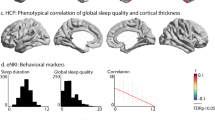
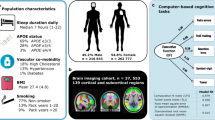
Abstract
Sleep duration, psychiatric disorders and dementias are closely interconnected in older adults. However, the underlying genetic mechanisms and brain structural changes are unknown. Using data from the UK Biobank for participants primarily of European ancestry aged 38–73 years, including 94% white people, we identified a nonlinear association between sleep, with approximately 7 h as the optimal sleep duration, and genetic and cognitive factors, brain structure, and mental health as key measures. The brain regions most significantly underlying this interconnection included the precentral cortex, the lateral orbitofrontal cortex and the hippocampus. Longitudinal analysis revealed that both insufficient and excessive sleep duration were significantly associated with a decline in cognition on follow up. Furthermore, mediation analysis and structural equation modeling identified a unified model incorporating polygenic risk score (PRS), sleep, brain structure, cognition and mental health. This indicates that possible genetic mechanisms and brain structural changes may underlie the nonlinear relationship between sleep duration and cognition and mental health.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
This project corresponds to UK Biobank application ID 19542. Neuroimaging, genotype and behavioral data from the UK Biobank dataset are available at https://biobank.ndph.ox.ac.uk/ by application. The variables used here are detailed in Supplementary Table 1. The previously published GWAS of sleep duration was downloaded from http://www.t2diabetesgenes.org/data/. European ancestral background LD scores from the 1000 Genomes Project were downloaded from https://alkesgroup.broadinstitute.org/LDSCORE/.
Code availability
MATLAB 2018b was used to perform nonlinear association analysis. FreeSurfer version 6.0 was used to process imaging data. PLINK 1.90 and PRSice (http://www.prsice.info) were used to perform genome-wide association analysis and calculate the PRS, respectively. lavaan 0.8 in R version 3.6.0 was used to perform longitudinal and mediation analyses and make the structural equation model. AER 1.2-9 in R version 3.6.0 was used to perform the interaction test; rms 6.2-0 was used to conduct restricted cubic spine analysis; GenomicSEM version 0.0.3 was used to calculate heritability and genetic correlation; two-lines test version 0.52 was used to identify the breakpoints of the nonlinear model. Scripts used to perform the analyses are available at https://github.com/yuzhulineu/UKB_sleep.
Change history
09 May 2022
A Correction to this paper has been published: https://doi.org/10.1038/s43587-022-00230-y
References
Stickgold, R. Sleep-dependent memory consolidation. Nature 437, 1272–1278 (2005).
Walker, M. P. & van der Helm, E. Overnight therapy? The role of sleep in emotional brain processing. Psychol. Bull. 135, 731–748 (2009).
Xie, L. L. et al. Sleep drives metabolite clearance from the adult brain. Science 342, 373–377 (2013).
He, Q., Zhang, P., Li, G. X., Dai, H. X. & Shi, J. P. The association between insomnia symptoms and risk of cardio-cerebral vascular events: a meta-analysis of prospective cohort studies. Eur. J. Prev. Cardiol. 24, 1071–1082 (2017).
Spira, A. P., Chen-Edinboro, L. P., Wu, M. N. & Yaffe, K. Impact of sleep on the risk of cognitive decline and dementia. Curr. Opin. Psychiatry 27, 478–483 (2014).
Sabia, S. et al. Association of sleep duration in middle and old age with incidence of dementia. Nat. Commun. 12, 2289 (2021).
Ikehara, S. et al. Association of sleep duration with mortality from cardiovascular disease and other causes for Japanese men and women: the JACC study. Sleep 32, 295–301 (2009).
Westwood, A. J. et al. Prolonged sleep duration as a marker of early neurodegeneration predicting incident dementia. Neurology 88, 1172–1179 (2017).
Crowley, K. Sleep and sleep disorders in older adults. Neuropsychol. Rev. 21, 41–53 (2011).
Ohayon, M. M., Carskadon, M. A., Guilleminault, C. & Vitiello, M. V. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep 27, 1255–1273 (2004).
Leng, Y. et al. Self-reported sleep patterns in a British population cohort. Sleep Med. 15, 295–302 (2014).
Gulia, K. K. & Kumar, V. M. Sleep disorders in the elderly: a growing challenge. Psychogeriatrics 18, 155–165 (2018).
Irwin, M. R. & Vitiello, M. V. Implications of sleep disturbance and inflammation for Alzheimer’s disease dementia. Lancet Neurol. 18, 296–306 (2019).
