Abstract
Cerebral amyloid angiopathy (CAA) is a common disease in older adults that contributes to dementia1,2,3. In CAA, amyloid beta (Aβ) is deposited along either capillaries (type 1) or vessel walls (type 2)4, with the underlying pathophysiology incompletely understood5. Here, we developed imaging and analysis tools based on regularized optimal mass transport (rOMT) theory6,7 to characterize cerebrospinal fluid (CSF) flow dynamics and glymphatic transport in a transgenic CAA type 1 rat model. We discovered that, in CAA, CSF moves more rapidly along the periarterial spaces that serve as influx routes to the glymphatic system. The observation of high-speed CSF flow currents in CAA was unexpected given the build-up of microvascular Aβ. However, velocity flux vector analysis revealed that CSF currents in CAA are partly diverted away from the brain, resulting in overall decreased glymphatic transport. Imaging at the neck showed that drainage to the deep cervical lymph nodes occurs along the carotid arteries and is time delayed in CAA, implying that upstream connections to the meningeal lymphatics were altered. Based on our findings we propose that, in CAA, both glymphatic transport and lymphatic drainage are compromised and that both systems represent therapeutic targets for treatment of CAA-related cognitive decline and dementia.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Statistical source data files depicting the quantification values mentioned in the text or plotted in graphs shown in Figs. 1 and 2 and Extended Data Figs. 3, 5 and 6 are available in the online version of this paper. rOMT processed speed map and Péclet map datasets generated from WT and rTg-DI rats analyzed in the current study are available at https://zenodo.org/record/5809664#.Yczwyy2ZNBw
Code availability
The rOMT code used for analysis of DCE-MRI data is available at https://zenodo.org/record/5809635#.YczwqS2ZNBw. Custom codes used for preprocessing of DCE-MRI datasets for glymphatic analysis are available at https://zenodo.org/record/5809482#.YczwgC2ZNBw
Change history
19 April 2022
A Correction to this paper has been published: https://doi.org/10.1038/s43587-022-00223-x
24 November 2023
A Correction to this paper has been published: https://doi.org/10.1038/s43587-023-00544-5
References
Pfeifer, L. A., White, L. R., Ross, G. W., Petrovitch, H. & Launer, L. J. Cerebral amyloid angiopathy and cognitive function: the HAAS autopsy study. Neurology 58, 1629–1634 (2002).
Matthews, F. E. et al. Epidemiological pathology of dementia: attributable-risks at death in the Medical Research Council Cognitive Function and Ageing Study. PLoS Med. 6, e1000180 (2009).
Banerjee, G. et al. Cognitive impairment before intracerebral hemorrhage is associated with cerebral amyloid angiopathy. Stroke 49, 40–45 (2018).
Thal, D. R. et al. Two types of sporadic cerebral amyloid angiopathy. J. Neuropathol. Exp. Neurol. 61, 282–293 (2002).
Attems, J., Jellinger, K., Thal, D. R. & Van Nostrand, W. Review: sporadic cerebral amyloid angiopathy. Neuropathol. Appl. Neurobiol. 37, 75–93 (2011).
Elkin, R. et al. GlymphVIS: visualizing glymphatic transport pathways using regularized optimal transport. Med. Image Comput. Comput. Assist. Inter. 11070, 844–852 (2018).
Koundal, S. et al. Optimal mass transport with Lagrangian workflow reveals advective and diffusion driven solute transport in the lymphatic system. Sci. Rep. 10, 1990 (2020).
Rustenhoven, J. et al. Functional characterization of the dural sinuses as a neuroimmune interface. Cell 184, 1000–1016 (2021).
Alves de Lima, K., Rustenhoven, J. & Kipnis, J. Meningeal immunity and its function in maintenance of the central nervous system in health and disease. Annu. Rev. Immunol. 38, 597–620 (2020).
Kress, B. T. et al. Impairment of paravascular clearance pathways in the aging brain. Ann. Neurol. 76, 845–861 (2014).
Ma, Q., Ineichen, B. V., Detmar, M. & Proulx, S. T. Outflow of cerebrospinal fluid is predominantly through lymphatic vessels and is reduced in aged mice. Nat. Commun. 8, 1434 (2017).
Da Mesquita, S. et al. Functional aspects of meningeal lymphatics in ageing and Alzheimer’s disease. Nature 560, 185–191 (2018).
Peng, W. et al. Suppression of glymphatic fluid transport in a mouse model of Alzheimer’s disease. Neurobiol. Dis. 93, 215–225 (2016).
