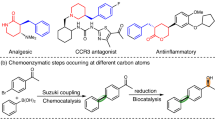
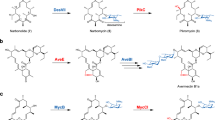
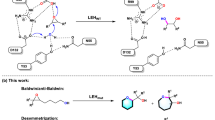
Abstract
Selective oxidation of ring C–H bonds is an attractive route to functionalized cyclic amines, which are versatile intermediates in drug synthesis and important fragment molecules in drug discovery. Here we report a combined substrate and enzyme engineering approach to achieve enantioselective functionalization of all unactivated C–H bonds of azepane, azocane, 7-azabicyclo[2.2.1]heptane and 8-azaspiro[4.5]decane by cytochrome P450BM3 (CYP102A1). Different N-modifying groups provide product diversity at high enantioselectivity (up to 99% e.e.) from a panel of just 48 variants of P450BM3. Substrate docking into molecular-dynamics-simulated structures of enzyme variants is shown to be useful for designing mutations to increase enantioselectivity by disfavouring binding poses leading to the unwanted enantiomer, and to increase enzymatic activity by disfavouring non-productive poses from ten or so variants per generation. The synthetic application of remote C–H activation within cyclic amines is exemplified by the synthesis of anisodamine via enantioselective hydroxylation of N-Boc-nortropinone.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data supporting the findings of this study are available within the paper and the Supplementary Information. Source data are provided with this paper. Crystallographic data for the structures reported in this Article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers CCDC 2159261 ((R)-4b) and 2159262 ((S)-6a). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/.
References
Vitaku, E., Smith, D. T. & Njardarson, J. T. Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among US FDA approved pharmaceuticals. J. Med. Chem. 57, 10257–10274 (2014).
Duke, S. O. et al. Natural toxins for use in pest management. Toxins 2, 1943–1962 (2010).
Murray, C. W. & Rees, D. C. The rise of fragment-based drug discovery. Nat. Chem. 1, 187–192 (2009).
Taylor, R. D., MacCoss, M. & Lawson, A. D. Rings in drugs. J. Med. Chem. 57, 5845–5859 (2014).
Wijdeven, M. A., Willemsen, J. & Rutjes, F. P. J. T. The 3-hydroxypiperidine skeleton: key element in natural product synthesis. Eur. J. Org. Chem. 2010, 2831–2844 (2010).
Nadin, A., Hattotuwagama, C. & Churcher, I. Lead-oriented synthesis: a new opportunity for synthetic chemistry. Angew. Chem. Int. Ed. 51, 1114–1122 (2012).
Vo, C. V. & Bode, J. W. Synthesis of saturated N-heterocycles. J. Org. Chem. 79, 2809–2815 (2014).
Allwood, D. M., Blakemore, D. C., Brown, A. D. & Ley, S. V. Metal-free coupling of saturated heterocyclic sulfonylhydrazones with boronic acids. J. Org. Chem. 79, 328–338 (2014).
Yang, Y., Lan, J. & You, J. Oxidative C–H/C–H coupling reactions between two (hetero)arenes. Chem. Rev. 117, 8787–8863 (2017).
Mitchell, E. A., Peschiulli, A., Lefevre, N., Meerpoel, L. & Maes, B. U. Direct α-functionalization of saturated cyclic amines. Chem. Eur. J. 18, 10092–10142 (2012).
Shaw, M. H., Shurtleff, V. W., Terrett, J. A., Cuthbertson, J. D. & MacMillan, D. W. Native functionality in triple catalytic cross-coupling: sp3 C–H bonds as latent nucleophiles. Science 352, 1304–1308 (2016).
Chen, W., Ma, L., Paul, A. & Seidel, D. Direct α-C–H bond functionalization of unprotected cyclic amines. Nat. Chem. 10, 165–169 (2018).
Lennox, A. J. J. et al. Electrochemical aminoxyl-mediated α-cyanation of secondary piperidines for pharmaceutical building block diversification. J. Am. Chem. Soc. 140, 11227–11231 (2018).