Ma, Y. J. et al. Association between sleep duration and cognitive decline. JAMA Netw. Open 3, e2013573 (2020).
Xu, W. et al. Sleep characteristics and cerebrospinal fluid biomarkers of Alzheimer’s disease pathology in cognitively intact older adults: the CABLE study. Alzheimers Dement. 16, 1146–1152 (2020).
Lo, J. C., Loh, K. K., Zheng, H., Sim, S. K. Y. & Chee, M. W. L. Sleep duration and age-related changes in brain structure and cognitive performance. Sleep 37, 1171–1178 (2014).
Kocevska, D. et al. The prospective association of objectively measured sleep and cerebral white matter microstructure in middle-aged and older persons. Sleep 42, zsz140 (2019).
Wu, Y. H. & Swaab, D. F. Disturbance and strategies for reactivation of the circadian rhythm system in aging and Alzheimer’s disease. Sleep Med. 8, 623–636 (2007).
Grumbach, P. et al. Sleep duration is associated with white matter microstructure and cognitive performance in healthy adults. Hum. Brain Mapp. 41, 4397–4405 (2020).
Shi, H. Y. et al. Sleep duration and snoring at midlife in relation to healthy aging in women 70 years of age or older. Nat. Sci. Sleep 13, 411–422 (2021).
El-Sheikh, M., Philbrook, L. E., Kelly, R. J., Hinnant, J. B. & Buckhalt, J. A. What does a good night’s sleep mean? Nonlinear relations between sleep and children’s cognitive functioning and mental health. Sleep 42, zsz078 (2019).
Kendall, K. M. et al. Cognitive performance among carriers of pathogenic copy number variants: analysis of 152,000 UK Biobank subjects. Biol. Psychiatry 82, 103–110 (2017).
Lutsey, P. L. et al. Sleep characteristics and risk of dementia and Alzheimer’s disease: the Atherosclerosis Risk in Communities Study. Alzheimers Dement. 14, 157–166 (2018).
Liang, Y., Qu, L. B. & Liu, H. Non-linear associations between sleep duration and the risks of mild cognitive impairment/dementia and cognitive decline: a dose–response meta-analysis of observational studies. Aging Clin. Exp. Res. 31, 309–320 (2019).
Helfrich, R. F., Mander, B. A., Jagust, W. J., Knight, R. T. & Walker, M. P. Old brains come uncoupled in sleep: slow wave–spindle synchrony, brain atrophy, and forgetting. Neuron 97, 221–230 (2018).
Klinzing, J. G., Niethard, N. & Born, J. Mechanisms of systems memory consolidation during sleep. Nat. Neurosci. 22, 1598–1610 (2019).
Olsson, M., Arlig, J., Hedner, J., Blennow, K. & Zetterberg, H. Sleep deprivation and cerebrospinal fluid biomarkers for Alzheimer’s disease. Sleep 41, zsy025 (2018).
Winer, J. R. et al. Sleep disturbance forecasts β-amyloid accumulation across subsequent years. Curr. Biol. 30, 4291–4298 (2020).
Holth, J. K. et al. The sleep–wake cycle regulates brain interstitial fluid tau in mice and CSF tau in humans. Science 363, 880–884 (2019).
Grandner, M. A. & Kripke, D. F. Self-reported sleep complaints with long and short sleep: a nationally representative sample. Psychosom. Med. 66, 239–241 (2004).
Kroenke, K., Spitzer, R. L. & Williams, J. B. The PHQ-9: validity of a brief depression severity measure. J. Gen. Intern. Med. 16, 606–613 (2001).
Gehrman, P. R. et al. Heritability of insomnia symptoms in youth and their relationship to depression and anxiety. Sleep 34, 1641–1646 (2011).
Gregory, A. M. et al. A longitudinal twin and sibling study of associations between insomnia and depression symptoms in young adults. Sleep 39, 1985–1992 (2016).
Freeman, D., Sheaves, B., Waite, F., Harvey, A. G. & Harrison, P. J. Sleep disturbance and psychiatric disorders. Lancet Psychiatry 7, 628–637 (2020).
Lim, A. S. P. et al. Regional neocortical gray matter structure and sleep fragmentation in older adults. Sleep 39, 227–235 (2016).
Spano, G. et al. Sleeping with hippocampal damage. Curr. Biol. 30, 523–529 (2020).
Fjell, A. M. et al. Self-reported sleep relates to hippocampal atrophy across the adult lifespan: results from the Lifebrain consortium. Sleep 43, zsz280 (2020).