Ding, X. B. et al. Impaired meningeal lymphatic drainage in patients with idiopathic Parkinson’s disease. Nat. Med. 27, 411–418 (2021).
Biffi, A. & Greenberg, S. M. Cerebral amyloid angiopathy: a systematic review. J. Clin. Neurol. 7, 1–9 (2011).
Wermer, M. J. H. & Greenberg, S. M. The growing clinical spectrum of cerebral amyloid angiopathy. Curr. Opin. Neurol. 31, 28–35 (2018).
Greenberg, S. M. Cerebral amyloid angiopathy: prospects for clinical diagnosis and treatment. Neurology 51, 690–694 (1998).
Albargothy, N. J. et al. Convective influx/glymphatic system: tracers injected into the CSF enter and leave the brain along separate periarterial basement membrane pathways. Acta Neuropathol. 136, 139–152 (2018).
Carare, R. O. et al. Solutes, but not cells, drain from the brain parenchyma along basement membranes of capillaries and arteries: significance for cerebral amyloid angiopathy and neuroimmunology. Neuropathol. Appl. Neurobiol. 34, 131–144 (2008).
Iliff, J. J. et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci. Transl. Med. 4, 147ra111 (2012).
Nedergaard, M. & Goldman, S. A. Brain drain. Sci. Am. 314, 44–49 (2016).
Davis, J. et al. A novel transgenic rat model of robust cerebral microvascular amyloid with prominent vasculopathy. Am. J. Pathol. 188, 2877–2889 (2018).
Lee, H. et al. Diffuse white matter loss in a transgenic rat model of cerebral amyloid angiopathy. J. Cereb. Blood Flow Metab. 41, 1103–1118 (2020).
Zhu, X., Hatfield, J., Sullivan, J. K., Xu, F. & Van Nostrand, W. E. Robust neuroinflammation and perivascular pathology in rTg-DI rats, a novel model of microvascular cerebral amyloid angiopathy. J. Neuroinflammation 17, 78 (2020).
Iliff, J. J. et al. Cerebral arterial pulsation drives paravascular CSF-interstitial fluid exchange in the murine brain. J. Neurosci. 33, 18190–18199 (2013).
Rennels, M. L., Blaumanis, O. R. & Grady, P. A. Rapid solute transport throughout the brain via paravascular fluid pathways. Adv. Neurol. 52, 431–439 (1990).
Zeppenfeld, D. M. et al. Association of perivascular localization of aquaporin-4 with cognition and Alzheimer disease in aging brains. JAMA Neurol. 74, 91–99 (2017).
Iliff, J. J. et al. Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J. Clin. Invest. 123, 1299–1309 (2013).
Boyle, P. A. et al. Cerebral amyloid angiopathy and cognitive outcomes in community-based older persons. Neurology 85, 1930–1936 (2015).
Esiri, M. et al. Cerebral amyloid angiopathy, subcortical white matter disease and dementia: literature review and study in OPTIMA. Brain Pathol. 25, 51–62 (2015).
Hughes, T. M. et al. Arterial stiffness and dementia pathology: Atherosclerosis Risk in Communities (ARIC)-PET Study. Neurology 90, e1248–e1256 (2018).
Riba-Llena, I. et al. Arterial stiffness is associated with basal ganglia enlarged perivascular spaces and cerebral small vessel disease load. Stroke 49, 1279–1281 (2018).
Benveniste, H. & Nedergaard, M. Cerebral small vessel disease: a glymphopathy? Curr. Opin. Neurobiol. 72, 15–21 (2021).
Deane, R. et al. LRP/amyloid beta-peptide interaction mediates differential brain efflux of Abeta isoforms. Neuron 43, 333–344 (2004).
Tarasoff-Conway, J. M. et al. Clearance systems in the brain-implications for Alzheimer disease. Nat. Rev. Neurol. 11, 457–470 (2015).
Benveniste, H. et al. Anesthesia with dexmedetomidine and low-dose isoflurane increases solute transport via the glymphatic pathway in rat brain when compared with high-dose isoflurane. Anesthesiology 127, 976–988 (2017).
Hablitz, L. M. et al. Increased glymphatic influx is correlated with high EEG delta power and low heart rate in mice under anesthesia. Sci. Adv. 5, eaav5447 (2019).
Lee, H. et al. The effect of body posture on brain glymphatic transport. J. Neurosci. 35, 11034–11044 (2015).
Hablitz, L. M. et al. Circadian control of brain glymphatic and lymphatic fluid flow. Nat. Commun. 11, 4411 (2020).
