides. Chem 6, 294–303 (2020).
Leigh, D. A. et al. Tying different knots in a molecular strand. Nature 584, 562–568 (2020).
Carpenter, J. P. et al. Controlling the shape and chirality of an eight-crossing molecular knot. Chem 7, 1534–1543 (2021).
Ashbridge, Z. et al. Vernier template synthesis of molecular knots. Science 375, 1035–1041 (2022).
Segawa, Y. et al. Topological molecular nanocarbons: All-benzene catenane and trefoil knot. Science 365, 272–276 (2019).
Safarowsky, O., Nieger, M., Fröhlich, R. & Vögtle, F. A molecular knot with twelve amide groups—one-step synthesis, crystal structure, chirality. Angew. Chem. Int. Ed. 39, 1616–1618 (2000).
Caprice, K., Pupier, M., Bauzá, A., Frontera, A. & Cougnon, F. B. L. Synchronized On/Off switching of four binding sites for water in a molecular Solomon link. Angew. Chem. Int. Ed. 58, 8053–8057 (2019).
Dang, L.-L., Feng, H.-J., Lin, Y.-J. & Jin, G.-X. Self-assembly of molecular figure-eight knots induced by quadruple stacking interactions. J. Am. Chem. Soc. 142, 18946–18954 (2020).
Ponnuswamy, N., Cougnon, F. B. L., Clough, J. M., Pantoş, G. D. & Sanders, J. K. M. Discovery of an organic trefoil knot. Science 338, 783–785 (2012).
Piguet, C., Bernardinelli, G. & Hopfgartner, G. Helicates as versatile supramolecular complexes. Chem. Rev. 97, 2005–2062 (1997).
Xi, X., Fang, Y., Dong, T. & Cui, Y. Bottom-up assembly from a helicate to homochiral micro- and mesoporous metal-organic frameworks. Angew. Chem. Int. Ed. 50, 1154–1158 (2011).
Gidron, O., Ebert, M.-O., Trapp, N. & Diederich, F. Chiroptical detection of nonchromophoric, achiral guests by enantiopure alleno-acetylenic helicages. Angew. Chem. Int. Ed. 53, 13614–13618 (2014).
Zou, Y.-Q. et al. Sterics and hydrogen bonding control stereochemistry and self-sorting in BINOL-based assemblies. J. Am. Chem. Soc. 143, 9009–9015 (2021).
Siddique, R. G. et al. Controlling the complexity and interconversion mechanisms in self-assembled [Fe2L3]4+ helicates and [Fe4L6]8+ cages. Angew. Chem. Int. Ed. 61, e202115555 (2022).
Ayme, J.-F. et al. Lanthanide template synthesis of a molecular trefoil knot. J. Am. Chem. Soc. 136, 13142–13145 (2014).
Marcos, V. et al. Allosteric initiation and regulation of catalysis with a molecular knot. Science 352, 1555–1559 (2016).
Leigh, D. A., Pritchard, R. G. & Stephens, A. J. A Star of David catenane. Nat. Chem. 6, 978–982 (2014).
Zhang, L. et al. Molecular trefoil knot from a trimeric circular helicate. J. Am. Chem. Soc. 140, 4982–4985 (2018).
Ayme, J.-F. et al. A synthetic molecular pentafoil knot. Nat. Chem. 4, 15–20 (2011).
Danon, J. J. et al. Braiding a molecular knot with eight crossings. Science 355, 159–162 (2017).
Brooker, S. Spin crossover with thermal hysteresis: practicalities and lessons learnt. Chem. Soc. Rev. 44, 2880–2892 (2015).
Senthil Kumar, K. & Ruben, M. Emerging trends in spin crossover (SCO) based functional materials and devices. Coord. Chem. Rev. 346, 176–205 (2017).
Halcrow, M. A. Spin-Crossover Materials: Properties and Applications (John Wiley & Sons, 2013).
Mikolasek, M. et al. Complete set of elastic moduli of a spin-crossover solid: spin-state dependence and mechanical actuation. J. Am. Chem. Soc. 140, 8970–8979 (2018).
Ruben, M., Rojo, J., Romero-Salguero, F. J., Uppadine, L. H. & Lehn, J.-M. Grid-type metal ion architectures: functional metallosupramolecular arrays. Angew. Chem. Int. Ed. 43, 3644–3662 (2004).
Singh, S. & Brooker, S. Correlations between ligand field Δo, spin crossover T1/2 and redox potential Epa in a family of five dinuclear helicates. Chem. Sci. 12, 10919–10929 (2021).
Aleshin, D. Y. et al. Unravelling of a [High Spin—Low Spin] ↔ [Low Spin—High Spin] equilibrium in spin-crossover iron(II) dinuclear helicates using paramagnetic NMR spectroscopy. Angew. Chem. Int. Ed. 61, e202110310 (2021).
Struch, N. et al. An octanuclear metallosupramolecular cage designed to exhibit spin-crossover behavior. Angew. Chem. Int. Ed. 56, 4930–4935 (2017).
Ferguson, A. et al. A face-capped [Fe4L4]8+ spin crossover tetrahedral cage. Chem. Commun. 49, 1597–1599 (2013).
Weselski, M., Książek, M., Mess, P., Kusz, J. & Bronisz, R. ‘Normal’ and ‘reverse’ spin crossover induced by two different structural events in iron(II) coordination polymer. Chem. Commun. 55, 7033–7036 (2019).
Wang, L.-F. et al. Spin-crossover modulation via single-crystal to single-crystal photochemical [2 + 2] reaction in Hofmann-type frameworks. Chem. Sci. 10, 7496–7502 (2019).
Guionneau, P., Marchivie, M., Bravic, G., Létard, J.-F. & Chasseau, D. Structuralaspects of spin crossover. Example of the [FeIILn(NCS)2] complexes. Top. Curr. Chem. 234, 97–128 (2004).
