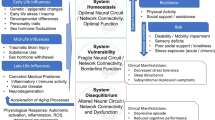
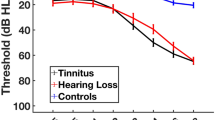
Abstract
Substantial evidence now links age-related hearing loss to incident major depressive disorder in older adults. However, research examining the neural circuits and behavioral mechanisms by which age-related hearing loss leads to depression is at an early phase. It is known that hearing loss has adverse structural and functional brain consequences, is associated with reduced social engagement and loneliness, and often results in tinnitus, which can independently affect cognitive control and emotion processing circuits. While pathways leading from these sequelae of hearing loss to affective dysregulation and depression are intuitive to hypothesize, few studies have yet been designed to provide conclusive evidence for specific pathophysiological mechanisms. Here we review the neurobiological and behavioral consequences of age-related hearing loss, present a model linking them to increased risk for major depressive disorder and suggest how future studies may facilitate the development of rationally designed therapeutic interventions for older adults with impaired hearing to reduce risk for depression and/or ameliorate depressive symptoms.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Change history
21 February 2021
In the version of this Review originally published, there was an erroneous empty box at the top left of Fig. 2; this has now been removed.
References
Livingston, G. et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 396, 413–446 (2020).
Lawrence, B. J. et al. Hearing loss and depression in older adults: a systematic review and meta-analysis. Gerontologist 60, e137–e154 (2020).
Brewster, K. K. et al. Age-related hearing loss and its association with depression in later life. Am. J. Geriatr. Psychiatry 26, 788–796 (2018).
Brewster, K. K. et al. Age-related hearing loss, late-life depression, and risk for incident dementia in older adults. J. Gerontol. A Biol. Sci. Med. Sci. https://doi.org/10.1093/gerona/glaa242 (2020).
Golub, J. S. et al. Association of audiometric age-related hearing loss with depressive symptoms among Hispanic individuals. JAMA Otolaryngol. Head Neck Surg. 145, 132–139 (2019).
Bigelow, R. T. et al. Association of hearing loss with psychological distress and utilization of mental health services among adults in the United States. JAMA Netw. Open 3, e2010986 (2020).
Kim, S. Y., Min, C., Lee, C. H., Park, B. & Choi, H. G. Bidirectional relation between depression and sudden sensorineural hearing loss: two longitudinal follow-up studies using a national sample cohort. Sci. Rep. 10, 1482 (2020).
Shukla, A. et al. Hearing loss, hearing aid use, and depressive symptoms in older adults—findings from the atherosclerosis risk in communities neurocognitive study (ARIC-NCS). J. Gerontol. B Psychol. Sci. Soc. Sci. 76, 518–523 (2021).
Golub, J. S. et al. Subclinical hearing loss is associated with depressive symptoms. Am. J. Geriatr. Psychiatry 28, 545–556 (2020).
Brewster, K. K. et al. A pilot randomized controlled trial of hearing aids to improve mood and cognition in older adults. Int. J. Geriatr. Psychiatry 35, 842–850 (2020).
Chien, W. & Lin, F. R. Prevalence of hearing aid use among older adults in the United States. Arch. Intern. Med. 172, 292–293 (2012).
Hardy, C. J. et al. Hearing and dementia. J. Neurol. 263, 2339–2354 (2016).
Slade, K., Plack, C. J. & Nuttall, H. E. The effects of age-related hearing loss on the brain and cognitive function. Trends Neurosci. 43, 810–821 (2020).
Wayne, R. V. & Johnsrude, I. S. A review of causal mechanisms underlying the link between age-related hearing loss and cognitive decline. Ageing Res. Rev. 23, 154–166 (2015).
van Agtmaal, M. J. M., Houben, A., Pouwer, F., Stehouwer, C. D. A. & Schram, M. T. Association of microvascular dysfunction with late-life depression: a systematic review and meta-analysis. JAMA Psychiatry 74, 729–739 (2017).