Millet, A., Larini, P., Clot, E. & Baudoin, O. Ligand-controlled β-selective C(sp3)–H arylation of N-Boc-piperidines. Chem. Sci. 4, 2241–2247 (2013).
Millet, A., Dailler, D., Larini, P. & Baudoin, O. Ligand-controlled α- and β-arylation of acyclic N-Boc amines. Angew. Chem. Int. Ed. 53, 2678–2682 (2014).
Topczewski, J. J., Cabrera, P. J., Saper, N. I. & Sanford, M. S. Palladium-catalysed transannular C–H functionalization of alicyclic amines. Nature 531, 220–224 (2016).
Zhang, J., Park, S. & Chang, S. Catalytic access to bridged sila-N-heterocycles from piperidines via cascade sp3 and sp2 C–Si bond formation. J. Am. Chem. Soc. 140, 13209–13213 (2018).
Chen, W., Paul, A., Abboud, K. A. & Seidel, D. Rapid functionalization of multiple C–H bonds in unprotected alicyclic amines. Nat. Chem. 12, 545–550 (2020).
Oeschger, R. et al. Diverse functionalization of strong alkyl C–H bonds by undirected borylation. Science 368, 736–741 (2020).
Trindade, A. F., Faulkner, E. L., Leach, A. G., Nelson, A. & Marsden, S. P. Fragment-oriented synthesis: β-elaboration of cyclic amine fragments using enecarbamates as platform intermediates. Chem. Commun. 56, 8802–8805 (2020).
Holland, H. L., Morris, T. A., Nava, P. J. & Zabic, M. A new paradigm for biohydroxylation by Beauveria bassiana ATCC 7159. Tetrahedron 55, 7441–7460 (1999).
Grogan, G. J. & Holland, H. L. The biocatalytic reactions of Beauveria spp. J. Mol. Catal. B 9, 1–32 (2000).
de Raadt, A., Griengl, H. & Weber, H. The concept of docking and protecting groups in biohydroxylation. Chem. Eur. J. 7, 27–31 (2001).
Sawayama, A. M. et al. A panel of cytochrome P450 BM3 variants to produce drug metabolites and diversify lead compounds. Chem. Eur. J. 15, 11723–11729 (2009).
Reinen, J. et al. Efficient screening of cytochrome P450 BM3 mutants for their metabolic activity and diversity toward a wide set of drug-like molecules in chemical space. Drug Metab. Dispos. 39, 1568–1576 (2011).
Ren, X. et al. Drug oxidation by cytochrome P450BM3: metabolite synthesis and discovering new P450 reaction types. Chem. Eur. J. 21, 15039–15047 (2015).
Fasan, R. Tuning P450 enzymes as oxidation catalysts. ACS Catal. 2, 647–666 (2012).
Roiban, G. D. & Reetz, M. T. Expanding the toolbox of organic chemists: directed evolution of P450 monooxygenases as catalysts in regio- and stereoselective oxidative hydroxylation. Chem. Commun. 51, 2208–2224 (2015).
Wei, Y., Ang, E. L. & Zhao, H. Recent developments in the application of P450 based biocatalysts. Curr. Opin. Chem. Biol. 43, 1–7 (2018).
Urlacher, V. B. & Girhard, M. Cytochrome P450 monooxygenases in biotechnology and synthetic biology. Trends Biotechnol. 37, 882–897 (2019).
Pham, S. Q., Pompidor, G., Liu, J., Li, X. D. & Li, Z. Evolving P450pyr hydroxylase for highly enantioselective hydroxylation at non-activated carbon atom. Chem. Commun. 48, 4618–4620 (2012).
Miura, Y. & Fulco, A. J. ω-1, ω-2 and ω-3 hydroxylation of long-chain fatty acids, amides and alcohols by a soluble enzyme system from Bacillus megaterium. Biochim. Biophys. Acta 388, 305–317 (1975).
