Abstract
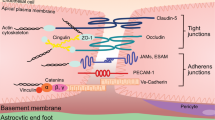
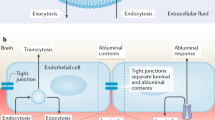
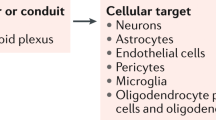
The blood–brain barrier (BBB) protects the central nervous system (CNS) from unregulated exposure to the blood and its contents. The BBB also controls the blood-to-brain and brain-to-blood permeation of many substances, resulting in nourishment of the CNS, its homeostatic regulation and communication between the CNS and peripheral tissues. The cells forming the BBB communicate with cells of the brain and in the periphery. This highly regulated interface changes with healthy aging. Here, we review those changes, starting with morphology and disruption. Transporter changes include those for amyloid beta peptide, glucose and drugs. Brain fluid dynamics, pericyte health and basement membrane and glycocalyx compositions are all altered with healthy aging. Carrying the ApoE4 allele leads to an acceleration of most of the BBB’s age-related changes. We discuss how alterations in the BBB that occur with healthy aging reflect adaptation to the postreproductive phase of life and may affect vulnerability to age-associated diseases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Biedl, A. & Kraus, R. Uber einer bisher unbekannte toxische Wirking der Gallensauren auf das zentralnervensystem. Zentralblatt Inn. Med. 19, 1185–1200 (1898).
Goldmann, E. E. Vitalfarbung am zentral-nervensystem. Abh. Preuss. Akad. Wiss., Phys.-Math. KL I, 1–60 (1913).
Neuwelt, E. et al. Strategies to advance translational research into brain barriers. Lancet Neurol. 7, 84–96 (2008).
Reese, T. S. & Karnovsky, M. J. Fine structural localization of a blood-brain barrier to endogenous peroxidase. J. Cell Biol. 34, 207–217 (1967).
Brightman, M. W. & Reese, T. S. Junctions between intimately apposed cell membranes in the vertebrate brain. J. Cell Biol. 40, 648–677 (1969).
Erickson, M. A. & Banks, W. A. Neuroimmune axes of the blood–brain barriers and blood–brain interfaces: bases for physiological regulation, disease states, and pharmacological interventions. Pharmacol. Rev. 70, 278–314 (2018).
Hawkins, B. T. & Davis, T. P. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol. Rev. 57, 173–185 (2005).
Daneman, R., Zhou, L., Kebede, A. A. & Barres, B. A. Pericytes are required for blood–brain barrier integrity during embryogenesis. Nature 468, 562–566 (2010).
Bell, R. D. et al. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron 68, 409–427 (2010).
Montagne, A. et al. APOE4 leads to blood–brain barrier dysfunction predicting cognitive decline. Nature 581, 71–76 (2020).
Stewart, P. A. et al. A quantitative analysis of blood-brain barrier ultrastructure in the aging human. Microvasc. Res. 33, 270–282 (1987).
Banks, W. A. The blood–brain barrier as an endocrine tissue. Nat. Rev. Endocrinol. 15, 444–455 (2019).
López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. The hallmarks of aging. Cell 153, 1194–1217 (2013).
Kennedy, B. K. et al. Geroscience: linking aging to chronic disease. Cell 159, 709–713 (2014).
Kiss, T. et al. Single-cell RNA sequencing identifies senescent cerebromicrovascular endothelial cells in the aged mouse brain. Geroscience 42, 429–444 (2020).
Stamatovic, S. M. et al. Decline in sirtuin-1 expression and activity plays a critical role in blood–brain barrier permeability in aging. Neurobiol. Dis. 126, 105–116 (2019).
Le Couteur, D. G. & Simpson, S. J. Adaptive senectitude: the prolongevity effects of aging. J. Gerontol. A Biol. Sci. Med Sci. 66, 179–182 (2011).
Cornford, E. M., Braun, L. D. & Oldendorf, W. H. Developmental modulations of blood–brain barrier permeability as an indicator of changing nutritional requirements in the brain. Pediatr. Res. 16, 324–328 (1982).
Mooradian, A. D. & Smith, T. L. The effect of age on lipid composition and order of rat cerebral microvessels. Neurochem. Res. 17, 233–237 (1992).