Diniz, B. S. et al. Oxidative stress markers imbalance in late-life depression. J. Psychiatr. Res. 102, 29–33 (2018).
Fetoni, A. R., Picciotti, P. M., Paludetti, G. & Troiani, D. Pathogenesis of presbycusis in animal models: a review. Exp. Gerontol. 46, 413–425 (2011).
Eckert, M. A., Vaden, K. I. Jr. & Dubno, J. R. Age-related hearing loss associations with changes in brain morphology. Trends Hear. https://doi.org/10.1177/2331216519857267 (2019).
Picciotti, P. et al. Age-dependent modifications of expression level of VEGF and its receptors in the inner ear. Exp. Gerontol. 39, 1253–1258 (2004).
Kalinec, G. M., Lomberk, G., Urrutia, R. A. & Kalinec, F. Resolution of cochlear inflammation: novel target for preventing or ameliorating drug-, noise- and age-related hearing loss. Front. Cell Neurosci. 11, 192 (2017).
Hegeman, J. M., Kok, R. M., van der Mast, R. C. & Giltay, E. J. Phenomenology of depression in older compared with younger adults: meta-analysis. Br. J. Psychiatry 200, 275–281 (2012).
Lin, F. R. et al. Association of hearing impairment with brain volume changes in older adults. Neuroimage 90, 84–92 (2014).
Peelle, J. E., Troiani, V., Grossman, M. & Wingfield, A. Hearing loss in older adults affects neural systems supporting speech comprehension. J. Neurosci. 31, 12638–12643 (2011).
Yang, M. et al. Brain structural and functional alterations in patients with unilateral hearing loss. Hear. Res. 316, 37–43 (2014).
Husain, F. T. et al. Neuroanatomical changes due to hearing loss and chronic tinnitus: a combined VBM and DTI study. Brain Res. 1369, 74–88 (2011).
Qian, Z. J., Chang, P. D., Moonis, G. & Lalwani, A. K. A novel method of quantifying brain atrophy associated with age-related hearing loss. Neuroimage Clin. 16, 205–209 (2017).
Boyen, K., Langers, D. R., de Kleine, E. & van Dijk, P. Gray matter in the brain: differences associated with tinnitus and hearing loss. Hear. Res. 295, 67–78 (2013).
Rutherford, B. R., Brewster, K., Golub, J. S., Kim, A. H. & Roose, S. P. Sensation and psychiatry: linking age-related hearing loss to late-life depression and cognitive decline. Am. J. Psychiatry 175, 215–224 (2018).
Belkhiria, C. et al. Cingulate cortex atrophy is associated with hearing loss in presbycusis with cochlear amplifier dysfunction. Front. Aging Neurosci. 11, 97 (2019).
Ren, F. et al. Gray matter atrophy is associated with cognitive impairment in patients with presbycusis: a comprehensive morphometric study. Front. Neurosci. 12, 744 (2018).
Glick, H. A. & Sharma, A. Cortical neuroplasticity and cognitive function in early-stage, mild-moderate hearing loss: evidence of neurocognitive benefit from hearing aid use. Front. Neurosci. 14, 93 (2020).
Alexopoulos, G. S. et al. Executive dysfunction and long-term outcomes of geriatric depression. Arch. Gen. Psychiatry 57, 285–290 (2000).
Erb, J. & Obleser, J. Upregulation of cognitive control networks in older adults’ speech comprehension. Front. Syst. Neurosci. 7, 116 (2013).
Wild, C. J. et al. Effortful listening: the processing of degraded speech depends critically on attention. J. Neurosci. 32, 14010–14021 (2012).
Tyler, L. K. et al. Preserving syntactic processing across the adult life span: the modulation of the frontotemporal language system in the context of age-related atrophy. Cereb. Cortex 20, 352–364 (2010).