Whitehouse, C. J. C., Bell, S. G. & Wong, L. L. P450BM3 (CYP102A1): connecting the dots. Chem. Soc. Rev. 41, 1218–1260 (2012).
Glieder, A., Farinas, E. T. & Arnold, F. H. Laboratory evolution of a soluble, self-sufficient, highly active alkane hydroxylase. Nat. Biotechnol. 20, 1135–1139 (2002).
Reetz, M. T., Bocola, M., Carballeira, J. D., Zha, D. & Vogel, A. Expanding the range of substrate acceptance of enzymes: combinatorial active-site saturation test. Angew. Chem. Int. Ed. 44, 4192–4196 (2005).
Jung, S. T., Lauchli, R. & Arnold, F. H. Cytochrome P450: taming a wild type enzyme. Curr. Opin. Biotechnol. 22, 809–817 (2011).
Kille, S., Zilly, F. E., Acevedo, J. P. & Reetz, M. T. Regio- and stereoselectivity of P450-catalysed hydroxylation of steroids controlled by laboratory evolution. Nat. Chem. 3, 738–743 (2011).
Seifert, A., Antonovici, M., Hauer, B. & Pleiss, J. An efficient route to selective bio-oxidation catalysts: an iterative approach comprising modeling, diversification, and screening, based on CYP102A1. ChemBioChem 12, 1346–1351 (2011).
Zhang, K., El Damaty, S. & Fasan, R. P450 fingerprinting method for rapid discovery of terpene hydroxylating P450 catalysts with diversified regioselectivity. J. Am. Chem. Soc. 133, 3242–3245 (2011).
Zhang, K., Shafer, B. M., Demars, M. D. 2nd, Stern, H. A. & Fasan, R. Controlled oxidation of remote sp3 C–H bonds in artemisinin via P450 catalysts with fine-tuned regio- and stereoselectivity. J. Am. Chem. Soc. 134, 18695–18704 (2012).
Narayan, A. R. et al. Enzymatic hydroxylation of an unactivated methylene C–H bond guided by molecular dynamics simulations. Nat. Chem. 7, 653–660 (2015).
Loskot, S. A., Romney, D. K., Arnold, F. H. & Stoltz, B. M. Enantioselective total synthesis of nigelladine A via late-stage C–H oxidation enabled by an engineered P450 enzyme. J. Am. Chem. Soc. 139, 10196–10199 (2017).
Acevedo-Rocha, C. G. et al. P450-catalyzed regio- and diastereoselective steroid hydroxylation: efficient directed evolution enabled by mutability landscaping. ACS Catal. 8, 3395–3410 (2018).
Li, Y. & Wong, L. L. Multi-functional oxidase activity of CYP102A1 (P450BM3) in the oxidation of quinolines and tetrahydroquinolines. Angew. Chem. Int. Ed. 58, 9551–9555 (2019).
Li, A. et al. Regio- and stereoselective steroid hydroxylation at the C7-position by cytochrome P450 monooxygenase mutants. Angew. Chem. Int. Ed. 59, 12499–12505 (2020).
Chen, W., Fisher, M. J., Leung, A., Cao, Y. & Wong, L. L. Oxidative diversification of steroids by nature-inspired scanning glycine mutagenesis of P450BM3 (CYP102A1). ACS Catal. 10, 8334–8343 (2020).
Paulsen, M. D. & Ornstein, R. L. Dramatic differences in the motions of the mouth of open and closed cytochrome P450BM3 by molecular dynamics simulations. Proteins Struct. Funct. Genet. 21, 237–243 (1995).
Pleiss, J. Systematic analysis of large enzyme families: identification of specificity- and selectivity-determining hotspots. ChemCatChem 6, 944–950 (2014).
Dodani, S. C. et al. Discovery of a regioselectivity switch in nitrating P450s guided by molecular dynamics simulations and Markov models. Nat. Chem. 8, 419–425 (2016).
Capoferri, L. et al. Insights into regioselective metabolism of mefenamic acid by cytochrome P450 BM3 mutants through crystallography, docking, molecular dynamics, and free energy calculations. Proteins Struct. Funct. Bioinf. 84, 383–396 (2016).