Kalaria, R. N. Cerebral vessels in ageing and Alzheimer’s disease. Pharmacol. Ther. 72, 193–214 (1996).
Sonntag, W. E., Eckman, D. M., Ingraham, J. & Riddle, D. R. in Brain Aging: Models, Methods, and Mechanisms (Ed. Riddle, D. R.) (CRC Press/Taylor & Francis, 2007).
Sonnen, J. A. et al. Ecology of the aging human brain. Arch. Neurol. 68, 1049–1056 (2011).
Besser, L. M. et al. The revised national Alzheimer’s Coordinating Center’s Neuropathology Form — available data and new analyses. J. Neuropathol. Exp. Neurol. 77, 717–726 (2018).
Rapoport, S. I., Ohno, K. & Pettigrew, K. D. Blood–brain barrier permeability in senescent rats. J. Gerontol. 34, 162–169 (1979).
Erickson, M. A. & Banks, W. A. Age-associated changes in the immune system and blood–brain barrier functions. Int. J. Mol. Sci. 20, 1632 (2019).
Erickson, M. A. & Banks, W. A. Blood–brain barrier dysfunction as a cause and consequence of Alzheimer’s disease. J. Cereb. Blood Flow. Metab. 33, 1500–1513 (2013).
Vorbrodt, A. W. & Dobrogowska, D. H. Immunocytochemical evaluation of blood–brain barrier to endogenous albumin in adult, newborn, and aged mice. Folia Histochem. Cytobiol. 32, 63–70 (1994).
Saunders, N. R., Dziegielewska, K. M., Mollgard, K. & Habgood, M. D. Markers for blood–brain barrier integrity: how appropriate is Evans blue in the twenty-first century and what are the alternatives? Front. Neurosci. 9, 1–15 (2015).
Garg, A. & Balthasar, J. P. Investigation of the influence of FcRn on the distribution of IgG to the brain. AAPS J. 11, 553–557 (2009).
Parrado-Fernandez, C. et al. Evidence for sex difference in the CSF/plasma albumin ratio in ~20 000 patients and 335 healthy volunteers. J. Cell. Mol. Med. 22, 5151–5154 (2018).
Chen, R. L. Is it appropriate to use albumin CSF/plasma ratio to assess blood brain barrier permeability? Neurobiol. Aging 32, 1338–1339 (2011).
Elahy, M. et al. Blood–brain barrier dysfunction developed during normal aging is associated with inflammation and loss of tight junctions but not with leukocyte recruitment. Immun. Ageing 12, 2 (2015).
Zhao, L. et al. Pharmacologically reversible zonation-dependent endothelial cell transcriptomic changes with neurodegenerative disease associations in the aged brain. Nat. Commun. 11, 4413 (2020).
Bien-Ly, N. et al. Lack of widespread BBB disruption in Alzheimer’s disease models: focus on therapeutic antibodies. Neuron 88, 289–297 (2015).
Yang, A. C. et al. Physiological blood–brain transport is impaired with age by a shift in transcytosis. Nature 583, 425–430 (2020).
Ivanov, A. I. Pharmacological inhibition of endocytic pathways: is it specific enough to be useful? Methods Mol. Biol. 440, 15–33 (2008).
Montagne, A. et al. Blood–brain barrier breakdown in the aging human hippocampus. Neuron 85, 296–302 (2015).
Verheggen, I. C. M. et al. Increase in blood–brain barrier leakage in healthy, older adults. Geroscience 42, 1183–1193 (2020).
Verheggen, I. C. M. et al. Imaging the role of blood-brain barrier disruption in normal cognitive ageing. Geroscience 42, 1751–1764 (2020).
Varatharaj, A. et al. Blood–brain barrier permeability measured using dynamic contrast-enhanced magnetic resonance imaging: a validation study. J. Physiol. 597, 699–709 (2019).
Moinuddin, A., Morley, J. E. & Banks, W. A. Regional variations in the transport of interleukin-1α across the blood–brain barrier in ICR and aging SAMP8 mice. Neuroimmunomodulation 8, 165–170 (2000).
Mooradian, A. D., Morin, A. M., Cipp, L. J. & Haspel, H. C. Glucose transport is reduced in the blood–brain barrier of aged rats. Brain Res. 551, 145–149 (1991).