Rosemann, S. & Thiel, C. M. Audio-visual speech processing in age-related hearing loss: stronger integration and increased frontal lobe recruitment. Neuroimage 175, 425–437 (2018).
Husain, F. T. et al. Discrimination task reveals differences in neural bases of tinnitus and hearing impairment. PLoS ONE 6, e26639 (2011).
Chen, Y. C. et al. Presbycusis disrupts spontaneous activity revealed by resting-state functional MRI. Front. Behav. Neurosci. 12, 44 (2018).
Lemke, U. & Besser, J. Cognitive load and listening effort: concepts and age-related considerations. Ear Hear. 37, 77S–84S (2016).
Pichora-Fuller, M. K. et al. Hearing impairment and cognitive energy: the Framework for Understanding Effortful Listening (FUEL). Ear Hear. 37, 5S–27S (2016).
Rudner, M. et al. Listening comprehension and listening effort in the primary school classroom. Front. Psychol. 9, 1193 (2018).
Rosemann, S. & Thiel, C. M. The effect of age-related hearing loss and listening effort on resting state connectivity. Sci. Rep. 9, 2337 (2019).
Fitzhugh, M. C., Hemesath, A., Schaefer, S. Y., Baxter, L. C. & Rogalsky, C. Functional connectivity of Heschl’s gyrus associated with age-related hearing loss: a resting-state fMRI study. Front. Psychol. 10, 2485 (2019).
Wang, X. et al. Altered regional and circuit resting-state activity associated with unilateral hearing loss. PLoS ONE 9, e96126 (2014).
Schmidt, S. A., Akrofi, K., Carpenter-Thompson, J. R. & Husain, F. T. Default mode, dorsal attention and auditory resting state networks exhibit differential functional connectivity in tinnitus and hearing loss. PLoS ONE 8, e76488 (2013).
Middlebrooks, J., Simon, J. Z., Popper, A. N. & Fay, R. R. (eds) The Auditory System at the Cocktail Party (Springer, 2017).
Russo, F. A., Ives, D. T., Goy, H., Pichora-Fuller, M. K. & Patterson, R. D. Age-related difference in melodic pitch perception is probably mediated by temporal processing: empirical and computational evidence. Ear Hear. 33, 177–186 (2012).
Dupuis, K. & Pichora-Fuller, M. K. Intelligibility of emotional speech in younger and older adults. Ear Hear. 35, 695–707 (2014).
Peelle, J. E. Listening effort: how the cognitive consequences of acoustic challenge are reflected in brain and behavior. Ear Hear. 39, 204–214 (2018).
LeDoux, J. E., Sakaguchi, A. & Reis, D. J. Subcortical efferent projections of the medial geniculate nucleus mediate emotional responses conditioned to acoustic stimuli. J. Neurosci. 4, 683–698 (1984).
Kumar, S., von Kriegstein, K., Friston, K. & Griffiths, T. D. Features versus feelings: dissociable representations of the acoustic features and valence of aversive sounds. J. Neurosci. 32, 14184–14192 (2012).
Wager, T. D., Davidson, M. L., Hughes, B. L., Lindquist, M. A. & Ochsner, K. N. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron 59, 1037–1050 (2008).
Husain, F. T., Carpenter-Thompson, J. R. & Schmidt, S. A. The effect of mild-to-moderate hearing loss on auditory and emotion processing networks. Front. Syst. Neurosci. 8, 10 (2014).
Christensen, J. A., Sis, J., Kulkarni, A. M. & Chatterjee, M. Effects of age and hearing loss on the recognition of emotions in speech. Ear Hear. 40, 1069–1083 (2019).
Picou, E. M. How hearing loss and age affect emotional responses to nonspeech sounds. J. Speech Lang. Hear. Res. 59, 1233–1246 (2016).
Zinchenko, A. et al. Modulation of cognitive and emotional control in age-related mild-to-moderate hearing loss. Front. Neurol. 9, 783 (2018).