Cheng, W., Rolls, E. T., Ruan, H. T. & Feng, J. F. Functional connectivities in the brain that mediate the association between depressive problems and sleep quality. JAMA Psychiatry 75, 1052–1061 (2018).
Mezick, E. J. et al. Intra-individual variability in sleep duration and fragmentation: associations with stress. Psychoneuroendocrinology 34, 1346–1354 (2009).
Okun, M. L. et al. Sleep variability, health-related practices, and inflammatory markers in a community dwelling sample of older adults. Psychosom. Med. 73, 142–150 (2011).
Vetter, C., Fischer, D., Matera, J. L. & Roenneberg, T. Aligning work and circadian time in shift workers improves sleep and reduces circadian disruption. Curr. Biol. 25, 907–911 (2015).
Mander, B. A., Winer, J. R. & Walker, M. P. Sleep and human aging. Neuron 94, 19–36 (2017).
Musiek, E. S., Xiong, D. D. & Holtzman, D. M. Sleep, circadian rhythms, and the pathogenesis of Alzheimer disease. Exp. Mol. Med. 47, e148 (2015).
Skene, D. J. & Swaab, D. F. Melatonin rhythmicity: effect of age and Alzheimer’s disease. Exp. Gerontol. 38, 199–206 (2003).
Jones, S. E. et al. Genetic studies of accelerometer-based sleep measures yield new insights into human sleep behaviour. Nat. Commun. 10, 1585 (2019).
Fry, A. et al. Comparison of sociodemographic and health-related characteristics of UK Biobank participants with those of the general population. Am. J. Epidemiol. 186, 1026–1034 (2017).
Davis, K. A. S. et al. Mental health in UK Biobank—development, implementation and results from an online questionnaire completed by 157 366 participants: a reanalysis. BJPsych Open 6, e18 (2020).
Desikan, R. S. et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31, 968–980 (2006).
Fischl, B. et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 33, 341–355 (2002).
Bycroft, C. et al. The UK Biobank resource with deep phenotyping and genomic data. Nature 562, 203–209 (2018).
Kang, J. et al. Increased brain volume from higher cereal and lower coffee intake: shared genetic determinants and impacts on cognition and metabolism. Cereb. Cortex https://doi.org/10.1093/cercor/bhac005 (2022).
Purcell, S. et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 81, 559–575 (2007).
Cooper, H. M., Hedges, L. V. & Valentine, J. C. The Handbook of Research Synthesis and Meta-Analysis 3rd edn (Russell Sage Foundation, 2019).
Simonsohn, U. Two lines: a valid alternative to the invalid testing of U-shaped relationships with quadratic regressions. Adv. Methods Pract. Psychol. Sci. 1, 538–555 (2018).
Kleiber, C. & Zeileis, A. Applied Econometrics with R (Springer, 2008).
Acknowledgements
This study used the UK Biobank Resource under application number 19542. We thank all participants and researchers from the UK Biobank. J.F. was supported by the National Key R&D Program of China (nos. 2018YFC1312900 and 2019YFA0709502), the Shanghai Municipal Science and Technology Major Project (no. 2018SHZDZX01), the ZJ Lab, Shanghai Center for Brain Science and Brain-Inspired Technology and the 111 Project (no. B18015). W.C. was supported by grants from the National Natural Sciences Foundation of China (no. 82071997) and the Shanghai Rising-Star Program (no. 21QA1408700).
Author information
Authors and Affiliations
Contributions
J.F. and W.C. proposed the study. Y.L., J.K. and W.Z. analyzed data. S.X. preprocessed data. W.C., J.F. and B.J.S. contributed to interpretation of results. Y.L. drafted the manuscript. B.J.S., C.L., J.Y. and W.C. edited the manuscript. Y.L., C.X. and W.C. contributed to visualization. All authors considered how to analyze data and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Aging thanks Naiara Demnitz, Cathryn Lewis and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Histograms of sleep duration in baseline and imaging assessment.
The sleep duration data from the baseline assessment (2006–2010, n = 498,277) and neuroimaging visit (2014+, n = 48,511) were used in the analyses. Sleep duration assessed at baseline was utilized to determine the association between cognitive function and online follow-up mental health assessments. Sleep duration assessed at the neuroimaging visit was used to determine the association with brain structure.
Extended Data Fig. 2 Covariates utilized in the statistical analyses.
Age, sex, body mass index, Townsend deprivation index, educational qualification, smoking status and drinking status were adjusted in all analyses. In addition, for analysis involving neuroimaging data and polygenetic risk score, intracranial volumes, neuroimaging scanning sites and PRS components were further added as covariates respectively.
