Abstract
Age-related regeneration failure in the central nervous system can occur as a result of a decline in remyelination efficacy. The responsiveness of myelin-forming cells to signals for remyelination is affected by aging-related epigenetic modification; however, the molecular mechanism is not fully clarified. In the present study, we report that the apelin receptor (APJ) mediates remyelination efficiency with age. APJ expression in myelin-forming cells is correlated with age-associated changes in remyelination efficiency, and the activation of APJ promotes remyelination through the translocation of myelin regulatory factor. APJ signaling activation promoted remyelination in both aged mice with toxin-induced demyelination and mice with experimental autoimmune encephalomyelitis. In human cells, APJ activation enhanced the expression of remyelination markers. Impaired oligodendrocyte function in aged animals can be reversibly reactivated; thus, the results demonstrate that dysfunction of the apelin–APJ system mediates remyelination failure in aged animals, and that their myelinating function can be reactivated by APJ activation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Source data underlying Fig. 2c,d and Extended Fig. 3b,c have been provided. The RNA-seq data were submitted to the database at DNA Data Bank of Japan under accession no. DRA009850 (https://ddbj.nig.ac.jp/DRASearch/study?acc=DRP005954). The data that support the findings of the present study are available from the corresponding author upon request.
References
Shields, S. A., Gilson, J. M., Blakemore, W. F. & Franklin, R. J. M. Remyelination occurs as extensively but more slowly in old rats compared to young rats following gliotoxin-induced CNS demyelination. Glia 28, 77–83 (1999).
Sim, F. J., Zhao, C., Penderis, J. & Franklin, R. J. M. The age-related decrease in CNS remyelination efficiency is attributable to an impairment of both oligodendrocyte progenitor recruitment and differentiation. J. Neurosci. 22, 2451–2459 (2002).
Wolswijk, G. Chronic stage multiple sclerosis lesions contain a relatively quiescent population of oligodendrocyte precursor cells. J. Neurosci. 18, 601–609 (1998).
Franklin, R. J. M. & Ffrench-Constant, C. Regenerating CNS myelin—from mechanisms to experimental medicines. Nat. Rev. Neurosci. 18, 753–769 (2017).
Shen, S. et al. Age-dependent epigenetic control of differentiation inhibitors is critical for remyelination efficiency. Nat. Neurosci. 11, 1024–1034 (2008).
Segel, M. et al. Niche stiffness underlies the ageing of central nervous system progenitor cells. Nature 573, 130–134 (2019).
Ruckh, J. M. et al. Rejuvenation of regeneration in the aging central nervous system. Cell Stem Cell 10, 96–103 (2012).
Cantuti-Castelvetri, L. et al. Defective cholesterol clearance limits remyelination in the aged central nervous system. Science 359, 684–688 (2018).
Stoffels, J. M. et al. Fibronectin aggregation in multiple sclerosis lesions impairs remyelination. Brain 136, 116–131 (2013).
Kuroda, M. et al. Peripherally derived FGF21 promotes remyelination in the central nervous system. J. Clin. Invest. 127, 3496–3509 (2017).
Hamaguchi, M. et al. Circulating transforming growth factor-β1 facilitates remyelination in the adult central nervous system. eLife 8, e41869 (2019).
Bansal, R., Stefansson, K. & Pfeiffer, S. E. Proligodendroblast antigen (POA), a developmental antigen expressed by A007/O4-positive oligodendrocyte progenitors prior to the appearance of sulfatide and galactocerebroside. J. Neurochem. 58, 2221–2229 (1992).
Tehrani, R. A. et al. Plasma levels of CTRP-3, CTRP-9 and apelin in women with multiple sclerosis. J. Neuroimmunol. 333, 576968 (2019).
Emery, B. & Lu, Q. R. Transcriptional and epigenetic regulation of oligodendrocyte development and myelination in the central nervous system. Cold Spring Harb. Perspect. Biol. 7, a020461 (2015).
Cahoy, J. D. et al. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J. Neurosci. 28, 264–278 (2008).
Bujalka, H. et al. MYRF is a membrane-associated transcription factor that autoproteolytically cleaves to directly activate myelin genes. PLoS Biol. 11, e1001625 (2013).
Azuma, Y. & Dasso, M. The role of Ran in nuclear function. Curr. Opin. Cell Biol. 12, 302–307 (2000).
Chapman, N. A., Dupré, D. J. & Rainey, J. K. The apelin receptor: physiology, pathology, cell signalling, and ligand modulation of a peptide-activated class A GPCR. Biochem. Cell Biol. 92, 431–440 (2014).
Magalon, K. et al. Olesoxime accelerates myelination and promotes repair in models of demyelination. Ann. Neurol. 71, 213–226 (2012).
Jeffery, N. D. & Blakemore, W. F. Locomotor deficits induced by experimental spinal cord demyelination are abolished by spontaneous remyelination. Brain 120, 27–37 (1997).
Takahashi, C., Muramatsu, R., Fujimura, H., Mochizuki, H. & Yamashita, T. Prostacyclin promotes oligodendrocyte precursor recruitment and remyelination after spinal cord demyelination. Cell Death Dis. 4, e795 (2013).
Najm, F. J. et al. Drug-based modulation of endogenous stem cells promotes functional remyelination in vivo. Nature 522, 216–220 (2015).
