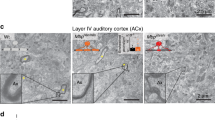
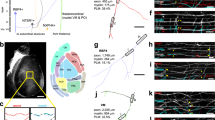
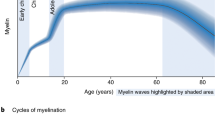
Abstract
Activity-dependent myelination (ADM) is a fundamental dimension of brain plasticity through which myelin changes as a function of neural activity. Mediated by structural changes in glia, ADM notably regulates axonal conduction velocity. Yet, it remains unclear how neural activity impacts myelination to orchestrate the timing of neural signalling, and how ADM shapes neural activity. We developed a model of spiking neurons enhanced with neuron–oligodendrocyte feedback and examined the relationship between ADM and neural activity. We found that ADM implements a homeostatic gain control mechanism that enhances neural firing rates and correlations through the temporal coordination of action potentials as axon lengths increase. Stimuli engage ADM plasticity to trigger bidirectional and reversible changes in conduction delays, as may occur during learning. Furthermore, ADM was found to enhance information transmission under various types of time-varying stimuli. These results highlight the role of ADM in shaping neural activity and communication.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Source data for Figs. 1–5 are available with this manuscript, via ref. 57 and publicly available on GitHub at https://github.com/Jeremie-Lefebvre/Talidou-et-al.-Nat-Comp-Sci-2022.
Code availability
Source codes for all results reported in this study are available with this manuscript via ref. 57 and publicly available on GitHub at https://github.com/Jeremie-Lefebvre/Talidou-et-al.-Nat-Comp-Sci-2022.
References
Deco, G., Jirsa, V., McIntosh, A. R., Sporns, O. & Kötter, R. Key role of coupling, delay, and noise in resting brain fluctuations. Proc. Natl Acad. Sci. USA 106, 10302–10307 (2009).
Dhamala, M., Jirsa, V. K. & Ding, M. Enhancement of neural synchrony by time delay. Phys. Rev. Lett. 92, 074104 (2004).
Cabral, J., Hugues, E., Sporns, O. & Deco, G. Role of local network oscillations in resting state functional connectivity. Neuroimage 57, 130–139 (2011).
Cabral, J., Hugues, E., Kringelbach, M. L. & Deco, G. Modeling the outcome of structural disconnection on resting-state functional connectivity. Neuroimage 62, 1342–1353 (2012).
Gibson, E. M. et al. Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science 344, 1252304 (2014).
Cullen, C. L. et al. Periaxonal and nodal plasticities modulate action potential conduction in the adult mouse brain. Cell Rep. 34, 108641 (2021).
Xin, W. & Chan, J. R. Myelin plasticity: sculpting circuits in learning and memory. Nat. Rev. Neurosci. 21, 682–694 (2020).
Steadman, P. E. et al. Disruption of oligodendrogenesis impairs memory consolidation in adult mice. Neuron 105, 150–164 (2020).
Pan, S., Mayoral, S. R., Choi, H. S., Chan, J. R. & Kheirbek, M. A. Preservation of a remote fear memory requires new myelin formation. Nat. Neurosci. 23, 487–499 (2020).
Stedehouder, J. et al. Local axonal morphology guides the topography of interneuron myelination in mouse and human neocortex. Elife 8, e48615 (2019).
Hughes, E. G., Orthmann-Murphy, J. L., Langseth, A. J. & Bergles, D. E. Myelin remodeling through experience-dependent oligodendrogenesis in the adult somatosensory cortex. Nat. Neurosci. 21, 696–706 (2018).
Auer, F., Vagionitis, S. & Czopka, T. Evidence for myelin sheath remodeling in the CNS revealed by in vivo imaging. Curr. Biol. 28, 549–559 (2018).
Young, K. M. et al. Oligodendrocyte dynamics in the healthy adult CNS: evidence for myelin remodeling. Neuron 77, 873–885 (2013).
Bells, S. et al. Changes in white matter microstructure impact cognition by disrupting the ability of neural assemblies to synchronize. J. Neurosci. 37, 8227–8238 (2017).
Scholz, J., Klein, M. C., Behrens, T. E. J. & Johansen-Berg, H. Training induces changes in white-matter architecture. Nat. Neurosci. 12, 1370–1371 (2009).