Ebert, M. C. C. J. C., Espinola, J. G., Lamoureux, G. & Pelletier, J. N. Substrate-specific screening for mutational hotspots using biased molecular dynamics simulations. ACS Catal. 7, 6786–6797 (2017).
Petrovic, D., Bokel, A., Allan, M., Urlacher, V. B. & Strodel, B. Simulation-guided design of cytochrome P450 for chemo- and regioselective macrocyclic oxidation. J. Chem. Inf. Model. 58, 848–858 (2018).
Li, Z. et al. Engineering cytochrome P450 enzyme systems for biomedical and biotechnological applications. J. Biol. Chem. 295, 833–849 (2020).
Peng, Y. Q. et al. A chemoenzymatic strategy for the synthesis of steroid drugs enabled by P450 monooxygenase-mediated steroidal core modification. ACS Catal. 12, 2907–2914 (2022).
Whitehouse, C. J. C. et al. Evolved CYP102A1 (P450BM3) variants oxidise a range of non-natural substrates and offer new selectivity options. Chem. Commun., 966–968 (2008).
Whitehouse, C. J. C. et al. Structural basis for the properties of two single-site proline mutants of CYP102A1 (P450BM3). ChemBioChem 11, 2549–2556 (2010).
Whitehouse, C. J. C. et al. Structure, electronic properties and catalytic behaviour of an activity-enhancing CYP102A1 (P450BM3) variant. Dalton Trans. 40, 10383–10396 (2011).
Ren, X., O’Hanlon, J. A., Morris, M., Robertson, J. & Wong, L. L. Synthesis of imidazolidin-4-ones via a cytochrome P450-catalyzed intramolecular C–H amination. ACS Catal. 6, 6833–6837 (2016).
O’Hanlon, J. A., Ren, X., Morris, M., Wong, L. L. & Robertson, J. Hydroxylation of anilides by engineered cytochrome P450BM3. Org. Biomol. Chem. 15, 8780–8787 (2017).
Li, C., Liu, Y., Pei, X.-Q. & Wu, Z.-L. Stereo-complementary bioreduction of saturated N-heterocyclic ketones. Process Biochem. 56, 90–97 (2017).
Sheehan, J. C. & Bloom, B. M. The synthesis of teloidinone and 6-hydroxytropinone. J. Am. Chem. Soc. 74, 3825–3828 (1952).
Stoll, A., Becker, B. & Jucker, E. Synthesis of α-hydroxysuccinaldehyde and 3,6-dihydroxytropane. Helv. Chim. Acta 35, 1263–1269 (1952).
Nedenskov, P. & Clauson-Kaas, N. Simplified preparation of 6-hydroxytropinone. Acta Chem. Scand. 8, 1295–1295 (1954).
Yu, Y. T. et al. Effectiveness of anisodamine for the treatment of critically ill patients with septic shock: a multicentre randomized controlled trial. Crit. Care 25, 349 (2021).
Majewski, M. & Lazny, R. Stereoselective synthesis of tropane alkaloids: physoperuvine and dihydroxytropanes. Synlett 785–786 (1996).
Majewski, M., Lazny, R. & Ulaczyk, A. Enantioselective ring opening of tropinone. A new entry into tropane alkaloids. Can. J. Chem. 75, 754–761 (1997).
Cramer, N., Laschat, S., Baro, A. & Frey, W. Enantioselective desymmetrization of tropinone derivatives by hydroboration. Synlett 2175–2177 (2003).
Kulkarni, K., Zhao, A. Y., Purcell, A. W. & Perlmutter, P. The enantioselective total synthesis and unambiguous proof of the absolute stereochemistry of pervilleine C. Synlett 2209–2212 (2008).
Mao, Z. Y., Huang, S. Y., Gao, L. H., Wang, A. E. & Huang, P. Q. A novel and versatile method for the enantioselective syntheses of tropane alkaloids. Sci. China Chem. 57, 252–264 (2014).