Banks, W. A., Moinuddin, A. & Morley, J. E. Regional transport of TNF-α across the blood–brain barrier in young ICR and young and aged SAMP8 mice. Neurobiol. Aging 22, 671–676 (2001).
Banks, W. A. & Kastin, A. J. Aging and the blood–brain barrier: changes in the carrier-mediated transport of peptides in rats. Neurosci. Lett. 61, 171–175 (1985).
Daniel, P. M., Love, E. R. & Pratt, O. E. The effect of age upon the influx of glucose into the brain. J. Physiol. 274, 141–148 (1978).
Mooradian, A. D. Blood–brain barrier choline transport is reduced in diabetic rats. Diabetes 36, 1094–1097 (1987).
Jaeger, L. B. et al. Testing the neurovascular hypothesis of Alzheimer’s disease: LRP-1 antisense reduces blood–brain barrier clearance, increases brain levels of amyloid-beta protein, and impairs cognition. J. Alzheimers Dis. 17, 553–570 (2009).
Ohata, M., Sundaram, U., Fredericks, W. R., London, E. D. & Rapoport, S. I. Regional cerebral blood flow during development and ageing of the rat brain. Brain 104, 319–332 (1981).
Ibáñez, V. et al. Resting state brain glucose metabolism is not reduced in normotensive healthy men during aging, after correction for brain atrophy. Brain Res. Bull. 63, 147–154 (2004).
Qato, D. M., Wilder, J., Schumm, L. P., Gillet, V. & Alexander, G. C. Changes in prescription and over-the-counter medication and dietary supplement use among older adults in the United States, 2005 vs 2011. JAMA Intern. Med. 176, 473–482 (2016).
ElDesoky, E. S. Pharmacokinetic–pharmacodynamic crisis in the elderly. Am. J. Ther. 14, 488–498 (2007).
Jansen, P. A. & Brouwers, J. R. Clinical pharmacology in old persons. Scientifica (Cairo) 2012, 723678 (2012).
Toornvliet, R. et al. Effect of age on functional P-glycoprotein in the blood–brain barrier measured by use of (R)-[11C]verapamil and positron emission tomography. Clin. Pharmacol. Ther. 79, 540–548 (2006).
van Assema, D. M. et al. P-glycoprotein function at the blood–brain barrier: effects of age and gender. Mol. Imaging Biol. 14, 771–776 (2012).
Pan, Y. & Nicolazzo, J. A. Impact of aging, Alzheimer’s disease and Parkinson’s disease on the blood–brain barrier transport of therapeutics. Adv. Drug Deliv. Rev. 135, 62–74 (2018).
Chiu, C. et al. P-glycoprotein expression and amyloid accumulation in human aging and Alzheimer’s disease: preliminary observations. Neurobiol. Aging 36, 2475–2482 (2015).
Yu, C. et al. Neuroinflammation activates Mdr1b efflux transport through NFκB: promoter analysis in BBB endothelia. Cell. Physiol. Biochem. 22, 745–756 (2008).
Salkeni, M. A., Lynch, J. L., Price, T. O. & Banks, W. A. Lipopolysaccharide impairs blood–brain barrier P-glycoprotein function in mice through prostaglandin- and nitric oxide-independent pathways and nitric oxide-independent pathways. J. Neuroimmune Pharmacol. 4, 276–282 (2009).
Pollak, T. A. et al. The blood–brain barrier in psychosis. Lancet Psychiatry 5, 79–92 (2018).
Xie, R., Hammarlund-Udenaes, M., de Boer, A. G. & de Lange, E. C. The role of P-glycoprotein in blood-brain barrier transport of morphine: transcortical microdialysis studies in mdr1a (–/–) and mdr1a (+/+) mice. Br. J. Pharmacol. 128, 563–568 (1999).
Storck, S. E. et al. The concerted amyloid-beta clearance of LRP1 and ABCB1/P-gp across the blood–brain barrier is linked by PICALM. Brain Behav. Immun. 73, 21–33 (2018).
Hartz, A. M., Miller, D. S. & Bauer, B. Restoring blood–brain barrier P-glycoprotein reduces brain amyloid-beta in a mouse model of Alzheimer’s disease. Mol. Pharmacol. 77, 715–723 (2010).
Pardridge, W. M. Drug transport across the blood–brain barrier. J. Cereb. Blood Flow. Metab. 32, 1959–1972 (2012).