Noori, R. et al. Activity-dependent myelination: a glial mechanism of oscillatory self-organization in large-scale brain networks. Proc. Natl Acad. Sci. USA 117, 13227–13237 (2020).
Liu, J. et al. Impaired adult myelination in the prefrontal cortex of socially isolated mice. Nat. Neurosci. 15, 1621–1623 (2012).
Makinodan, M., Rosen, K. M., Ito, S. & Corfas, G. A critical period for social experience-dependent oligodendrocyte maturation and myelination. Science 337, 1357–1360 (2012).
Dutta, D. J. et al. Regulation of myelin structure and conduction velocity by perinodal astrocytes. Proc. Natl Acad. Sci. USA 115, 11832–11837 (2018).
Kato, D., Wake, H. & Lee, P. R. et al. Motor learning requires myelination to reduce asynchrony and spontaneity in neural activity. Glia 68, 193–210 (2020).
Cheng, S. M. & Carr, C. E. Functional delay of myelination of auditory delay lines in the nucleus laminaris of the barn owl. Dev. Neurobiol. 67, 1957–1974 (2007).
Salami, M., Itami, C., Tsumoto, T. & Kimura, F. Change of conduction velocity by regional myelination yields constant latency irrespective of distance between thalamus and cortex. Proc. Natl Acad. Sci. USA 100, 6174–6179 (2003).
Tomassy, G. S. et al. Distinct profiles of myelin distribution along single axons of pyramidal neurons in the neocortex. Science 344, 319–324 (2014).
Morrison, A., Diesmann, M. & Gerstner, W. Phenomenological models of synaptic plasticity based on spike timing. Biol. Cybern. 98, 459–478 (2008).
Seidl, A. H. Regulation of conduction time along axons. Neuroscience 276, 126–134 (2014).
Aboitiz, F., Morales, D. & Montiel, J. The evolutionary origin of the mammalian isocortex: towards an integrated developmental and functional approach. Behav. Brain Sci. 26, 535–552 (2003).
Ma, Z., Turrigiano, G. G., Wessel, R. & Hengen, K. B. Cortical circuit dynamics are homeostatically tuned to criticality in vivo. Neuron 104, 655–664 (2019).
Atay, F. M. Distributed delays facilitate amplitude death of coupled oscillators. Phys. Rev. Lett. 91, 94101 (2003).
McNamara, B. & Wiesenfeld, K. Theory of stochastic resonance. Phys. Rev. A 39, 4854–4869 (1989).
Frank, T. D. Delay Fokker–Planck equations, perturbation theory, and data analysis for nonlinear stochastic systems with time delays. Phys. Rev. E 71, 031106 (2005).
Frank, T. D. Kramers–Moyal expansion for stochastic differential equations with single and multiple delays: applications to financial physics and neurophysics. Phys. Lett. A 360, 552–562 (2007).
Turrigiano, G. Homeostatic synaptic plasticity: local and global mechanisms for stabilizing neuronal function. Cold Spring Harb. Perspect. Biol. 4, a005736 (2012).
Tien, N. W. & Kerschensteiner, D. Homeostatic plasticity in neural development. Neural Dev 13, 9 (2018).
Zohary, E., Shadlen, M. N. & Newsome, W. T. Correlated neuronal discharge rate and its implications for psychophysical performance. Nature 370, 140–143 (1994).
Vyazovskiy, V. V., Olcese, U. & Lazimy, Y. M. et al. Cortical firing and sleep homeostasis. Neuron 63, 865–878 (2009).
Uhlhaas, P. J. & Wolf, S. Neural synchrony in brain disorders: relevance for cognitive dysfunctions and pathophysiology. Neuron 52, 155–168 (2006).
Knowles, J. K., Xu, H. & Soane, C. et al. Maladaptive myelination promotes generalized epilepsy progression. Nat. Neurosci. 25, 596–606 (2022).
Tan, A. Y., Chen, Y., Scholl, B., Seidemann, E. & Priebe, N. J. Sensory stimulation shifts visual cortex from synchronous to asynchronous states. Nature 509, 226–229 (2014).
Beggs, J., Timme, N., Being critical of criticality in the brain. Front. Physiol. 3: 163 (2012).
van Vreeswijk, C. & Sompolinsky, H. Chaos in neuronal networks with balanced excitatory and inhibitory activity. Science 274, 1724–1726 (1996).
