Abstract
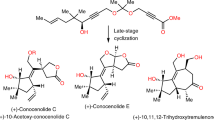
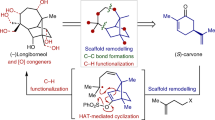
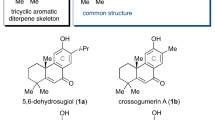
Nature has evolved exquisite synthetic pathways for terpenes by combining isoprenes into chains, folding them into carbocycles and then oxidizing and/or rearranging them into a vast range of complex molecules (>50,000 examples). Although laboratory syntheses can sometimes emulate elements of this process, additional approaches that can similarly emulate nature’s approach and lead to molecular diversity are desirable. Here we show that the synthesis of polycyclic terpene molecules can be achieved through a predictable and reliable process that starts from phenols. The synthetic route comprises three steps: prenylation, dearomatization and/or prenyl migration, and, finally, epoxidation and/or radical cyclization. Critically, the third step uses a cooperative bimetallic catalyst to mediate the cyclization of epoxy enones under hydrogenative conditions. Overall, this approach leads to the stereocontrolled formation of bicyclic-, linear-, angular-, clovane- and propellane-based terpene structures with functional groups that allow further manipulation. For example, these motifs can be repurposed for ring contractions to access additional terpene-based architectures. Of note, several natural products were prepared through a formal total synthesis using this approach; these routes are as concise as, and often shorter than, previously reported syntheses.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Full experimental procedures, compound characterization and spectral data for all the new materials can all be found in the Supplementary Information. Crystallographic data for the 14 structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers CCDC 2117563 (60), 2117564 (74), 2117565 (62), 2117566 (57), 2117567 (13), 2117568 (14), 2117569 (51), 2117570 (Supplementary54), 2117571 (38), 2117572 (66), 2117573 (11), 2117574 (84), 2117575 (12) and 2117576 (Supplementary55). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/.
References
Christianson, D. W. Structural and chemical biology of terpenoid cyclases. Chem. Rev. 117, 11570–11648 (2017).
Fischbach, M. & Clardy, J. One pathway, many products. Nat. Chem. Biol. 3, 353–355 (2007).
Harms, V., Kirschning, A. & Dickschat, J. S. Nature-driven approaches to non-natural terpene analogues. Nat. Prod. Rep. 37, 1080–1097 (2020).
Kanda, Y. et al. Two-phase synthesis of taxol. J. Am. Chem. Soc. 142, 10526–10533 (2020).
Wildermuth, R. et al. A modular synthesis of tetracyclic meroterpenoid antibiotics. Nat. Commun. 8, 2083 (2017).
Maruoka, K., Sato, J., Banno, H. & Yamamoto, H. Organoaluminum-promoted rearrangement of allyl phenyl ethers. Tetrahedron Lett. 31, 377–380 (1990).
Giese, B. Formation of CC bonds by addition of free radicals to alkenes. Angew. Chem. Int. Ed. 22, 753–764 (1983).
Streuff, J. & Gansäuer, A. Metal-catalyzed β-functionalization of Michael acceptors through reductive radical addition reactions. Angew. Chem. Int. Ed. 54, 14232–14242 (2015).
Büschleb, M. et al. Synthetic strategies toward natural products containing contiguous stereogenic quaternary carbon atoms. Angew. Chem. Int. Ed. 55, 4156–4186 (2016).
Morcillo, S. P. et al. Ti(III)-catalyzed cyclizations of ketoepoxypolyprenes: control over the number of rings and unexpected stereoselectivities. J. Am. Chem. Soc. 136, 6943–6951 (2014).
Lo, J. C. et al. Fe-catalyzed C–C bond construction from olefins via radicals. J. Am. Chem. Soc. 139, 2484–2503 (2017).
Hu, P. et al. Quaternary-centre-guided synthesis of complex polycyclic terpenes. Nature 569, 703–707 (2019).
Xia, Z. et al. Re2O7 catalyzed dienone–phenol rearrangement. RSC Adv. 5, 38499–38502 (2015).