Cserr, H. F. & Knopf, P. M. Cervical lymphatics, the blood–brain barrier and the immunoreactivity of the brain: a new view. Immunol. Today 13, 507–512 (1992).
Kida, S., Pantazis, A. & Weller, R. O. CSF drains directly from the subarachnoid space into nasal lymphatics in the rat. Anatomy, histology and immunological significance. Neuropathol. Appl. Neurobiol. 19, 480–488 (1993).
Davson, H. & Segal, M. B. in Physiology of the CSF and Blood-Brain Barriers 489–523 (CRC Press, 1996).
Cserr, H. F. in Hydrocephalus (eds K. Shapiro, A. Marmarou, & H. Portnoy) 59–68 (Raven Press, 1984).
Cserr, H. F. & Berman, B. J. Iodide and thiocyanate efflux from brain following injection into rat caudate nucleus. Am. J. Physiol. 4, F331–F337 (1978).
Ray, L., Iliff, J. J. & Heys, J. J. Analysis of convective and diffusive transport in the brain interstitium. Fluids Barriers CNS 16, 6 (2019).
Iliff, J. J. et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci. Transl. Med. 4, 147ra111 (2012).
Iliff, J. J. et al. Cerebral arterial pulsation drives paravascular CSF–interstitial fluid exchange in the murine brain. J. Neurosci. 33, 18190–18199 (2013).
Wright, E. M. Transport processes in the formation of the cerebrospinal fluid. Rev. Physiol. Biochem. Pharmacol. 83, 1–34 (1978).
Johanson, C. E. in Neuromethods; The Neuronal Microenvironment (eds Boulton, A. A., Baker, G. B. & Walz, W.) 33–104 (The Humana Press, 1988).
Ichimura, T., Fraser, P. A. & Cserr, H. F. Distribution of extracellular tracers in perivascular spaces of the rat brain. Brain Res. 545, 103–113 (1991).
Preston, J. E. Aging choroid plexus–cerebrospinal fluid system. Microsc. Res. Tech. 52, 31–37 (2001).
Ma, Q., Ineichen, B. V., Detmar, M. & Proulx, S. T. Outflow of cerebrospinal fluid is predominantly through lymphatic vessels and is reduced in aged mice. Nat. Commun. 8, 1434 (2017).
Kress, B. T. et al. Impairment of paravascular clearance pathways in the aging brain. Ann. Neurol. 76, 845–861 (2014).
Xie, L. et al. Sleep drives metabolite clearance from the adult brain. Science 342, 373–377 (2013).
Avolio, A. P. et al. Effects of aging on changing arterial compliance and left ventricular load in a northern Chinese urban community. Circulation 68, 50–58 (1983).
Tsao, C. W. et al. Relations of arterial stiffness and endothelial function to brain aging in the community. Neurology 81, 984–991 (2013).
Lee, H. Y. & Oh, B. H. Aging and arterial stiffness. Circ. J. 74, 2257–2262 (2010).
Iliff, J. J. et al. Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J. Neurosci. 34, 16180–16193 (2014).
Palmer, A. L. & Ousman, S. S. Astrocytes and aging. Front. Aging Neurosci. 10, 337 (2018).
Li, Y. et al. Aging neurovascular unit and potential role of DNA damage and repair in combating vascular and neurodegenerative disorders. Front. Neurosci. 13, 778 (2019).
Kubotera, H. et al. Astrocytic endfeet re-cover blood vessels after removal by laser ablation. Sci. Rep. 9, 1263 (2019).
Alvarez, J. I. et al. The Hedgehog pathway promotes blood–brain barrier integrity and CNS immune quiescence. Science 334, 1727–1731 (2011).
Allahyari, R. V., Clark, K. L., Shepard, K. A. & Garcia, A. D. R. Sonic hedgehog signaling is negatively regulated in reactive astrocytes after forebrain stab injury. Sci. Rep. 9, 565 (2019).
Scheibel, A. & Fried, I. in Aging of the Brain Vol. 22 (eds Alger, D. et al.) (Raven Press, 1983).
Jamieson, J. J., Linville, R. M., Ding, Y. Y., Gerecht, S. & Searson, P. C. Role of iPSC-derived pericytes on barrier function of iPSC-derived brain microvascular endothelial cells in 2D and 3D. Fluids Barriers CNS 16, 15 (2019).