Wang, M. X., Ray, L., Tanaka, K. F., Iliff, J. J. & Heys, J. Varying perivascular astroglial endfoot dimensions along the vascular tree maintain perivascular-interstitial flux through the cortical mantle. Glia 69, 715–728 (2021).
Szentistvanyi, I., Patlak, C. S., Ellis, R. A. & Cserr, H. F. Drainage of interstitial fluid from different regions of rat brain. Am. J. Physiol. 246, F835–F844 (1984).
Yeo, K. P. et al. Efficient aortic lymphatic drainage is necessary for atherosclerosis regression induced by ezetimibe. Sci. Adv. 6, eabc2697 (2020).
Yagmurlu, K. et al. Anatomical features of the deep cervical lymphatic system and intrajugular lymphatic vessels in humans. Brain Sci. 10, 953 (2020).
Kim, M. J. et al. Comparing the organs and vasculature of the head and neck in five murine species. Vivo 31, 861–871 (2017).
Szabo, K. The cranial venous system in the rat: anatomical pattern and ontogenetic development. II. Dorsal drainage. Ann. Anat. 177, 313–322 (1995).
Weller, R. O., Subash, M., Preston, S. D., Mazanti, I. & Carare, R. O. Perivascular drainage of amyloid-beta peptides from the brain and its failure in cerebral amyloid angiopathy and Alzheimer’s disease. Brain Pathol. 18, 253–266 (2008).
Aspelund, A. et al. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J. Exp. Med. 212, 991–999 (2015).
Louveau, A. et al. Structural and functional features of central nervous system lymphatic vessels. Nature 523, 337–341 (2015).
Antila, S. et al. Development and plasticity of meningeal lymphatic vessels. J. Exp. Med. 214, 3645–3667 (2017).
Benveniste, H. et al. Glymphatic cerebrospinal fluid and solute transport quantified by MRI and PET imaging. Neuroscience 474, 63–79 (2021).
Bolinger, L., Prammer, M. G. & Leigh, J. S. A multiple-frequency coil with a highly uniform B1 field. J. Magn. Reson. 81, 162–166 (1989).
Tustison, N. J. et al. N4ITK: improved N3 bias correction. IEEE Trans. Med. Imaging 29, 1310–1320 (2010).
Koundal, S. et al. Brain morphometry and longitudinal relaxation time of spontaneously hypertensive rats (SHRs) in early and intermediate stages of hypertension investigated by 3D VFA-SPGR MRI. Neuroscience 404, 14–26 (2019).
Papp, E. A., Leergaard, T. B., Calabrese, E., Johnson, G. A. & Bjaalie, J. G. Waxholm space atlas of the Sprague Dawley rat brain. Neuroimage 97, 374–386 (2014).
Mortensen, K. N. et al. Impaired glymphatic transport in spontaneously hypertensive rats. J. Neurosci. 39, 6365–6377 (2019).
Acknowledgements
We thank P. Brown (Magnetic Resonance Research Center) at Yale University for coil development and support. We also thank L. Zhao for editing our manuscript. This work was supported by a grant from the National Institutes of Health/National Institute on Aging (no. AG053991 to H.B., A.T. and W.E.V.N.), Cure Alzheimer’s Fund and A.T. acknowledges support from AFOSR grant FA955-20-1-0029.
Author information
Authors and Affiliations
Contributions
X.C., R.E. and A.T. designed all the computational fluid dynamics algorithms based on regularized optimal mass transport, performed all rOMT analysis of the data and wrote the rOMT Supplementary Methods. X.L. and S.K. performed all brain glymphatic MRI experiments and morphometric analysis of brain data. S.K. and H.B. designed, performed and analyzed all MRI experiments on lymph node drainage. X.Z., B.M. and F.X. performed immunohistochemistry and assisted with blinded data analysis and quantifications. F.D. assisted with statistical design and data analysis. M.P. assisted with analysis of LN time activity data. H.L. assisted with quantitative MRI data analysis and helped design and perform initial glymphatic whole-brain experiments. J.K. provided intellectual contribution and interpretation of lymph node data, and participated in manuscript writing. A.T. designed the mathematical rOMT framework, provided intellectual contribution, oversaw rOMT data analysis and contributed to writing the manuscript. W.E.V.N. designed the biological components of the experiments with H.B., created the rTg-DI rat model and supplied this strain and WT littermates for the study; and provided intellectual contributions and participated in manuscript writing. H.B. designed all experiments, oversaw data analysis and interpretation and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
H.B. received research support from PureTech. J.K. is a member of the scientific advisory group for PureTech. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Aging thanks Steve Greenberg, Bryn Martin, Douglas Kelley and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
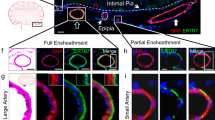
Extended Data Fig. 1 Progressive accumulation of microvascular amyloid and astrocytes in rTg-DI rats.
