Abstract
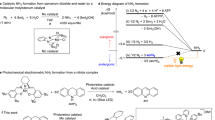
Although metal complexes are known to split dinitrogen at ambient temperature and pressure, the synthesis of ammonia from these compounds with H2 as the terminal reductant is rarely achieved. Here we report a photocatalytic ammonia synthesis from a N2-derived terminal molybdenum nitride by using H2 as the terminal reductant. An iridium hydride photocatalyst mediates the reaction on irradiation with blue light. A molybdenum pentahydride was identified as the principal metal product to arise after ammonia release. Conversion of the molybdenum pentahydride back to the terminal molybdenum nitride was accomplished in three steps and completed a synthetic cycle for NH3 formation from N2 and H2. Mechanistic investigations support a pathway that involves photoexcitation of the iridium hydride and a subsequent energy transfer rather than electron transfer. Deuterium labelling confirmed H2 as the source of the N–H bonds. This photodriven, proton-coupled electron transfer allows the use of H2 as the terminal reductant for the catalytic formation of NH3 from N2 using metal catalysts.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The data that support the findings of this study are included with the article and Supplementary Information. Crystallographic data for the structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers CCDC 2068569 ([(depe)2Mo(O)(F)][BF4]) and CCDC 2068568 ([(depe)2Mo(H)2(MeCN)2][BF4]2). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures.
References
Smil, V. in Enriching the Earth: Fritz Haber, Carl Bosch, and the Transformation of World Food Production (MIT Press, 2008).
Smil, V. Global population and the nitrogen cycle. Sci. Am. 277, 76–81 (1997).
Chen, J. G. et al. Beyond fossil fuel-driven nitrogen transformations. Science 360, eaar6611 (2018).
Elishav, O. et al. Progress and prospective of nitrogen-based alternative fuels. Chem. Rev. 120, 5352–5436 (2020).
Gambarotta, S. Dinitrogen fixation and activation after 30 years: a puzzle still unsolved. J. Organomet. Chem. 500, 117–126 (1995).
Pool, J. A., Lobkovsky, E. & Chirik, P. J. Hydrogenation and cleavage of dinitrogen to ammonia with a zirconium complex. Nature 427, 527–530 (2004).
Reiners, M. et al. NH3 formation from N2 and H2 mediated by molecular tri-iron complexes. Nat. Chem. 12, 740–746 (2020).
Nishibayashi, Y., Iwai, S. & Hidai, M. Bimetallic system for nitrogen fixation: ruthenium-assisted protonation of coordinated N2 on tungsten with H2. Science 279, 540–542 (1998).
Nishibayashi, Y., Takemoto, S., Iwai, S. & Hidai, M. Formation of ammonia in the reactions of a tungsten dinitrogen with ruthenium dihydrogen complexes under mild reaction conditions. Inorg. Chem. 39, 5946–5957 (2000).
Nishibayashi, Y., Wakiii, I., Hirata, K., DuBois, M. R. & Hidai, M. Protonation of coordinated N2 on tungsten with H2 mediated by sulfido-bridged dinuclear molybdenum complexes. Inorg. Chem. 40, 578–580 (2001).
Hidai, M. & Mizobe, Y. Recent advances in the chemistry of dinitrogen complexes. Chem. Rev. 95, 1115–1133 (1995).
Hidai, M., Tominari, K. & Uchida, Y. Preparation and properties of dinitrogen–molybdenum complexes. J. Am. Chem. Soc. 94, 110–114 (1972).
Kim, S., Loose, F. & Chirik, P. J. Beyond ammonia: nitrogen–element bond forming reactions with coordinated dinitrogen. Chem. Rev. 120, 5637–5681 (2020).
Yandulov, D. V. & Schrock, R. R. Catalytic reduction of dinitrogen to ammonia at a single molybdenum center. Science 301, 76–78 (2003).
Schrock, R. R. Catalytic reduction of dinitrogen to ammonia at a single molybdenum center. Acc. Chem. Res. 38, 955–962 (2005).