Nikolakopoulou, A. M. et al. Pericyte loss leads to circulatory failure and pleiotrophin depletion causing neuron loss. Nat. Neurosci. 22, 1089–1098 (2019).
Sweeney, M. D., Zhao, Z., Montagne, A., Nelson, A. R. & Zlokovic, B. V. Blood–brain barrier: from physiology to disease and back. Physiol. Rev. 99, 21–78 (2019).
Goodall, E. F. et al. Age-associated mRNA and miRNA expression changes in the blood–brain barrier. Int. J. Mol. Sci. 20, 3097 (2019).
He, L. et al. Analysis of the brain mural cell transcriptome. Sci. Rep. 6, 35108 (2016).
Fjorder, A. S. et al. Haploinsufficiency of ARHGAP42 is associated with hypertension. Eur. J. Hum. Genet. 27, 1296–1303 (2019).
Reed, M. J., Damodarasamy, M. & Banks, W. A. The extracellular matrix of the blood–brain barrier: structural and functional roles in health, aging, and Alzheimer’s disease. Tissue Barriers 7, 1651157 (2019).
Xu, L., Nirwane, A. & Yao, Y. Basement membrane and blood–brain barrier. Stroke Vasc. Neurol. 4, 78–82 (2019).
Gastfriend, B. D., Palecek, S. P. & Shusta, E. V. Modeling the blood–brain barrier: beyond the endothelial cells. Curr. Opin. Biomed. Eng. 5, 6–12 (2018).
Liu, S., Agalliu, D., Yu, C. & Fisher, M. The role of pericytes in blood-brain barrier function and stroke. Curr. Pharm. Des. 18, 3653–3662 (2012).
Mitchell, G. F. et al. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: the Framingham Heart Study. Hypertension 43, 1239–1245 (2004).
Rizzoni, D. et al. Vascular aging and disease of the small vessels. High. Blood Press. Cardiovasc. Prev. 26, 183–189 (2019).
Kurtz, A. & Oh, S. J. Age related changes of the extracellular matrix and stem cell maintenance. Prev. Med. 54, S50–S56 (2012).
Scioli, M. G., Bielli, A., Arcuri, G., Ferlosio, A. & Orlandi, A. Ageing and microvasculature. Vasc. Cell 6, 19 (2014).
Xi, Y. P., Nette, E. G., King, D. W. & Rosen, M. Age-related changes in normal human basement membrane. Mech. Ageing Dev. 19, 315–324 (1982).
Hawkes, C. A. et al. Perivascular drainage of solutes is impaired in the ageing mouse brain and in the presence of cerebral amyloid angiopathy. Acta Neuropathol. 121, 431–443 (2011).
Hawkes, C. A. et al. Regional differences in the morphological and functional effects of aging on cerebral basement membranes and perivascular drainage of amyloid-beta from the mouse brain. Aging Cell 12, 224–236 (2013).
Keable, A. et al. Deposition of amyloid beta in the walls of human leptomeningeal arteries in relation to perivascular drainage pathways in cerebral amyloid angiopathy. Biochim. Biophys. Acta 1862, 1037–1046 (2016).
Uspenskaia, O., Liebetrau, M., Herms, J., Danek, A. & Hamann, G. F. Aging is associated with increased collagen type IV accumulation in the basal lamina of human cerebral microvessels. BMC Neurosci. 5, 37 (2004).
Vasudevan, A. et al. Basement membrane protein nidogen-1 shapes hippocampal synaptic plasticity and excitability. Hippocampus 20, 608–620 (2010).
Candiello, J., Cole, G. J. & Halfter, W. Age-dependent changes in the structure, composition and biophysical properties of a human basement membrane. Matrix Biol. 29, 402–410 (2010).
Sykova, E., Mazel, T. & Simonova, Z. Diffusion constraints and neuron-glia interaction during aging. Exp. Gerontol. 33, 837–851 (1998).
Ceafalan, L. C. et al. Age-related ultrastructural changes of the basement membrane in the mouse blood–brain barrier. J. Cell. Mol. Med. 23, 819–827 (2019).
Kutuzov, N., Flyvbjerg, H. & Lauritzen, M. Contributions of the glycocalyx, endothelium, and extravascular compartment to the blood–brain barrier. Proc. Natl Acad. Sci. USA 115, E9429–e9438 (2018).