Belkhiria, C. et al. Insula and amygdala atrophy are associated with functional impairment in subjects with presbycusis. Front. Aging Neurosci. 12, 102 (2020).
Keesom, S. M. & Hurley, L. M. Silence, solitude, and serotonin: neural mechanisms linking hearing loss and social isolation. Brain Sci. https://doi.org/10.3390/brainsci10060367 (2020).
Cruz, O. L., Kasse, C. A., Sanchez, M., Barbosa, F. & Barros, F. A. Serotonin reuptake inhibitors in auditory processing disorders in elderly patients: preliminary results. Laryngoscope 114, 1656–1659 (2004).
Peelle, J. E. Methodological challenges and solutions in auditory functional magnetic resonance imaging. Front. Neurosci. 8, 253 (2014).
Picou, E. M. et al. Hearing, Emotion, Amplification, Research, and Training workshop: current understanding of hearing loss and emotion perception and priorities for future research. Trends Hear. https://doi.org/10.1177/2331216518803215 (2018).
Mick, P., Kawachi, I. & Lin, F. R. The association between hearing loss and social isolation in older adults. Otolaryngol. Head Neck Surg. 150, 378–384 (2014).
Pichora-Fuller, M. K., Mick, P. & Reed, M. Hearing, cognition, and healthy aging: social and public health implications of the links between age-related declines in hearing and cognition. Semin. Hear. 36, 122–139 (2015).
Arlinger, S. Negative consequences of uncorrected hearing loss—a review. Int. J. Audio. 42, 2S17–20S17 (2003).
Perissinotto, C. M., Stijacic Cenzer, I. & Covinsky, K. E. Loneliness in older persons: a predictor of functional decline and death. Arch. Intern. Med. 172, 1078–1083 (2012).
Wang, H. X., Karp, A., Winblad, B. & Fratiglioni, L. Late-life engagement in social and leisure activities is associated with a decreased risk of dementia: a longitudinal study from the Kungsholmen Project. Am. J. Epidemiol. 155, 1081–1087 (2002).
Glass, T. A., De Leon, C. F., Bassuk, S. S. & Berkman, L. F. Social engagement and depressive symptoms in late life: longitudinal findings. J. Aging Health 18, 604–628 (2006).
Sung, Y. K., Li, L., Blake, C., Betz, J. & Lin, F. R. Association of hearing loss and loneliness in older adults. J. Aging Health 28, 979–994 (2016).
Mick, P. & Pichora-Fuller, M. K. Is hearing loss associated with poorer health in older adults who might benefit from hearing screening? Ear Hear. 37, e194–e201 (2016).
Aylaz, R., Akturk, U., Erci, B., Ozturk, H. & Aslan, H. Relationship between depression and loneliness in elderly and examination of influential factors. Arch. Gerontol. Geriatr. 55, 548–554 (2012).
Hamalainen, A., Phillips, N., Wittich, W., Pichora-Fuller, M. K. & Mick, P. Sensory-cognitive associations are only weakly mediated or moderated by social factors in the Canadian Longitudinal Study on Aging. Sci. Rep. 9, 19660 (2019).
Kanai, R. et al. Brain structure links loneliness to social perception. Curr. Biol. 22, 1975–1979 (2012).
Nakagawa, S. et al. White matter structures associated with loneliness in young adults. Sci. Rep. 5, 17001 (2015).
Duzel, S. et al. Structural brain correlates of loneliness among older adults. Sci. Rep. 9, 13569 (2019).
Inagaki, T. K. et al. Yearning for connection? Loneliness is associated with increased ventral striatum activity to close others. Soc. Cogn. Affect. Neurosci. 11, 1096–1101 (2016).
Cacioppo, J. T., Norris, C. J., Decety, J., Monteleone, G. & Nusbaum, H. In the eye of the beholder: individual differences in perceived social isolation predict regional brain activation to social stimuli. J. Cogn. Neurosci. 21, 83–92 (2009).