Tanaka, H., Nishibayashi, Y. & Yoshizawa, K. Interplay between theory and experiment for ammonia synthesis catalyzed by transition metal complexes. Acc. Chem. Res. 49, 987–995 (2016).
Nesbit, M. A., Oyala, P. H. & Peters, J. C. Characterization of the earliest intermediate of Fe–N2 protonation: CW and pulse EPR detection of an Fe–NNH species and its evolution to Fe–NNH2+. J. Am. Chem. Soc. 141, 8116–8127 (2019).
Chalkley, M. J., Del Castillo, T. J., Matson, B. D., Roddy, J. P. & Peters, J. C. Catalytic N2-to-NH3 conversion by Fe at lower driving force: a proposed role for metallocene-mediated PCET. ACS Cent. Sci. 3, 217–223 (2017).
Chalkley, M. J., Oyala, P. H. & Peters, J. C. Cp* noninnocence leads to a remarkably weak C–H bond via metallocene protonation. J. Am. Chem. Soc. 141, 4721–4729 (2019).
Bezdek, M. J., Pappas, I. & Chirik, P. J. in Nitrogen Fixation (ed. Nishibayashi, Y.) 1–22 (Springer, 2017).
Bezdek, M. J. & Chirik, P. J. A fresh approach to synthesizing ammonia from air and water. Nature 568, 464–466 (2019).
Nishibayashi, Y. Recent progress in transition-metal-catalyzed reduction of molecular dinitrogen under ambient reaction conditions. Inorg. Chem. 54, 9234–9247 (2015).
Eizawa, A. & Nishibayashi, Y. Catalytic nitrogen fixation using molybdenum–dinitrogen complexes as catalysts. Top. Organomet. Chem. 60, 153–170 (2017).
Kuriyama, S. & Nishibayashi, Y. in Nitrogen Fixation (ed. Nishibayashi, Y.) 215–234 (Springer, 2017).
MacLeod, K. C. & Holland, P. L. Recent developments in the homogeneous reduction of dinitrogen by molybdenum and iron. Nat. Chem. 5, 559–565 (2013).
Chalkley, M. J., Drover, M. W. & Peters, J. C. Catalytic N2-to-NH3 (or -N2H4) conversion by well-defined molecular coordination complexes. Chem. Rev. 120, 5582–5636 (2020).
Anderson, J. S., Rittle, J. & Peters, J. C. Catalytic conversion of nitrogen to ammonia by an iron model complex. Nature 501, 84–87 (2013).
Creutz, S. E. & Peters, J. C. Catalytic reduction of N2 to NH3 by an Fe–N2 complex featuring a C-atom anchor. J. Am. Chem. Soc. 136, 1105–1115 (2014).
Wise, C. F., Agarwal, R. G. & Mayer, J. M. Determining proton-coupled standard potentials and X–H bond dissociation free energies in nonaqueous solvents using open-circuit potential measurements. J. Am. Chem. Soc. 142, 10681–10691 (2020).
Chalkley, M. J. & Peters, J. C. Relating N–H bond strengths to the overpotential for catalytic nitrogen fixation. Eur. J. Inorg. Chem. 2020, 1353–1357 (2020).
Hetterscheid, D. G. H., Hanna, B. S. & Schrock, R. R. Molybdenum triamidoamine systems. reactions involving dihydrogen relevant to catalytic reduction to dinitrogen. Inorg. Chem. 48, 8569–8577 (2009).
Bezdek, M., Guo, S. & Chirik, P. J. Coordination-induced weakening of ammonia, water, and hydrazine X–H bonds in a molybdenum complex. Science 354, 730–733 (2016).
Laplaza, C. E. & Cummins, C. C. Dinitrogen cleavage by a three-coordinate molybdenum(III) complex. Science 268, 861–863 (1995).
Ashida, Y., Arashiba, K., Nakajima, K. & Nishibayashi, Y. Molybdenum-catalysed ammonia production with samarium diiodide and alcohols or water. Nature 568, 536–540 (2019).