Ando, Y. et al. Brain-specific ultrastructure of capillary endothelial glycocalyx and its possible contribution for blood brain barrier. Sci. Rep. 8, 17523 (2018).
Iozzo, R. V. & Schaefer, L. Proteoglycan form and function: a comprehensive nomenclature of proteoglycans. Matrix Biol. 42, 11–55 (2015).
Wight, T. N. A role for proteoglycans in vascular disease. Matrix Biol. 71-72, 396–420 (2018).
Reitsma, S., Slaaf, D. W., Vink, H., van Zandvoort, M. A. & oude Egbrink, M. G. The endothelial glycocalyx: composition, functions, and visualization. Pflugers Arch. 454, 345–359 (2007).
Wight, T. N. Vessel proteoglycans and thrombogenesis. Prog. Hemost. Thromb. 5, 1–39 (1980).
Gao, L. & Lipowsky, H. H. Composition of the endothelial glycocalyx and its relation to its thickness and diffusion of small solutes. Microvasc. Res. 80, 394–401 (2010).
Luft, J. H. Fine structures of capillary and endocapillary layer as revealed by ruthenium red. Fed. Proc. 25, 1773–1783 (1966).
McClatchey, P. M., Schafer, M., Hunter, K. S. & Reusch, J. E. The endothelial glycocalyx promotes homogenous blood flow distribution within the microvasculature. Am. J. Physiol. Heart Circ. Physiol. 311, H168–H176 (2016).
Mulivor, A. W. & Lipowsky, H. H. Role of glycocalyx in leukocyte–endothelial cell adhesion. Am. J. Physiol. Heart Circ. Physiol. 283, H1282–H1291 (2002).
Yao, Y., Rabodzey, A. & Dewey, C. F. Jr. Glycocalyx modulates the motility and proliferative response of vascular endothelium to fluid shear stress. Am. J. Physiol. Heart Circ. Physiol. 293, H1023–H1030 (2007).
Danielli, J. F. Capillary permeability and oedema in the perfused frog. J. Physiol. 98, 109–129 (1940).
Vink, H. & Duling, B. R. Capillary endothelial surface layer selectively reduces plasma solute distribution volume. Am. J. Physiol. Heart Circ. Physiol. 278, H285–H289 (2000).
Henry, C. B. & Duling, B. R. TNF-α increases entry of macromolecules into luminal endothelial cell glycocalyx. Am. J. Physiol. Heart Circ. Physiol. 279, H2815–H2823 (2000).
Martens, R. J., Vink, H., van Oostenbrugge, R. J. & Staals, J. Sublingual microvascular glycocalyx dimensions in lacunar stroke patients. Cerebrovasc. Dis. 35, 451–454 (2013).
Hempel, C., Hyttel, P. & Kurtzhals, J. A. Endothelial glycocalyx on brain endothelial cells is lost in experimental cerebral malaria. J. Cereb. Blood Flow. Metab. 34, 1107–1110 (2014).
Haeren, R. H. et al. Assessment and imaging of the cerebrovascular glycocalyx. Curr. Neurovasc. Res. 13, 249–260 (2016).
Yoon, J. H., Lee, E. S. & Jeong, Y. In vivo imaging of the cerebral endothelial glycocalyx in mice. J. Vasc. Res. 54, 59–67 (2017).
Zuurbier, C. J., Demirci, C., Koeman, A., Vink, H. & Ince, C. Short-term hyperglycemia increases endothelial glycocalyx permeability and acutely decreases lineal density of capillaries with flowing red blood cells. J. Appl. Physiol. 99, 1471–1476 (2005).
Nieuwdorp, M. et al. Endothelial glycocalyx damage coincides with microalbuminuria in type 1 diabetes. Diabetes 55, 1127–1132 (2006).
Machin, D. R. et al. Advanced age results in a diminished endothelial glycocalyx. Am. J. Physiol. Heart Circ. Physiol. 315, H531–H539 (2018).
Methia, N. et al. ApoE deficiency compromises the blood brain barrier especially after injury. Mol. Med. 7, 810–815 (2001).
Hafezi-Moghadam, A., Thomas, K. L. & Wagner, D. D. ApoE deficiency leads to a progressive age-dependent blood–brain barrier leakage. Am. J. Physiol. Cell Physiol. 292, C1256–C1262 (2007).