Wong, N. M. et al. Loneliness in late-life depression: structural and functional connectivity during affective processing. Psychol. Med. 46, 2485–2499 (2016).
Feng, C., Wang, L., Li, T. & Xu, P. Connectome-based individualized prediction of loneliness. Soc. Cogn. Affect. Neurosci. 14, 353–365 (2019).
Bhatt, J. M., Lin, H. W. & Bhattacharyya, N. Prevalence, severity, exposures, and treatment patterns of tinnitus in the United States. JAMA Otolaryngol. Head Neck Surg. 142, 959–965 (2016).
Tyler, R. S. Tinnitus Handbook (Singular, 2000).
Nondahl, D. M. et al. Tinnitus and its risk factors in the Beaver Dam Offspring Study. Int. J. Audio. 50, 313–320 (2011).
Yakunina, N., Lee, W. H., Ryu, Y. J. & Nam, E. C. Tinnitus suppression effect of hearing aids in patients with high-frequency hearing loss: a randomized double-blind controlled trial. Otol. Neurotol. 40, 865–871 (2019).
De Ridder, D. et al. An integrative model of auditory phantom perception: tinnitus as a unified percept of interacting separable subnetworks. Neurosci. Biobehav. Rev. 44, 16–32 (2014).
Trevis, K. J. et al. Identification of a neurocognitive mechanism underpinning awareness of chronic tinnitus. Sci. Rep. 7, 15220 (2017).
Araneda, R. et al. A key role of the prefrontal cortex in the maintenance of chronic tinnitus: an fMRI study using a Stroop task. Neuroimage Clin. 17, 325–334 (2018).
Heeren, A. et al. Tinnitus specifically alters the top-down executive control sub-component of attention: evidence from the Attention Network Task. Behav. Brain Res. 269, 147–154 (2014).
Lee, S. Y. et al. Neurocognition of aged patients with chronic tinnitus: focus on mild cognitive impairment. Clin. Exp. Otorhinolaryngol. 13, 8–14 (2020).
Golm, D., Schmidt-Samoa, C., Dechent, P. & Kroner-Herwig, B. Neural correlates of tinnitus related distress: an fMRI-study. Hear. Res. 295, 87–99 (2013).
Davies, J. E., Gander, P. E. & Hall, D. A. Does chronic tinnitus alter the emotional response function of the amygdala?: A sound-evoked fMRI study. Front. Aging Neurosci. 9, 31 (2017).
Carpenter-Thompson, J. R., Akrofi, K., Schmidt, S. A., Dolcos, F. & Husain, F. T. Alterations of the emotional processing system may underlie preserved rapid reaction time in tinnitus. Brain Res. 1567, 28–41 (2014).
Rauschecker, J. P., Leaver, A. M. & Muhlau, M. Tuning out the noise: limbic-auditory interactions in tinnitus. Neuron 66, 819–826 (2010).
Chen, Y. C. et al. Tinnitus distress is linked to enhanced resting-state functional connectivity from the limbic system to the auditory cortex. Hum. Brain Mapp. 38, 2384–2397 (2017).
Chen, Y. C. et al. Amygdala functional disconnection with the prefrontal-cingulate-temporal circuit in chronic tinnitus patients with depressive mood. Prog. Neuropsychopharmacol. Biol. Psychiatry 79, 249–257 (2017).
Nondahl, D. M. et al. The impact of tinnitus on quality of life in older adults. J. Am. Acad. Audio. 18, 257–266 (2007).
Salazar, J. W. et al. Depression in patients with tinnitus: a systematic review. Otolaryngol. Head Neck Surg. 161, 28–35 (2019).
Bhatt, J. M., Bhattacharyya, N. & Lin, H. W. Relationships between tinnitus and the prevalence of anxiety and depression. Laryngoscope 127, 466–469 (2017).
Lewis, J. E., Stephens, S. D. & McKenna, L. Tinnitus and suicide. Clin. Otolaryngol. Allied Sci. 19, 50–54 (1994).