Nugent, W. A. & RajanBabu, T. V. Transition-metal-centered radicals in organic synthesis. Titanium(III)-induced cyclization of epoxy olefins. J. Am. Chem. Soc. 110, 8561–8562 (1988).
Zhang, Z., Richrath, R. B. & Gansäuer, A. Merging catalysis in single electron steps with photoredox catalysis-efficient and sustainable radical chemistry. ACS Catal. 9, 3208–3212 (2019).
Gansäuer, A., Bluhm, H. & Pierobon, M. Emergence of a novel catalytic radical reaction: titanocene-catalyzed reductive opening of epoxides. J. Am. Chem. Soc. 120, 12849–12859 (1998).
Rosales, A. et al. Ti(III)-catalyzed, concise synthesis of marine furanospongian diterpenes. RSC Adv. 2, 12922–12925 (2012).
Silva, L. F. Construction of cyclopentyl units by ring contraction reactions. Tetrahedron 58, 9137–9161 (2002).
Li, L., Chen, Z., Zhang, X. & Jia, Y. Divergent strategy in natural product total synthesis. Chem. Rev. 118, 3752–3832 (2018).
Le Bideau, F., Kousara, M., Chen, L., Wei, L. & Dumas, F. Tricyclic sesquiterpenes from marine origin. Chem. Rev. 117, 6110–6159 (2017).
Hung, K., Hu, X. & Maimone, T. J. Total synthesis of complex terpenoids employing radical cascade processes. Nat. Prod. Rep. 35, 174–202 (2018).
Gansäuer, A. & Narayan, S. Titanocene-catalysed electron transfer-mediated opening of epoxides. Adv. Synth. Catal. 344, 465–475 (2002).
Barrero, A. F., Quílez del Moral, J. F., Sánchez, E. M. & Arteaga, J. F. Titanocene-mediated radical cyclization: an emergent method towards the synthesis of natural products. Eur. J. Org. Chem. 7, 1627–1641 (2006).
Justicia, J. et al. Bioinspired terpene synthesis: a radical approach. Chem. Soc. Rev. 40, 3525–3537 (2011).
Saito, S. & Yamamoto, H. Efficient conjugate reduction of α,β-unsaturated carbonyl compounds by complexation with aluminum tris(2,6-diphenylphenoxide). J. Org. Chem. 61, 2928–2929 (1996).
Saito, S. & Yamamoto, H. Designer Lewis acid catalysts—bulky aluminium reagents for selective organic synthesis. Chem. Commun. 1997, 1585–1592 (1997).
Lei, X., Dai, M., Hua, Z. & Danishefsky, S. J. Biomimetic total synthesis of tricycloillicinone and mechanistic studies toward the rearrangement of prenyl phenyl ethers. Tetrahedron Lett. 49, 6383–6385 (2008).
Homer, J. A. et al. Enantioselective para-Claisen rearrangement for the synthesis of illicium-derived prenylated phenylpropanoids. Org. Lett. 23, 3248–3252 (2021).
Guérard, K. C. et al. Oxidative 1,2- and 1,3-alkyl shift processes: developments and applications in synthesis. J. Org. Chem. 77, 2121–2133 (2012).
Hou, H. et al. Total syntheses of the tetracyclic cyclopiane diterpenes conidiogenone, conidiogenol, and conidiogenone B. Angew. Chem. Int. Ed. 55, 4456–4460 (2016).
Maruoka, K., Banno, H. & Yamamoto, H. Enantioselective activation of ethers by chiral organoaluminum reagents: application to asymmetric Claisen rearrangement. Tetrahedron Asymmetry 2, 663–666 (1991).
Hoffmann, R. W. Allylic 1,3-strain as a controlling factor in stereoselective transformations. Chem. Rev. 89, 1841–1860 (1989).
Yao, C., Dahmen, T., Gansäuer, A. & Norton, J. Anti-Markovnikov alcohols via epoxide hydrogenation through cooperative catalysis. Science 364, 764–767 (2019).
