Abstract
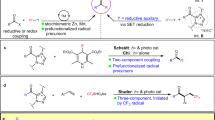
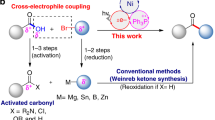
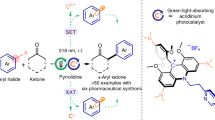
The development of new strategies and concepts towards the synthetic utilization of carbonyl compounds is of pivotal relevance. Nevertheless, the generation of ketyl radicals requires relatively harsh conditions and a large excess of reductants, which represents a long-standing, unsolved problem that hampers the broad application of ketyl coupling processes. Several catalytic approaches have been developed to generate ketyl radicals and successfully applied in reductive cyclizations and two-component cross-coupling reactions. However, catalytic multicomponent reactions that involve a ketyl radical remain rare, but are in high demand owing to their ability to rapidly generate complexity in molecules. Here we report a multicomponent, redox-neutral photocatalytic manifold that combines readily available aldehydes, feedstock 1,3-butadiene and various nucleophiles to build architecturally complex and functionally diverse homoallylic alcohols in one pot. This operationally straightforward method exhibits a wide functional group tolerance, enables the synthesis of drug-like architectures that are not readily accessible by other methods and is applied towards key intermediates of several natural products, which makes the strategy of broad interest in areas such as synthetic and medicinal chemistry.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Materials and methods, detailed optimization studies, experimental procedures, mechanistic studies, DFT calculation details and NMR spectra are available in the Supplementary Information and from the corresponding authors upon reasonable request. NMR spectroscopy data in JCAMP-DX format, Cartesian coordinates of the DFT-optimized structures, step-by-step set-up pictures and ultraviolet–visible and fluorescence spectroscopy data are available at Zenodo under the Creative Commons Attribution 4.0 International license: https://doi.org/10.5281/zenodo.6425457.
References
Wender, P. A. & Miller, B. L. Synthesis at the molecular frontier. Nature 460, 197–201 (2009).
Young, I. S. & Baran, P. S. Protecting-group-free synthesis as an opportunity for invention. Nat. Chem. 1, 193–205 (2009).
Rotstein, B. H., Zaretsky, S., Rai, V. & Yudin, A. K. Small heterocycles in multicomponent reactions. Chem. Rev. 114, 8323–8359 (2014).
Touré, B. B. & Hall, D. G. Natural product synthesis using multicomponent reaction strategies. Chem. Rev. 109, 4439–4486 (2009).
Ganem, B. Strategies for Innovation in multicomponent reaction design. Acc. Chem. Res. 42, 463–472 (2009).
Fazakerley, N. J., Helm, M. D. & Procter, D. J. Total synthesis of (+)-pleuromutilin. Chem. Eur. J. 19, 6718–6723 (2013).
Farney, E. P., Feng, S. S., Schäfers, F. & Reisman, S. E. Total synthesis of (+)-pleuromutilin. J. Am. Chem. Soc. 140, 1267–1270 (2018).
Cha, J. Y., Yeoman, J. T. S. & Reisman, S. E. A concise total synthesis of (−)-maoecrystal Z. J. Am. Chem. Soc. 133, 14964–14967 (2011).
Pan, S. et al. Enantioselective total synthesis of (+)-steenkrotin A and determination of its absolute configuration. Chem. Eur. J. 22, 959–970 (2016).
Classen, M. J., Böcker, M. N. A., Roth, R., Amberg, W. M. & Carreira, E. M. Enantioselective total synthesis of (+)-euphorikanin A. J. Am. Chem. Soc. 143, 8261–8265 (2021).
Roth, H. G., Romero, N. A. & Nicewicz, D. A. Experimental and calculated electrochemical potentials of common organic molecules for applications to single-electron redox chemistry. Synlett 27, 714–723 (2015).
Parsaee, F. et al. Radical philicity and its role in selective organic transformations. Nat. Rev. Chem. 5, 486–499 (2021).
Streuff, J. The electron-way: metal-catalyzed reductive umpolung reactions of saturated and α,β-unsaturated carbonyl derivatives. Synthesis 45, 281–307 (2013).
Molander, G. A. & Harris, C. R. Sequencing reactions with samarium(II) iodide. Chem. Rev. 96, 307–338 (1996).
Szostak, M., Fazakerley, N. J., Parmar, D. & Procter, D. J. Cross-coupling reactions using samarium(II) iodide. Chem. Rev. 114, 5959–6039 (2014).