Extended Data Fig. 3 Nonlinear association between sleep duration and cortical area and thickness.
Cortical regions with their a) area and b) thickness significantly and nonlinearly associated with sleep duration adjusted for sleep duration with intracranial volume, age, sex, sex, body mass index, Townsend deprivation index, educational qualification, smoking status and drinking status, imaging scanning sites (Bonferroni corrected, p < 0.05). F-tests were utilized to access statistical significance and derive F-statistics and corresponding one-sided p values adjusted for multiple comparisons.
Extended Data Fig. 4 Sex difference of the association between sleep duration and mental health, cognitive function and brain structure.
The nonlinear association between sleep duration and anxiety symptom was more significant in female participants (F female = 622.6, n = 533, 2878, 15240, 36239, 26712, 4712 and 1000 participants respectively; F male = 417.2, n = 347, 2126, 12587, 29710, 18677, 3229 and 665 participants respectively), whereas mania symptoms showed more significant association with sleep duration for male participants (F female = 140.3, n = 550, 2928, 15466, 36672, 26988, 4774 and 1025 participants respectively; F male = 145.0 respectively, n = 354, 2148, 12673, 29893, 18780, 3252 and 670 participants respectively).Fluid intelligence were found to have a greater nonlinear association with sleep duration in females compared with males (F female = 272.7, n = 940, 3981, 16606, 32724, 25625, 5051 and 1502 participants respectively; F male = 205.4, n = 673, 3144, 14934, 29192, 20019, 4018 and 1223 participants respectively) while pair matching were more associated with sleep duration in males (F female = 85.8, n = 3087, 11892, 48704, 98567, 79070, 15934 and 4922 participants respectively; F male = 104.1, n = 2367, 9356, 44236, 88501, 61182, 12315 and 3980 participants respectively). For brain structure, female participants demonstrated a more significant association between sleep duration and cortical volumes (rh, F female = 29.1, n = 192, 991, 4221, 8375, 5746, 1158 and 249 participants respectively; F male = 14.7, n = 118, 631, 3445, 7523, 5592, 1231 and 238 participants respectively) while cortical thickness was more significantly associated with sleep duration for males (F female = 2.89, n = 192, 991, 4221, 8375, 5746, 1158 and 249 participants respectively; F male = 20.0, n = 118, 631, 3445, 7523, 5592, 1231 and 238 participants respectively). Lines are fitted nonlinear model indicating fitted mean value and error bar is standard error of the mean.
Extended Data Fig. 5 Histograms of the change of variables over time in the longitudinal analysis.
Baseline sleep duration is 0.031 hours longer than the follow-up sleep duration (std = 0.94). At baseline, participants were more depressed compared with the measurement at follow-up (difference = 0.0069, std = 0.11). Fluid intelligence of participants at baseline was also higher than at follow-up (difference = 0.043, std = 1.73).
Extended Data Fig. 6 Structural equation model, longitudinal analysis and mediation analysis for participants with more than 7 hours sleep.
a. The longitudinal association between the sleep duration, depression and fluid intelligence revealed by cross-lagged panel model. The baseline sleep duration (β= 0.025, p = 1.3 × 10−5) and depressive symptom (β= −0.023, p = 1.3 × 10−5) was significantly associated with fluid intelligence in the follow-up. b. Mediation analysis. The mediation models were conducted to analyze the direct relationship between sleep duration and fluid intelligence, with sleep duration, brain structure and both of them as mediator respectively. Brain regions significantly mediated the association between sleep duration and fluid intelligence (β= −0.0046, p = 1.4 × 10−5). These figures utilized participants with sleep duration more than 7 hours. c. Full frame model. Standardized coefficients were showed in the figure. PRS was significantly associated with mental health (β = −0.034, p = 4.7 × 10−5). Brain volumes were a better predictor of cognitive function (β= −0.198, p < 1.0 × 10−20) compared to mental health (β = 0.048, p = 3.5 × 10−6). Sleep duration was the most significant predictor of mental health (β = 0.167, p < 1.0 × 10−20) and brain regions (β= −0.044, p < 1.0 × 10−20). Latent variable including brain structure, mental health and cognitive function were estimated in the model which showed in the figure with orange, green and blue box respectively. Wald tests were utilized to derive the two-sided p value adjusted for multiple comparisons (FDR correction). * represented p < 0.05, ** represented p < 0.01 and *** represented p < 0.001.