Donahue, J. E. & Johanson, C. E. Apolipoprotein E, amyloid-beta, and blood–brain barrier permeability in Alzheimer disease. J. Neuropathol. Exp. Neurol. 67, 261–270 (2008).
Kulminski, A. M. et al. Age, gender, and cancer but not neurodegenerative and cardiovascular diseases strongly modulate systemic effect of the Apolipoprotein E4 allele on lifespan. PLoS Genet. 10, e1004141 (2014).
Halliday, M. R. et al. Relationship between cyclophilin a levels and matrix metalloproteinase 9 activity in cerebrospinal fluid of cognitively normal apolipoprotein e4 carriers and blood–brain barrier breakdown. JAMA Neurol. 70, 1198–1200 (2013).
Montagne, A., Zhao, Z. & Zlokovic, B. V. Alzheimer’s disease: a matter of blood–brain barrier dysfunction? J. Exp. Med. 214, 3151–3169 (2017).
Nishitsuji, K., Hosono, T., Nakamura, T., Bu, G. & Michikawa, M. Apolipoprotein E regulates the integrity of tight junctions in an isoform-dependent manner in an in vitro blood–brain barrier model. J. Biol. Chem. 286, 17536–17542 (2011).
Alata, W., Ye, Y., St-Amour, I., Vandal, M. & Calon, F. Human apolipoprotein E ε4 expression impairs cerebral vascularization and blood–brain barrier function in mice. J. Cereb. Blood Flow. Metab. 35, 86–94 (2015).
Bell, R. D. et al. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature 485, 512–516 (2012).
Johnson, L. A. et al. Apolipoprotein E4 mediates insulin resistance-associated cerebrovascular dysfunction and the post-prandial response. J. Cereb. Blood Flow. Metab. 39, 770–781 (2019).
Rhea, E. M., Torres, E. R. S., Raber, J. & Banks, W. A. Insulin BBB pharmacokinetics in young ApoE male and female transgenic mice. PLoS ONE 15, e0228455 (2020).
Yamazaki, Y. et al. ApoE (Apolipoprotein E) in brain pericytes regulates endothelial function in an isoform-dependent manner by modulating basement membrane components. Arterioscler. Thromb. Vasc. Biol. 40, 128–144 (2020).
Nahirney, P. C., Reeson, P. & Brown, C. E. Ultrastructural analysis of blood–brain barrier breakdown in the peri-infarct zone in young adult and aged mice. J. Cereb. Blood Flow. Metab. 36, 413–425 (2016).
Bors, L. et al. Age-dependent changes at the blood–brain barrier. A comparative structural and functional study in young adult and middle aged rats. Brain Res. Bull. 139, 269–277 (2018).
Zipser, B. D. et al. Microvascular injury and blood-brain barrier leakage in Alzheimer’s disease. Neurobiol. Aging 28, 977–986 (2007).
Zlokovic, B. V. Cerebrovascular effects of apolipoprotein E: implications for Alzheimer disease. JAMA Neurol. 70, 440–444 (2013).
Salloway, S. et al. Effect of APOE genotype on microvascular basement membrane in Alzheimer’s disease. J. Neurol. Sci. 203-204, 183–187 (2002).
Bachmeier, C. et al. Apolipoprotein E isoform-specific effects on lipoprotein receptor processing. Neuromolecular Med. 16, 686–696 (2014).
Deane, R. et al. ApoE isoform-specific disruption of amyloid beta peptide clearance from mouse brain. J. Clin. Investig. 118, 4002–4013 (2008).
Vandal, M. et al. Reduction in DHA transport to the brain of mice expressing human APOE4 compared to APOE2. J. Neurochem. 129, 516–526 (2014).
Tachibana, M., Yamazaki, Y., Liu, C. C., Bu, G. & Kanekiyo, T. Pericyte implantation in the brain enhances cerebral blood flow and reduces amyloid-beta pathology in amyloid model mice. Exp. Neurol. 300, 13–21 (2018).
Paul, G. et al. Safety and tolerability of intracerebroventricular PDGF-BB in Parkinson’s disease patients. J. Clin. Investig. 125, 1339–1346 (2015).