Prier, C. K., Rankic, D. A. & MacMillan, D. W. C. Visible light photoredox catalysis with transition metal complexes: applications in organic synthesis. Chem. Rev. 113, 5322–5363 (2013).
Yan, M., Kawamata, Y. & Baran, P. S. Synthetic organic electrochemical methods since 2000: on the verge of a renaissance. Chem. Rev. 117, 13230–13319 (2017).
Tarantino, K. T., Liu, P. & Knowles, R. R. Catalytic ketyl–olefin cyclizations enabled by proton-coupled electron transfer. J. Am. Chem. Soc. 135, 10022–10025 (2013).
Rono, L. J., Yayla, H. G., Wang, D. Y., Armstrong, M. F. & Knowles, R. R. Enantioselective photoredox catalysis enabled by proton-coupled electron transfer: development of an asymmetric aza-pinacol cyclization. J. Am. Chem. Soc. 135, 17735–17738 (2013).
Lu, Z., Shen, M. & Yoon, T. P. [3+2] cycloadditions of aryl cyclopropyl ketones by visible light photocatalysis. J. Am. Chem. Soc. 133, 1162–1164 (2011).
Lee, K. N., Lei, Z. & Ngai, M.-Y. β-selective reductive coupling of alkenylpyridines with aldehydes and imines via synergistic Lewis acid/photoredox catalysis. J. Am. Chem. Soc. 139, 5003–5006 (2017).
Ye, C.-X. et al. Dual catalysis for enantioselective convergent synthesis of enantiopure vicinal amino alcohols. Nat. Commun. 9, 410 (2018).
Cao, G.-M. et al. Visible-light photoredox-catalyzed umpolung carboxylation of carbonyl compounds with CO2. Nat. Commun. 12, 3306 (2021).
Qi, L. & Chen, Y. Polarity-reversed allylations of aldehydes, ketones, and imines enabled by Hantzsch ester in photoredox catalysis. Angew. Chem. Int. Ed. 55, 13312–13315 (2016).
Wang, L., Lear, J. M., Rafferty, S. M., Fosu, S. C. & Nagib, D. A. Ketyl radical reactivity via atom transfer catalysis. Science 362, 225–229 (2018).
Rafferty, S. M., Rutherford, J. E., Zhang, L., Wang, L. & Nagib, D. A. Cross-selective aza-pinacol coupling via atom transfer catalysis. J. Am. Chem. Soc. 143, 5622–5628 (2021).
Hu, P. et al. Electroreductive olefin–ketone coupling. J. Am. Chem. Soc. 142, 20979–20986 (2020).
Zhang, X. et al. Reductive arylation of aliphatic and aromatic aldehydes with cyanoarenes by electrolysis for the synthesis of alcohols. Org. Lett. 23, 3472–3476 (2021).
Zhang, S. et al. Electrochemical arylation of aldehydes, ketones, and alcohols: from cathodic reduction to convergent paired electrolysis. Angew. Chem. Int. Ed. 60, 7275–7282 (2021).
Péter, Á., Agasti, S., Knowles, O., Pye, E. & Procter, D. J. Recent advances in the chemistry of ketyl radicals. Chem. Soc. Rev. 50, 5349–5365 (2021).
Xia, Q., Dong, J., Song, H. & Wang, Q. Visible-light photocatalysis of the ketyl radical coupling reaction. Chem. Eur. J. 25, 2949–2961 (2019).
Ulich, L. H. & Adams, R. The reaction between acid halides and aldehydes. III. J. Am. Chem. Soc. 43, 660–667 (1921).
Yang, Z.-P. & Fu, G. C. Convergent catalytic asymmetric synthesis of esters of chiral dialkyl carbinols. J. Am. Chem. Soc. 142, 5870–5875 (2020).
Huang, H.-M., Bellotti, P., Erchinger, J. E., Paulisch, T. O. & Glorius, F. Radical carbonyl umpolung arylation via dual nickel catalysis. J. Am. Chem. Soc. 144, 1899–1909 (2022).
Huang, H.-M., Bellotti, P., Ma, J., Dalton, T. & Glorius, F. Bifunctional reagents in organic synthesis. Nat. Rev. Chem. 5, 301–321 (2021).
Chuentragool, P., Kurandina, D. & Gevorgyan, V. Catalysis with palladium complexes photoexcited by visible light. Angew. Chem. Int. Ed. 58, 11586–11598 (2019).
Juliá, F., Constantin, T. & Leonori, D. Applications of halogen-atom transfer (XAT) for the generation of carbon radicals in synthetic photochemistry and photocatalysis. Chem. Rev. 122, 2292–2352 (2022).