Zhao, L. et al. Switching the magnetic hysteresis of an [FeII–NC–WV]-based coordination polymer by photoinduced reversible spin crossover. Nat. Chem. 13, 698–704 (2021).
Duriska, M. B. et al. A nanoscale molecular switch triggered by thermal, light, and guest perturbation. Angew. Chem. Int. Ed. 48, 2549–2552 (2009).
Tang, M. et al. Molecular-strain engineering of double-walled tetrahedra. Chem 7, 2160–2174 (2021).
Pasteur, L. Mémoire sur la relation qui peut exister entre la forme crystalline et la composition chimique, et sur la cause de la polarisation rotatoire. Ann. Chim. Phys. Sér. 3 24, 442–459 (1848).
Roberts, D. A., Pilgrim, B. S., Sirvinskaite, G., Ronson, T. K. & Nitschke, J. R. Covalent post-assembly modification triggers multiple structural transformations of a tetrazine-edged Fe4L6 tetrahedron. J. Am. Chem. Soc. 140, 9616–9623 (2018).
Askevold, B., Khusniyarov, M. M., Herdtweck, E., Meyer, K. & Schneider, S. A square-planar ruthenium(II) complex with a low-spin configuration. Angew. Chem. Int. Ed. 49, 7566–7569 (2010).
Smith, M. E. & Andersen, R. A. Me5C5Ni(acac): a monomeric, paramagnetic, 18-electron, spin-equilibrium molecule. J. Am. Chem. Soc. 118, 11119–11128 (1996).
Wu, D.-Y., Sato, O., Einaga, Y. & Duan, C.-Y. A spin-crossover cluster of iron(II) exhibiting a mixed-spin structure and synergy between spin transition and magnetic interaction. Angew. Chem. Int. Ed. 48, 1475–1478 (2009).
Bousseksou, A., Molnár, G., Real, J. A. & Tanaka, K. Spin crossover and photomagnetism in dinuclear iron(II) compounds. Coord. Chem. Rev. 251, 1822–1833 (2007).
Acknowledgements
We are grateful for financial support from the National Natural Science Foundation of China (grant nos. 22171232 and 21971211), the Natural Science Foundation of Zhejiang Province (grant no. 2022XHSJJ007), the Qiantang River Talent Foundation (grant no. QJD1902029) and Westlake University. We thank X. Lu and X. Shi, X. Miao, Z. Chen and C. Zhang for their help in recording NMR spectra, X-ray data collection of diffraction dots, CD spectroscopy and magnetic measurement, respectively. We thanks X. Lin and C. Wu for their very helpful discussion on magnetism. This research was supported by both the Instrumentation and Service Center for Molecular Science and the Instrumentation and Service Center for Physical Science, as well as by Westlake University HPC Center. We also thank the staff of the BL17B beamline of National Facility for Protein Science in Shanghai at Shanghai Synchrotron Radiation Facility for assistance during data collection.
Author information
Authors and Affiliations
Contributions
Z.L. and L.W. conceived the idea, designed the research and produced the manuscript. L.W. and Z.L. carried out experiments and analysed the data. L.W. contributed to NMR spectroscopic analysis. L.W., M.T. and L.J. contributed to X-ray crystallographic analyses. Y.C. and J.L. contributed to mass spectrometric analyses. Z.L. is the principal investigator of the Laboratory for Supramolecular Organic Functional Assemblies and supervised the research. L.B., S.W. and Y.L. discussed and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
Z.L. and L.W. are inventors on a Chinese patent application (Application No. CN202210972052.X). The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks Paul Kruger and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Alison Stoddart, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Experimental procedures and characterization data. Supplementary Discussion, Figs. 1–79, Tables 1–19 and refs. 1–18
Supplementary Video 1
A video of the X-ray crystal structure of TK3.
Supplementary Video 2
A video of the X-ray crystal structure of TK4.
Supplementary Video 3
A video of the X-ray crystal structure of TK4D.
Supplementary Video 4
A video of the X-ray crystal structure of TK6.
Supplementary Video 5
A video of the X-ray crystal structure of TK7.
Supplementary Data 1
Crystallographic data for TK3-100K (CCDC 2143936).
Supplementary Data 2
Crystallographic data for TK3-273K (CCDC 2143937).
Supplementary Data 3
Crystallographic data for TK4-100K (CCDC 2143938).
Supplementary Data 4
Crystallographic data for TK4-273K (CCDC 2143939).
Supplementary Data 5
Crystallographic data for TK4D (CCDC 2143944).
Supplementary Data 6
Crystallographic data for Λ-(+)-TK6-100K (CCDC 2143940).
Supplementary Data 7
Crystallographic data for Λ-(+)-TK6-273K (CCDC 2143941).
Supplementary Data 8
Crystallographic data for Δ-(−)-TK6-100K (CCDC 2143942).
Supplementary Data 9
Crystallographic data for Δ-(−)-TK6-273K (CCDC 2143943).
Supplementary Data 10
Crystallographic data for TK7 (CCDC 2151636).
Source data
Source Data Fig. 3
Source data for CD spectra.
Source Data Fig. 5
Source data for VT NMR spectra and VT magnetism.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, L., Tang, M., Jiang, L. et al. Synthesis of contra-helical trefoil knots with mechanically tuneable spin-crossover properties. Nat. Synth 2, 17–25 (2023). https://doi.org/10.1038/s44160-022-00173-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44160-022-00173-7
This article is cited by
-
Synthesis of covalent organic pillars as molecular nanotubes with precise length, diameter and chirality
Nature Synthesis (2023)
-
High yielding stimuli-responsive contra-helical trefoil knots
Science China Chemistry (2023)