Brain sections from ventral hippocampus from (a) 3months (M), (b) 6 M and (c) 12 M wild-type rats and age-matched rTg-DI rats (d-f). The brain sections were labeled with Amylo-Glo to detect fibrillar amyloid (blue), rabbit polyclonal antibody to detect cerebral microvessels (red), and goat polyclonal antibody to GFAP to identify astrocytes (green). Scale bars = 50 µm. Note that an increased number of perivascular astrocytes is evident in rTg-DI rats as early as 3 M. This experiment was independently repeated twice with similar results.
Extended Data Fig. 2 Increased perivascular microglia in rTg-DI rats.
Brain sections from ventral hippocampus of (a) 3-month (M), (b) 6 M and (c) 12 wild-type rats and age-matched rTg-DI rats (d-f). The brain sections were labeled with Amylo-Glo to detect fibrillar amyloid (blue), rabbit polyclonal antibody to detect cerebral microvessels (red), and goat polyclonal antibody to Iba-1 to identify microglia (green). Scale bars = 50 µm. Note that increased number of microglia cells are evident in rTg-DI rats as early as 3 M. This experiment was independently repeated twice with similar results.
Extended Data Fig. 3 CSF and tissue volume changes across age and strain.
a Graph with quantification of CSF compartment volumes of the 3-month, (M) 6 M and 12 M WT (light blue bars) cohort and corresponding rTg-DI rat cohorts (blue bars). Each dot above the bar represents the value obtained from one rat. Note: WT n = 9, 10, 8 at 3,6 and 12 months; rTg-DI n = 9, 9, 10 at 3, 6, and 12 months, respectively from 3 independent experiments. Data are mean ± s.e.m. Statistical analysis with two-way ANOVA with independent variables including strain (rTg-DI vs WT rats), time (age: 3, 6, 12 M) and the time x strain interaction were fit to compare the mean differences of different outcomes between rTg-DI and WT rats, between different time points within each strain of rats. A p-value of less than 0.05 was chosen to indicate statistical significance and no adjustment of multiple testing was considered. **p-value = 0.004. b Graph with quantification tissue compartment volumes of the 3 M 6 M and 12 M WT (light blue bars) and rTg-DI rat (blue bars) cohorts. Each dot above the bar represents the value obtained from one rat. Note: WT n = 9, 10, 8 at 3,6 and 12 months; rTg-DI n = 9, 9, 10 at 3, 6, and 12 months, respectively from 3 independent experiments. Data are mean ± s.e.m. Statistical analysis same as in b. *p-value = 0.038, **p-value = 0.021, ***p-value = 0.015.
Extended Data Fig. 4 Significantly greater spatial distribution of glymphatic transport observed in WT compared to rTg-DI rats.
a Spatially normalized population averaged color coded speed maps of 12-month (M) old WT (N = 8) and 12 M rTg-DI (N = 10) rats are shown overlaid onto population averaged proton density weighted anatomical MRI brain templates. b For the 12 M WT (N = 8) and 12 M rTg-DI (N = 10) cohorts, statistical parametric maps (color coded for p-values) were calculated at p-value < 0.05 and overlaid onto the MRI brain images to display anatomical areas with significantly more speed in WT rats in comparison to rTg-DI rats or the reverse comparison. Note that the p-value map is uncorrected via the false-discovery rate procedure. Scale bars = 2 mm. Anatomical levels of the axially displayed anatomical templates are given by their nearest Bregma distance. L-HypoT = left hypothalamus; Thal = thalamus; vHip = ventral hippocampus; GN = geniculate nucleus; R-Ctx = retro-splenial cortex; O-Ctx = Occipital cortex. Scale bar = 3 mm.