Extended Data Fig. 7 Mediation analysis.
a. Three mediation analysis were conducted between PRS and sleep duration, 1) PRS →depressive symptoms→brain structure →sleep, 2) PRS →depressive symptoms→sleep, 3) PRS→brain structure →sleep. Depressive symptoms and brain structure serially mediated the association between PRS and sleep duration (β=4.14 × 10−5, p = 0.044). Specifically, with depression significantly associated with PRS (β = −0.033, p = 1.6 × 10−4) and brain volumes positively associated with depression (β = −0.028, p = 1.4 × 10−3), and in addition, brain volumes significantly associated with sleep duration (β = 0.044, p = 1.4 × 10−6). Meanwhile, depressive symptoms and brain structure also separately significantly mediated the association between PRS and sleep duration (β2 = 0.004, p = 3.3 × 10−4, β3 = 0.001, p = 0.01). b. Three mediation pathway analyses were conducted for the cognitive function of fluid intelligence for participants with less than 8 hours sleep duration, 1) sleep duration→brain structure→ depression→ fluid intelligence, 2) sleep duration→ brain structure → fluid intelligence, 3) sleep duration→ depression→ fluid intelligence. Sleep duration showed a significant positive association with fluid intelligence in the model (β = 0.062, p = 2 × 10−15). The serial mediation pathway via brain structure and depression was not significant (β1 = 3 × 10−5, p = 0.06), but brain structure and depression were separately significant mediators for this association. Brain structure accounted for the association between sleep duration and fluid intelligence (β2 = 0.009, p = 2 × 10−7; β3 = 0.004, p = 5.6 × 10−5). Wald tests were utilized to derive the two-sided p value adjusted for multiple comparisons (FDR correction). * represented p < 0.05, ** represented p < 0.01 and *** represented p < 0.001.
Extended Data Fig. 8 Mediation analysis between sleep duration and cognitive functions.
For participants with sleep duration ≤ 7 hours, brain structure related to sleep significantly mediated the association between sleep duration and numeric memory (path β= 0.006, p = 1.4 × 10−11), trail making (path β= −0.003, p = 7.8 × 10−7), prospective memory (path β= −8.8 × 10−4, p = 0.02) and tower rearranging (path β= 0.004, p = 9.5 × 10−9). Meanwhile, sleep duration and brain regions related to sleep significantly mediated the association between PRS of sleep and symbol digit substitution (path β = 1.5 × 10−4, p = 0.001). Specifically, with sleep duration significantly associated with PRS (β = 0.058, p = 4.5 × 10−10) and brain volumes positively associated with sleep duration (β = 0.053, p = 1.1 × 10−8), and in addition, brain volumes significantly associated with symbol digit substitution (β = 0.049, p = 1.1 × 10−7). Sleep duration (β = 0.003, p = 6.3 × 10−5) and brain volumes (β = 0.002, p = 4.2 × 10−3) also separately mediated the association between PRS and symbol digit substitution. The association between these cognitive functions and sleep duration were also significantly mediated by brain structure related to sleep for participants with sleep duration > 7 hours, including symbol digit substitution (path β= −0.002, p = 0.019), numeric memory (path β= −0.003, p = 0.004) and trail making (path β= 0.002, p = 0.017). Reaction time and sleep duration were also mediated by brain structure for participants with sleep duration > 7 hours (path β= 0.001, p = 0.031). Wald tests were utilized to derive the two-sided p value adjusted for multiple comparisons (FDR correction). * represented p < 0.05, ** represented p < 0.01 and *** represented p < 0.001.
Supplementary information
Supplementary Information
Supplementary Figs. 1–8 and Supplementary Tables 1–13
Rights and permissions
About this article
Cite this article
Li, Y., Sahakian, B.J., Kang, J. et al. The brain structure and genetic mechanisms underlying the nonlinear association between sleep duration, cognition and mental health. Nat Aging 2, 425–437 (2022). https://doi.org/10.1038/s43587-022-00210-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s43587-022-00210-2
This article is cited by
-
Shorter self-reported sleep duration is associated with worse virtual spatial navigation performance in men
Scientific Reports (2024)
-
The Impact of Social Media Use on Sleep and Mental Health in Youth: a Scoping Review
Current Psychiatry Reports (2024)
-
Association of sleep duration and risk of mental disorder: a systematic review and meta-analysis
Sleep and Breathing (2024)
-
The brain structure, immunometabolic and genetic mechanisms underlying the association between lifestyle and depression
Nature Mental Health (2023)
-
Mediating role of depressive symptoms on the relationship between sleep duration and cognitive function
Scientific Reports (2023)