Rebreyend, C. & de Bruin, B. Photolytic N2 splitting: a road to sustainable NH3 production? Angew. Chem. Int. Ed. 54, 42–44 (2015).
Bruch, Q. J. et al. Dinitrogen reduction to ammonium at rhenium utilizing light and proton-coupled electron transfer. J. Am. Chem. Soc. 141, 20198–20208 (2019).
Pappas, I. & Chirik, P. J. Ammonia synthesis by hydrogenolysis of titanium–nitrogen bonds using proton coupled electron transfer. J. Am. Chem. Soc. 137, 3498–3501 (2015).
Pappas, I. & Chirik, P. J. Catalytic proton coupled electron transfer from metal hydrides to titanocene amides, hydrazines and imides: determination of thermodynamic parameters relevant to nitrogen fixation. J. Am. Chem. Soc. 138, 3498–3501 (2016).
Margulieux, G. W., Kim, S. & Chirik, P. J. Determination of the N–H bond dissociation free energy in a pyridine(diimine)molybdenum complex prepared by proton-coupled electron transfer. Inorg. Chem. 59, 15394–15401 (2020).
Forrest, S. J. K., Schluschaß, B., Yuzik-Klimova, E. Y. & Schneider, S. Nitrogen fixation via splitting into nitrido complexes. Chem. Rev. 121, 6522–6587 (2021).
Askevold, B. et al. Ammonia formation by metal–ligand cooperative hydrogenolysis of a nitrido ligand. Nat. Chem. 3, 532–537 (2011).
Schendzielorz, F. S., Finger, M., Volkmann, C., Würtele, C. & Schneider, S. A terminal osmium(IV) nitride: ammonia formation and ambiphilic reactivity. Angew. Chem. Int. Ed. 55, 11417–11420 (2016).
Konnick, M. M., Bischof, S. M., Ess, D. H., Periana, R. A. & Hashiguchi, B. G. Base accelerated generation of N2 and NH3 from an osmium nitride. J. Mol. Catal. A 382, 1–7 (2014).
Kim, S., Zhong, H., Park, Y., Loose, F. & Chirik, P. J. Catalytic hydrogenation of a manganese(V) nitride to ammonia. J. Am. Chem. Soc. 142, 9518–9524 (2020).
Wang, D., Loose, F., Chirik, P. J. & Knowles, R. R. N–H bond formation in a manganese(V) nitride yields ammonia by light-driven proton-coupled electron transfer. J. Am. Chem. Soc. 141, 4995–4799 (2019).
Loose, F. et al. Evaluation of excited state bond weakening for ammonia synthesis from a manganese nitride: stepwise proton coupled electron transfer is preferred over hydrogen atom transfer. Chem. Commun. 55, 5595–5598 (2019).
Schreier, M. R., Pfund, B., Guo, X. & Wenger, O. S. Photo-triggered hydrogen atom transfer from an iridium hydride complex to unactivated olefins. Chem. Sci. 11, 8582–8594 (2020).
Park, Y. et al. Visible light enables catalytic formation of weak chemical bonds with molecular hydrogen. Nat. Chem. 13, 969–976 (2021).
Katayama, A. et al. Dinitrogen–molybdenum complex induces dinitrogen cleavage by one-electron oxidation. Angew. Chem. Int. Ed. 58, 11279–11284 (2019).
Hu, Y. et al. Synthesis, electrochemistry, and reactivity of new iridium(III) and rhodium(III) hydrides. Organometallics 31, 5058–5064 (2012).
Kim, S., Loose, F., Bezdek, M., Wang, X. & Chirik, P. J. Hydrogenation of N-heteroarenes using rhodium precatalysts: reductive elimination leads to formation of multimetallic clusters. J. Am. Chem. Soc. 141, 17900–17908 (2019).
Archer, L. J. & George, T. A. The effect of the wavelength of light upon the extent of dinitrogen exchange in bis(dinitrogen) complexes of molybdenum and tungsten. Inorg. Chim. Acta 44, L129–L132 (1980).