Fodor, G. & Kovacs, O. The stereochemistry of the tropane alkaloids. 3. The configuration of scopolamine and of valeroidine. J. Chem. Soc. 2341–2344 (1953).
Cramer, N., Laschat, S. & Baro, A. Enzymatic resolution of tropinone derivatives. Synlett 2178–2181 (2003).
Niu, Y. Y. et al. The absolute configuration plays an important role in muscarinic activity of BGT-A and its analogs. Bioorgan. Med. Chem. 16, 10251–10256 (2008).
Fodor, G. & Soti, F. Correlation of valeroidine with S-(−)-methoxysuccinic acid and of mono-tigloyltropane and ditigloyltropane-3.6-diol with its R-(+)-antimer. Tetrahedron Lett. 1917–1921 (1964).
Munoz, M. A., Munoz, O. & Joseph-Nathan, P. Absolute configuration determination and conformational analysis of (−)-(3S,6S)-3α,6β-diacetoxytropane using vibrational circular dichroism and DFT techniques. Chirality 22, 234–241 (2010).
Fodor, G., Toth, J. & Vincze, I. W. Stereochemistry of tropane alkaloids. 13. The absolute configuration and a simplified syntheses of valeroidine. J. Chem. Soc. 3219–3221 (1961).
Wu, T. et al. Preparative separation of four isomers of synthetic anisodamine by HPLC and diastereomer crystallization. Chirality 31, 11–20 (2019).
Acknowledgements
This work was supported by the Biotechnology and Biological Sciences Research Council, UK (BB/V003445/1). Y.Z. acknowledges a University of Oxford–China Scholarship Council Graduate Scholarship. M.W. and P.H.-L. acknowledge the EPSRC Centre for Doctoral Training in Synthesis for Biology and Medicine (EP/L015838/1) for graduate studentships. For the purpose of open access, the authors have applied a creative commons attribution (CC BY) licence to any author-accepted manuscript version arising.
Author information
Authors and Affiliations
Contributions
Y.Z. performed enzyme and substrate engineering, activity screening, preparative-scale substrate oxidations, product characterization, MD simulations, substrate docking and docking-guided mutagenesis, and wrote the paper. Z.X. performed the stereoselective synthesis of anisodamine and wrote the paper. Y.L., E.J. and P.H.-L. carried out activity screening and product characterization experiments. M.W. performed activity screening, product characterization and steps in the synthesis of anisodamine. K.E.C. determined the crystal structures to assign the absolute configurations of two products and wrote the paper. J.R. conceived and guided the project, designed the synthesis of anisodamine and wrote the paper. L.L.W. conceived and guided the project, designed the steps in IDGM and wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks Rita Bernhardt, Rudi Fasan, Nicholas Turner and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary handling editor Alison Stoddart, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Figs. 1–27, Tables 1–17, product characterization data and NMR spectra.
Supplementary Data 1
Crystallographic data for compound 4b, CCDC 2159261.
Supplementary Data 2
Structure factors for 4b, CCDC 2159261.
Supplementary Data 3
Crystallographic data for compound 6a, CCDC 2159262.
Supplementary Data 4
Structure factors for compound 6a, CCDC 2159262.
Source data
Source Data Fig. 2
Original Ortep figures for crystal structures.
Source Data Fig. 3
Source data for Fig. 3a,b.
Source Data Fig. 3
Original ray-traced figures from Pymol for Fig. 3c–f.
Source Data Fig. 4
Source data for Fig. 4a and Fig. 4b.
Source Data Fig. 4
Original ray-traced figures from Pymol for Fig. 4c,d.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, Y., Xiong, Z., Li, Y. et al. Enantioselective oxidation of unactivated C–H bonds in cyclic amines by iterative docking-guided mutagenesis of P450BM3 (CYP102A1). Nat. Synth 1, 936–945 (2022). https://doi.org/10.1038/s44160-022-00166-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44160-022-00166-6