Chen, J. et al. Synthesis, characterization, and catalytic activity of bimetallic Ti/Cr complexes. Organometallics 39, 4592–4598 (2020).
Srikrishna, A. & Mahesh, K. An enantiospecific approach to irregular Ligusticum grayi sesquiterpenes: synthesis of cis-preisothapsa-2,8(12)-diene. Synlett 17, 2537–2540 (2011).
Stevens, K. E. & Paquette, L. A. Stereocontrolled total synthesis of (±)-Δ9(12)-capnellene. Tetrahedron Lett. 22, 4393–4396 (1981).
Kutney, J. P. & Chen, Y. H. The chemistry of thujone. XVII. The synthesis of ambergris fragrances and related analogues. Can. J. Chem. 72, 1570–1581 (1994).
Ho, T. S. & Jana, G. H. Total synthesis of 9-isocyanoneopupukeanane. J. Org. Chem. 24, 8965–8967 (1999).
Baker, B. A., Bošković, Ž. V. & Lipshutz, B. H. (BDP)CuH: a ‘hot’ Stryker’s reagent for use in achiral conjugate reductions. Org. Lett. 10, 289–292 (2008).
Qin, T. et al. Nickel-catalyzed Barton decarboxylation and Giese reactions: a practical take on classic transforms. Angew. Chem. Int. Ed. 56, 260–265 (2017).
Curran, D. P. & Shen, W. Tandem transannular radical cyclizations. Total syntheses of (±)-modhephene and (±)-epi-modhephene. Tetrahedron 49, 755–770 (1993).
Schmiedel, V. M. et al. Synthesis and structure revision of dichrocephones A and B. Angew. Chem. Int. Ed. 57, 2419–2422 (2018).
Acknowledgements
We thank A. Filatov, K. Jesse and S. Whitmeyer (University of Chicago) for X-ray analyses of our crystalline intermediates, and J. Kurutz and C. Jin Qin for assistance with the NMR spectroscopy and mass spectrometry, respectively (University of Chicago). This work was supported by funds from the University of Chicago and the NIH (R01-124295A).
Author information
Authors and Affiliations
Contributions
S.A.S. and F.S. conceived the project, and S.A.S. and J.R.N. directed the research. F.S. designed, carried out and analysed all experiments described in the main article, except for those studies on the catalytic approach to the epoxy-enone cyclizations (which were performed by C.Y.). S.A.S. and F.S. composed the manuscript and the Supplementary Information; all the authors commented on the manuscript and the Supplementary Information.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks the anonymous reviewers for their contribution to the peer review of this work. Peter Seavill was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Sections A–T, experimental details, Schemes 1–3, and Tables 1 and 2.
Supplementary Data 1
Crystallographic data of compound 11, CCDC 2117573.
Supplementary Data 2
Crystallographic data of compound 12, CCDC 2117575.
Supplementary Data 3
Crystallographic data of compound 13, CCDC 2117567.
Supplementary Data 4
Crystallographic data of compound 14, CCDC 2117568.
Supplementary Data 5
Crystallographic data of compound 38, CCDC 2117571.
Supplementary Data 6
Crystallographic data of compound 51, CCDC 2117569.
Supplementary Data 7
Crystallographic data of compound 57, CCDC 2117566.
Supplementary Data 8
Crystallographic data of compound 60, CCDC 2117563.
Supplementary Data 9
Crystallographic data of compound 62, CCDC 2117565.
Supplementary Data 10
Crystallographic data of compound 66, CCDC 2117572.
Supplementary Data 11
Crystallographic data of compound 74, CCDC 2117564.
Supplementary Data 12
Crystallographic data of compound 84, CCDC 2117574.
Supplementary Data 13
Crystallographic data of compound S54, CCDC 2117570
Supplementary Data 14
Crystallographic data of compound S55, CCDC 2117576.
Rights and permissions
About this article
Cite this article
Salahi, F., Yao, C., Norton, J.R. et al. The synthesis of diverse terpene architectures from phenols. Nat. Synth 1, 313–321 (2022). https://doi.org/10.1038/s44160-022-00051-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44160-022-00051-2