Tshepelevitsh, S. et al. On the basicity of organic bases in different media. Eur. J. Org. Chem. 2019, 6735–6748 (2019).
Cotton, F. A., Eglin, J. L. & Wiesinger, K. J. Synthesis and characterization of molybdenum species: dinuclear and mononuclear species of the molecular formulas [Mo2(O2CCH3)2(LL)2][BF4]2 and [Mo(O)(F)(LL)2][BF4] where LL = bis-phosphine. The use of [Mo2(NCCH3)10][BF4]4 as a source for the [Mo2]4+ core. Inorg. Chim. Acta 195, 11–23 (1992).
Bendix, J. & Bøgevig, A. Synthesis and characterization of a stable trans-dioxo tungsten(IV) complex and series of mono-oxo molybdenum(IV) and tungsten(IV) complexes. Structural and electronic effects of π-bonding in trans-[M(O)(X)(dppe)2]+/0 systems. Inorg. Chem. 37, 5992–6001 (1998).
Wiedner, E. S. et al. Thermodynamic hydricity of transition metal hydrides. Chem. Rev. 116, 8655–8692 (2016).
Barrett, S. M., Pitman, C. L., Walden, A. G. & Miller, A. J. M. Photo-switchable hydride transfer from iridium to 1-methylnicotinamide rationalized by thermochemical cycles. J. Am. Chem. Soc. 136, 14718–14721 (2014).
Pitman, C. L. & Miller, A. J. M. Molecular photoelectrocatalysts for visible light-driven hydrogen evolution from neutral water. ACS Catal. 4, 2727–2733 (2014).
Che, C.-M., Lam, H.-W., Tong, W.-F., Lai, T.-F. & Lau, T.-C. Model reactions for nitrogen fixation. Photo-induced formation and X-ray crystal structure of [Os2(NH3)8(MeCN)2(N2)]5+ from [OsVI(NH3)4N]3+. J. Chem. Soc. Chem. Commun. 1989, 1883–1884 (1989).
Pivovarov, A. P. et al. Photochemical reaction of phosphine hydride complexes of molybdenum and tungsten with molecular nitrogen. Bull. Acad. Sci. USSR Div. Chem. Sci. 30, 947–952 (1981).
Acknowledgements
This research was supported by the US Department of Energy, Office of Science, Office of Basic Energy Sciences, Catalysis Science Program, under award DE-SC0006498 (P.J.C., S.K., Y.P. and J.K.). S.K. thanks Samsung Scholarship for partial financial support.
Author information
Authors and Affiliations
Contributions
S.K. and P.J.C. designed the experiments and prepared the manuscript. S.K. conducted the majority of the experiments. Y.P. contributed to the preparation of iridium hydrides, the detection of ND3 and density functional theory (DFT) computational studies. J.K. contributed reproducibility experiments during revision. T.P.P. collected and solved the crystal structures using data from X-ray diffraction.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks Hideki Masuda and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Peter Seavill, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–48, Tables 1 and 2, discussions and experimental details.
Supplementary Data 1
Crystallographic data of [(depe)2Mo(H)2(MeCN)2][BF4]2, CCDC 2068568.
Supplementary Data 2
Crystallographic data of [(depe)2Mo(O)(F)][BF4], CCDC 2068569.
Rights and permissions
About this article
Cite this article
Kim, S., Park, Y., Kim, J. et al. Ammonia synthesis by photocatalytic hydrogenation of a N2-derived molybdenum nitride. Nat. Synth 1, 297–303 (2022). https://doi.org/10.1038/s44160-022-00044-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44160-022-00044-1
This article is cited by
-
Mechanistic study for efficient nitrogen fixation
Nature Synthesis (2023)
-
Prospects and challenges for nitrogen-atom transfer catalysis
Nature Reviews Chemistry (2023)
-
Prospects and challenges of green ammonia synthesis
Nature Synthesis (2023)
-
Light side of nitrogen fixation
Nature Synthesis (2022)
-
Catalytic nitrogen fixation using visible light energy
Nature Communications (2022)