Dahlmann, M., Grub, J. & Löser, E. Butadiene in Ullmann’s Encyclopedia of Industrial Chemistry (Wiley-VCH, 2011).
Huang, H.-M. et al. Catalytic radical generation of π-allylpalladium complexes. Nat. Catal. 3, 393–400 (2020).
Shing Cheung, K. P., Kurandina, D., Yata, T. & Gevorgyan, V. Photoinduced palladium-catalyzed carbofunctionalization of conjugated dienes proceeding via radical-polar crossover scenario: 1,2-aminoalkylation and beyond. J. Am. Chem. Soc. 142, 9932–9937 (2020).
Trost, B. M. & Crawley, M. L. Asymmetric transition-metal-catalyzed allylic alkylations: applications in total synthesis. Chem. Rev. 103, 2921–2944 (2003).
Maekawa, H., Yamamoto, Y., Shimada, H., Yonemura, K. & Nishiguchi, I. Mg-promoted mixed pinacol coupling. Tetrahedron Lett. 45, 3869–3872 (2004).
Kunkely, H. & Vogler, A. Photo-oxidation of bis[1,2-bis(diphenylphosphino)ferrocene]-palladium(0) in CCl4 induced by ferrocene to solvent charge transfer excitation. J. Organomet. Chem. 559, 215–217 (1998).
Koy, M., Bellotti, P., Katzenburg, F., Daniliuc, C. G. & Glorius, F. Synthesis of all-carbon quaternary centers by palladium-catalyzed olefin dicarbofunctionalization. Angew. Chem. Int. Ed. 59, 2375–2379 (2020).
Bellotti, P., Koy, M., Gutheil, C., Heuvel, S. & Glorius, F. Three-component three-bond forming cascade via palladium photoredox catalysis. Chem. Sci. 12, 1810–1817 (2021).
Ladouceur, S., Fortin, D. & Zysman-Colman, E. Enhanced luminescent iridium(III) complexes bearing aryltriazole cyclometallated ligands. Inorg. Chem. 50, 11514–11526 (2011).
Hanss, D., Freys, J. C., Bernardinelli, G. & Wenger, O. S. Cyclometalated iridium(III) complexes as photosensitizers for long-range electron transfer: occurrence of a coulomb barrier. Eur. J. Inorg. Chem. 2009, 4850–4859 (2009).
Uoyama, H., Goushi, K., Shizu, K., Nomura, H. & Adachi, C. Highly efficient organic light-emitting diodes from delayed fluorescence. Nature 492, 234–238 (2012).
Tamayo, A. B. et al. Synthesis and characterization of facial and meridional tris-cyclometalated Iridium(III) complexes. J. Am. Chem. Soc. 125, 7377–7387 (2003).
McIntosh, J. M. 3-Sulfolene in Encyclopedia of Reagents for Organic Synthesis (John Wiley & Sons, Ltd, 2001).
Pitzer, L., Schäfers, F. & Glorius, F. Rapid assessment of the reaction-condition-based sensitivity of chemical transformations. Angew. Chem. Int. Ed. 58, 8572–8576 (2019).
Tsubomura, T. et al. Strongly luminescent palladium(0) and platinum(0) diphosphine complexes. Inorg. Chem. 47, 481–486 (2008).
Kakizoe, D. et al. Photophysical properties of simple palladium(0) complexes bearing triphenylphosphine derivatives. Inorg. Chem. 60, 9516–9528 (2021).
Tsubomura, T. & Sakai, K. Photochemical reactions of palladium(0) and platinum(0) phosphine complexes. Coord. Chem. Rev. 171, 107–113 (1998).
Kancherla, R. et al. Oxidative addition to palladium(0) made easy through photoexcited-state metal catalysis: experiment and computation. Angew. Chem. Int. Ed. 58, 3412–3416 (2019).
Li, F. et al. Photocatalytic generation of π-allyltitanium complexes via radical intermediates. Angew. Chem. Int. Ed. 60, 1561–1566 (2021).
Chen, J. et al. Photoinduced copper-catalyzed asymmetric C–O cross-coupling. J. Am. Chem. Soc. 143, 13382–13392 (2021).
Huang, H.-M. et al. Three-component, interrupted radical Heck/allylic substitution cascade involving unactivated alkyl bromides. J. Am. Chem. Soc. 142, 10173–10183 (2020).