Shimizu, F. et al. Pericyte-derived glial cell line-derived neurotrophic factor increase the expression of claudin-5 in the blood–brain barrier and the blood–nerve barrier. Neurochem. Res. 37, 401–409 (2012).
Salameh, T. S., Shah, G. N., Price, T. O., Hayden, M. R. & Banks, W. A. Blood–brain barrier disruption and neurovascular unit dysfunction in diabetic mice: protection with the mitochondrial carbonic anhydrase inhibitor topiramate. J. Pharmacol. Exp. Ther. 359, 452–459 (2016).
May, J. M., Jayagopal, A., Qu, Z. C. & Parker, W. H. Ascorbic acid prevents high glucose-induced apoptosis in human brain pericytes. Biochem. Biophys. Res. Commun. 452, 112–117 (2014).
Soto, I. et al. APOE stabilization by exercise prevents aging neurovascular dysfunction and complement induction. PLoS Biol. 13, e1002279 (2015).
Latimer, C. S. et al. Reversal of glial and neurovascular markers of unhealthy brain aging by exercise in middle-aged female mice. PLoS ONE 6, e26812 (2011).
Bok, E. et al. Dietary restriction and neuroinflammation: a potential mechanistic link. Int. J. Mol. Sci. 20, 464 (2019).
McCullough, M. J., Gyorkos, A. M. & Spitsbergen, J. M. Short-term exercise increases GDNF protein levels in the spinal cord of young and old rats. Neuroscience 240, 258–268 (2013).
Belaya, I. et al. Astrocyte remodeling in the beneficial effects of long-term voluntary exercise in Alzheimer’s disease. J. Neuroinflammation 17, 271 (2020).
Lundquist, A. J., Parizher, J., Petzinger, G. M. & Jakowec, M. W. Exercise induces region-specific remodeling of astrocyte morphology and reactive astrocyte gene expression patterns in male mice. J. Neurosci. Res. 97, 1081–1094 (2019).
Berthiaume, A. A., Hartmann, D. A., Majesky, M. W., Bhat, N. R. & Shih, A. Y. Pericyte structural remodeling in cerebrovascular health and homeostasis. Front. Aging Neurosci. 10, 210 (2018).
Rhea, E. M., Raber, J. & Banks, W. A. ApoE and cerebral insulin: trafficking, receptors, and resistance. Neurobiol. Dis. 137, 104755 (2020).
Zhao, N. et al. Apolipoprotein E4 impairs neuronal insulin signaling by trapping insulin receptor in the endosomes. Neuron 96, 115–129 (2017).
Wilhelmus, M. M. et al. Apolipoprotein E genotype regulates amyloid-beta cytotoxicity. J. Neurosci. 25, 3621–3627 (2005).
Sweeney, M. D., Ayyadurai, S. & Zlokovic, B. V. Pericytes of the neurovascular unit: key functions and signaling pathways. Nat. Neurosci. 19, 771–783 (2016).
Blanchard, J. W. et al. Reconstruction of the human blood–brain barrier in vitro reveals a pathogenic mechanism of APOE4 in pericytes. Nat. Med. 26, 952–963 (2020).
Acknowledgements
This work was supported by the Department of Veterans Affairs and NIH R01 AG046619.
Author information
Authors and Affiliations
Contributions
All authors contributed to the ideas, literature reviews, and writing and editing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Aging thanks Jeffrey Iliff, Patric Turowski and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Banks, W.A., Reed, M.J., Logsdon, A.F. et al. Healthy aging and the blood–brain barrier. Nat Aging 1, 243–254 (2021). https://doi.org/10.1038/s43587-021-00043-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s43587-021-00043-5
This article is cited by
-
Peripheral blood amyloid-β involved in the pathogenesis of Alzheimer’s disease via impacting on peripheral innate immune cells
Journal of Neuroinflammation (2024)
-
Border-associated macrophages in the central nervous system
Journal of Neuroinflammation (2024)
-
Proteomics of mouse brain endothelium uncovers dysregulation of vesicular transport pathways during aging
Nature Aging (2024)
-
Gastrointestinal and brain barriers: unlocking gates of communication across the microbiota–gut–brain axis
Nature Reviews Gastroenterology & Hepatology (2024)
-
From the Mind to the Spine: The Intersecting World of Alzheimer’s and Osteoporosis
Current Osteoporosis Reports (2024)