Isaacson, J. S. & Scanziani, M. How inhibition shapes cortical activity. Neuron 72, 231–43 (2011).
Bialek, W., de Ruyter van Steveninck, R.R., Rieke, F. & Warland, D. Spikes: Exploring the Neural Code (MIT Press, 1996).
de Ruyter van Steveninck, R. R., Lewen, G. D., Strong, S. P., Koberle, R. & Bialek, W. Reproducibility and variability in neural spike trains. Science 275, 1805–1808 (1997).
Mount, C. W. & Monje, M. Wrapped to adapt: experience-dependent myelination. Neuron 95, 743–756 (2017).
Fields, R. D. A new mechanism of nervous system plasticity: activity-dependent myelination. Nat. Rev. Neurosci. 16, 756–767 (2015).
Watt, A. J. & Desai, N. S. Homeostatic plasticity and STDP: keeping a neuronas cool in a fluctuating world. Front. Synaptic Neurosci. 2, 5 (2010).
Almeida, R. G. & Lyons, D. A. On myelinated axon plasticity and neuronal circuit formation and function. J. Neurosci. 37, 10023–10034 (2017).
Klingseisen, A. & Lyons, D. A. Axonal regulation of central nervous system myelination: structure and function. Neuroscientist 24, 7–21 (2018).
Shadlen, M. N. & Newsome, W. T. The variable discharge of cortical neurons: implications for connectivity, computation, and information coding. J. Neurosci. 18, 3870–3896 (1998).
Hebb D. O. The Organization of Behavior (Wiley, 1949).
Lefebvre, J., Hutt, A., Knebel, J. F., Whittingstall, K. & Murray, M. M. Stimulus statistics shape oscillations in nonlinear recurrent neural networks. J. Neurosci. 35, 2895–2903 (2015).
Hutt, A., Mierau, A. & Lefebvre, J. Dynamic control of synchronous activity in networks of spiking neurons. PLoS ONE 11, e0161488 (2016).
Horsthemke, W., & Lefever, R., Noise-Induced Transitions: Theory and Applications in Physics, Chemistry, and Biology (Springer, 1984).
Tchumatchenko, T., Malyshev, A., Geisel, T., Volgushev, M. & Wolf, F. Correlations and synchrony in threshold neuron models. Phys. Rev. Lett. 104, 058102 (2010).
T. Tchumatchenko, T. Geisel, M. Volgushev, and F. Wolf, Spike correlations – what can they tell about synchrony? Front. Neurosci. 5: 68 (2011).
C., Laing, & G. J. Lord, Stochastic Methods in Neuroscience (Oxford, 2009).
Talidou, A., Frankland, P.W., Mabbott, D., & Lefebvre, J. Homeostatic coordination and up-regulation of neural activity by activity-dependent myelination. Zenodo https://doi.org/10.5281/zenodo.6943969 (2022)
Acknowledgements
We thank the National Research Council of Canada (NSERC grant RGPIN-2017-06662, J.L.) as well as the Canadian Institute of Health Research (CIHR grant NO PJT-156164, P.W.F., D.M. and J.L.) for funding.
Author information
Authors and Affiliations
Contributions
J.L. designed the study. J.L. and A.T. performed the mathematical derivations and simulations. J.L., A.T., P.W.F. and D.M. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Computational Science thanks Maurice Chacron, Thomas R. Knösche and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Handling editor: Ananya Rastogi, in collaboration with the Nature Computational Science team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information
Supplementary discussion, Tables 1 and 2 and Figs. 1–3.
Source data
Source Data Fig. 1
Data for time series plotted.
Source Data Fig. 2
Data for time series, associated metrics and statistics.
Source Data Fig. 3
Statistical and theoretical data.
Source Data Fig. 4
Data for time series, associated metrics and statistics.
Source Data Fig. 5
Data for time series, associated metrics and statistics.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Talidou, A., Frankland, P.W., Mabbott, D. et al. Homeostatic coordination and up-regulation of neural activity by activity-dependent myelination. Nat Comput Sci 2, 665–676 (2022). https://doi.org/10.1038/s43588-022-00315-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s43588-022-00315-z
This article is cited by
-
Alterations in subcortical magnetic susceptibility and disease-specific relationship with brain volume in major depressive disorder and schizophrenia
Translational Psychiatry (2024)
-
The role of ADM in brain function
Nature Computational Science (2022)