Consiglio, G. & Waymouth, R. M. Enantioselective homogeneous catalysis involving transition-metal-allyl intermediates. Chem. Rev. 89, 257–276 (1989).
Kurandina, D., Parasram, M. & Gevorgyan, V. Visible light-induced room-temperature Heck reaction of functionalized alkyl halides with vinyl arenes/heteroarenes. Angew. Chem. Int. Ed. 56, 14212–14216 (2017).
Wang, G.-Z., Shang, R., Cheng, W.-M. & Fu, Y. Irradiation-induced Heck reaction of unactivated alkyl halides at room temperature. J. Am. Chem. Soc. 139, 18307–18312 (2017).
Zhou, W.-J. et al. Visible-light-driven palladium-catalyzed radical alkylation of C−H bonds with unactivated alkyl bromides. Angew. Chem. Int. Ed. 56, 15683–15687 (2017).
Zhao, G., Yao, W., Mauro, J. N. & Ngai, M.-Y. Excited-state palladium-catalyzed 1,2-spin-center shift enables selective C-2 reduction, deuteration, and iodination of carbohydrates. J. Am. Chem. Soc. 143, 1728–1734 (2021).
Kim, D., Lee, G. S., Kim, D. & Hong, S. H. Direct C(sp2)–H alkylation of unactivated arenes enabled by photoinduced Pd catalysis. Nat. Commun. 11, 5266 (2020).
Luo, Y.-C., Tong, F.-F., Zhang, Y., He, C.-Y. & Zhang, X. Visible-light-induced palladium-catalyzed selective defluoroarylation of trifluoromethylarenes with arylboronic acids. J. Am. Chem. Soc. 143, 13971–13979 (2021).
Huang, H.-M., Bellotti, P. & Glorius, F. Transition metal-catalysed allylic functionalization reactions involving radicals. Chem. Soc. Rev. 49, 6186–6197 (2020).
Huang, H.-M., Bellotti, P., Chen, P.-P., Houk, K. N. & Glorius, F. Allylic C(sp3)–H arylation of olefins via ternary catalysis. Nat. Synth. 1, 59–68 (2022).
Flynn, A. B. & Ogilvie, W. W. Stereocontrolled synthesis of tetrasubstituted olefins. Chem. Rev. 107, 4698–4745 (2007).
Kazmaier, U. Transition Metal Catalyzed Enantioselective Allylic Substitution in Organic Synthesis vol. 38 (Springer, 2012).
Norcott, P. & McErlean, C. S. P. Synthesis of highly enantio-enriched heliespirones A and C by a diastereoselective aromatic Claisen rearrangement. Aust. J. Chem. 71, 366–372 (2018).
Nakajima, N., Uoto, K., Yonemitsu, O. & Hata, T. Facile total synthesis of carbonolides by Witting–Hoener macro-cyclization and stereoselective epoxidation. Chem. Pharm. Bull. 39, 64–74 (1991).
Craig, D. C., Edwards, G. L. & Muldoon, C. A. A stereoselective approach to 2,6-disubstituted tetrahydropyrans by conjugate addition reactions of vinyl sulfones. Synlett 1997, 1318–1320 (1997).
Acknowledgements
We thank the Alexander von Humboldt Foundation and the start-up funding from ShanghaiTech University (H.-M.H.), the Deutsche Forschungsgemeinschaft (Leibniz Award, F.G.; SBF 858, P.B.) and the Institute for Basic Science (IBS-R010-D1, S.K.) of the Republic of Korea for generous financial support. We thank Prof. S. Chang (KAIST) for the kind support of this work, and M.J. Milner, J. E. Erchinger and S. Heuvel (WWU Münster) are acknowledged for experimental assistance.
Author information
Authors and Affiliations
Contributions
H.-M.H. and F.G. conceived the project and supervised the research. H.-M.H. and P.B. performed all of the experiments and analysed all of the data. X.Z. performed the cyclic voltammetry data acquisition and analysis. S.K. performed the DFT calculations. H.-M.H., P.B., S.K. and F.G. co-wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: Peter Seavill, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Sections 1–14, experimental details, Figs. 1–30, Tables 1–11 and NMR spectra.
Rights and permissions
About this article
Cite this article
Huang, HM., Bellotti, P., Kim, S. et al. Catalytic multicomponent reaction involving a ketyl-type radical. Nat. Synth 1, 464–474 (2022). https://doi.org/10.1038/s44160-022-00085-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44160-022-00085-6
This article is cited by
-
Carbonyl cross-metathesis via deoxygenative gem-di-metal catalysis
Nature Chemistry (2024)