Extended Data Fig. 5 Heart rate changes across age and strains.
a Graph with quantification of the mean heart rate recorded of the anesthetized rats during MRI imaging from 3-month (M) 6 M and 12 M WT (light blue bars) and age-matched rTg-DI rats (blue bars). Each dot above the bar represents the mean heart rate recorded over the 2–3 h imaging period from one rat. Data are mean ± s.e.m. Note: WT n = 9, 10, 8 animals examined at 3,6 and 12 M, respectively, as independent experiments; rTg-DI n = 9, 9, 10 animals examined at 3,6 and 12 M, respectively, as independent experiments. Statistical analysis with two-way ANOVA with independent variables including strain (rTg-DI vs WT rats), time (age: 3, 6, 12 M) and the time x strain interaction were fit to compare the mean differences of different outcomes between rTg-DI and WT rats, between different time points within each strain of rats. A p-value of less than 0.05 was chosen to indicate statistical significance and no adjustment of multiple testing was considered. *p-value = 0.030, **p-value = 0.013, ****p-value < 0.0001.
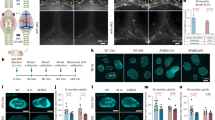
Extended Data Fig. 6 Perivascular AQP4 polarization of capillaries is impaired with evolving CAA.
Changes in APQ4 localization was evaluated in WT and CAA rats by immunofluorescence. a-c: Representative slices at the level of the ventral hippocampus from 3 M (a), 6 M (b) and 12 M (c) WT rats showing strong perivascular AQP4 expression and localization across all age cohorts. d-f Corresponding brain slices at the level from age-matched rTg-DI rats demonstrating that the localization of perivascular AQP4 changes with evolving CAA pathology and is down-regulated in relation to the vasculature resulting in higher tissue ‘background’ AQP4 expression in 6 M (e) and 12 M (f) rTg-DI rats in comparison to 3 M rTg-DI rats (D). Scale bar = 500 μ g-i: Graphs of quantification of AQP4 expression in perivascular domains surrounding capillaries in WT and rTg-DI rats. At 3 M there are no differences in perivascular AQP4 expression across the strains (g), however, at 6 M and 12 M the polarization index is decreased in rTg-DI compared to WT inferring more dispersed expression away from the capillary (h, i). Each dot represents the polarization from one capillary in the ventral hippocampus, with n = 20 capillaries/rat and n = 4 for 3 M and 6 M groups and 20capillaries/rat and n = 5 for the 12 M group from three independent experiments. Horizontal bars indicate mean ± s.e.m.; two-tailed Mann-Whitney U test. *p-value < 0.05, ****p-value < 0.0001.
Extended Data Fig. 7 Drainage from CNS to cervical lymph nodes in normal SD rats.
a Graphs of time signal changes in individual right-sided (dashed blue lines) and left-sided (blue lines) deep cervical lymph nodes (dcLN) derived from independent experiments of n = 6 normal Sprague Dawley (SD) rats. dcLN data from one rat was excluded to excessive vascular motion artefacts. Blue line indicates the mean peak time. b Corresponding graphs of time signal changes in individual right-sided (dashed magenta lines) and left-sided (magenta lines) parotid lymph nodes from the same cohort of normal SD rats. c Corresponding graphs of the time signal changes observed in the submandibular cervical lymph nodes (average of 2-3 nodes/rat) from the same cohort of normal SD rats. d-f: Velocity flux vectors – color coded for magnitude – from three different SD rats overlaid onto anatomical masks of the carotid arteries and dcLN showing the direction of solute drainage along the external carotid artery and within the carotid bifurcation towards the dcLN (black boxes). Scale bars = 1 mm.
Supplementary information
Supplementary Information
Supplementary Figs. 1–4 and legends.
Source data
Source Data Fig. 1.
Statistical source data.
Source Data Fig. 2.
Statistical source data.
Source Data Extended Data Fig. 3.
Statistical source data.
Source Data Extended Data Fig. 5.
Statistical source data.
Source Data Extended Data Fig. 6.
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, X., Liu, X., Koundal, S. et al. Cerebral amyloid angiopathy is associated with glymphatic transport reduction and time-delayed solute drainage along the neck arteries. Nat Aging 2, 214–223 (2022). https://doi.org/10.1038/s43587-022-00181-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s43587-022-00181-4
This article is cited by
-
Unbalanced regularized optimal mass transport with applications to fluid flows in the brain
Scientific Reports (2024)
-
Identification of direct connections between the dura and the brain
Nature (2024)
-
Border-associated macrophages promote cerebral amyloid angiopathy and cognitive impairment through vascular oxidative stress
Molecular Neurodegeneration (2023)
-
Current views on meningeal lymphatics and immunity in aging and Alzheimer’s disease
Molecular Neurodegeneration (2023)
-
The pitfalls of interpreting hyperintense FLAIR signal as lymph outside the human brain
Nature Communications (2023)