Beukes, E. W., Andersson, G., Allen, P. M., Manchaiah, V. & Baguley, D. M. Effectiveness of guided internet-based cognitive behavioral therapy vs face-to-face clinical care for treatment of tinnitus: a randomized clinical trial. JAMA Otolaryngol. Head Neck Surg. 144, 1126–1133 (2018).
Johnson, R. M., Brummett, R. & Schleuning, A. Use of alprazolam for relief of tinnitus. A double-blind study. Arch. Otolaryngol. Head Neck Surg. 119, 842–845 (1993).
Robinson, S. K., Viirre, E. S. & Stein, M. B. Antidepressant therapy in tinnitus. Hear. Res. 226, 221–231 (2007).
Sziklai, I., Szilvassy, J. & Szilvassy, Z. Tinnitus control by dopamine agonist pramipexole in presbycusis patients: a randomized, placebo-controlled, double-blind study. Laryngoscope 121, 888–893 (2011).
Pereira-Jorge, M. R. et al. Anatomical and functional MRI changes after one year of auditory rehabilitation with hearing aids. Neural Plast. 2018, 9303674 (2018).
Cardon, G. & Sharma, A. Somatosensory cross-modal reorganization in adults with age-related, early-stage hearing loss. Front. Hum. Neurosci. 12, 172 (2018).
Campbell, J. & Sharma, A. Cross-modal re-organization in adults with early stage hearing loss. PLoS ONE 9, e90594 (2014).
Pichora-Fuller, M. K. & Levitt, H. Speech comprehension training and auditory and cognitive processing in older adults. Am. J. Audio. 21, 351–357 (2012).
Claes, A. J. et al. The Repeatable Battery for the Assessment of Neuropsychological Status for Hearing impaired individuals (RBANS-H) before and after cochlear implantation: a protocol for a prospective, longitudinal cohort study. Front. Neurosci. 10, 512 (2016).
Brewster, K. K. et al. Age-related hearing loss, neuropsychological performance, and incident dementia in older adults. J. Alzheimers Dis. https://doi.org/10.3233/JAD-200908 (2021).
US Congress, Senate. Over-the-Counter Hearing Aid Act of 2017 S.670, 115th Congress (2017); https://www.congress.gov/bill/115th-congress/senate-bill/670
Morimoto, S. S. et al. Targeting cognitive control deficits with neuroplasticity-based computerized cognitive remediation in patients with geriatric major depression: a randomized, double-blind, controlled trial. Am. J. Geriatr. Psychiatry 28, 971–980 (2020).
Warringa, L. T. L., Henke, C. E., Pronk, M., Kramer, S. E. & Stam, M. Relationships between coping behaviors and social loneliness in adults with self-reported hearing problems. Ear Hear. 41, 1040–1050 (2020).
Acknowledgements
The manuscript was supported by the NIMH grant T32 MH020004-22.
Author information
Authors and Affiliations
Contributions
All authors (K.K.B., J.S.G. and B.R.R.) contributed to the literature search, data interpretation and writing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
K.K.B. and B.R.R. have no competing interests or financial disclosures to report. J.S.G. received travel expenses for industry-sponsored meetings (Cochlear, Advanced Bionics and Oticon Medical), consulting fees or honoraria (Oticon Medical, Auditory Insight, Optinose, Abbott and Decibel Therapeutics), and his department received unrestricted educational grants (Storz, Stryker, Acclarent, 3NT and Decibel Therapeutics).
Additional information
Peer review information Nature Aging thanks Matthew Amans, Blake Lawrence, Helen Nuttall and Kathy Pichora-Fuller for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Brewster, K.K., Golub, J.S. & Rutherford, B.R. Neural circuits and behavioral pathways linking hearing loss to affective dysregulation in older adults. Nat Aging 1, 422–429 (2021). https://doi.org/10.1038/s43587-021-00065-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s43587-021-00065-z