Stangel, M., Kuhlmann, T., Matthews, P. M. & Kilpatrick, T. J. Achievements and obstacles of remyelinating therapies in multiple sclerosis. Nat. Rev. Neurol. 13, 742–754 (2017).
Sun, J., Zhou, H., Bai, F., Zhang, Z. & Ren, Q. Remyelination: a potential therapeutic strategy for Alzheimer’s disease? J. Alzheimers Dis. 58, 597–612 (2017).
Sozmen, E. G. et al. Nogo receptor blockade overcomes remyelination failure after white matter stroke and stimulates functional recovery in aged mice. Proc. Natl Acad. Sci. USA 113, E8453–E8462 (2016).
Li, Y. et al. Olfactory ensheathing cell transplantation into spinal cord prolongs the survival of mutant SOD1(G93A) ALS rats through neuroprotection and remyelination. Anat. Rec. 294, 847–857 (2011).
Stariha, R. L. & Kim, S. U. Protein kinase C and mitogen-activated protein kinase signalling in oligodendrocytes. Microsc. Res. Tech. 52, 680–688 (2001).
Kumar, S. et al. Estrogen receptor β ligand therapy activates PI3K/Akt/mTOR signaling in oligodendrocytes and promotes remyelination in a mouse model of multiple sclerosis. Neurobiol. Dis. 56, 131–144 (2013).
Fyffe-Maricich, S. L., Karlo, J. C., Landreth, G. E. & Miller, R. H. The ERK2 mitogen-activated protein kinase regulates the timing of oligodendrocyte differentiation. J. Neurosci. 31, 843–850 (2011).
Yoon, S. et al. protein 3 phosphorylation links the Ras and PI3-kinase pathways to nucleocytoplasmic transport. Mol. Cell 29, 362–375 (2008).
Wake, H., Lee, P. R. & Fields, R. D. Control of local protein synthesis and initial events in myelination by action potentials. Science 333, 1647–1651 (2011).
Kidoya, H. & Takakura, N. Biology of the apelin-APJ axis in vascular formation. J. Biochem. 152, 125–131 (2012).
Arai, K. & Lo, E. H. An oligovascular niche: cerebral endothelial cells promote the survival and proliferation of oligodendrocyte precursor cells. J. Neurosci. 29, 4351–4355 (2009).
Murza, A., Belleville, K., Longpré, J. M., Sarret, P. & Marsault, É. Stability and degradation patterns of chemically modified analogs of apelin-13 in plasma and cerebrospinal fluid. Biopolymers 102, 297–303 (2014).
Villeda, S. A. et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature 477, 90–94 (2011).
Villeda, S. A. et al. Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice. Nat. Med. 20, 659–663 (2014).
Katsimpardi, L. et al. Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science 344, 630–634 (2014).
Rai, R. et al. Downregulation of the apelinergic axis accelerates aging, whereas its systemic restoration improves the mammalian healthspan. Cell Rep. 21, 1471–1480 (2017).
Vinel, C. et al. The exerkine apelin reverses age-associated sarcopenia. Nat. Med. 24, 1360–1371 (2018).
Olszanecka-Glinianowicz, M. et al. Circulating apelin level in relation to nutritional status in polycystic ovary syndrome and its association with metabolic and hormonal disturbances. Clin. Endocrinol. 79, 238–242 (2013).
Ishida, J. et al. Regulatory roles for APJ, a seven-transmembrane receptor related to angiotensin-type 1 receptor in blood pressure in vivo. J. Biol. Chem. 279, 26274–26279 (2004).
Sharma, B. et al. Alternative progenitor cells compensate to rebuild the coronary vasculature in Elabela- and Apj-deficient hearts. Dev. Cell 42, 655–666 (2017).
Michalski, J. P., Anderson, C., Beauvais, A., De Repentigny, Y. & Kothary, R. The proteolipid protein promoter drives expression outside of the oligodendrocyte lineage during embryonic and early postnatal development. PLoS ONE 6, e19772 (2011).
McCarthy, D. J., Chen, Y. & Smyth, G. K. Differential expression analysis of multifactor RNA-Seq experiments with respect to biological variation. Nucleic Acids Res. 40, 4288–4297 (2012).
Muramatsu, R. et al. Angiogenesis induced by CNS inflammation promotes neuronal remodeling through vessel-derived prostacyclin. Nat. Med. 18, 1658–1664 (2012).
Muramatsu, R. et al. RGMa modulates T cell responses and is involved in autoimmune encephalomyelitis. Nat. Med. 17, 488–494 (2011).
Acknowledgements
We thank H. Tsunematsu, K. Matoba, M. Hamaguchi, K. Inoue and T. Sano for support experiments. This work was supported by a Grant-in-Aid of Scientific Research from the Japan Society for the Promotion of Science to (grant no. 19H03554 to R.M.), AMED (grant no. JP20gm6210020 to R.M.), and Scientific Research from the Japan Society for the Promotion of Science (grant no. 17H06178 to T.Y.).
Author information
Authors and Affiliations
Contributions
M.I. and R.M. performed the experiments. R.M. coordinated the research and wrote the manuscript. Y. Kato conducted RNA-seq analysis. A.U. supported in vivo experiments. S.T. conducted flow cytometry. B.S., H.K., N.T. and AF contributed transgenic mice experiments. Y. Kawahara helped RNA-seq analysis. H.F., H.M. and M.T. contributed human sample experiments. T.Y. advised research direction.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Aging thanks Xianhua Piao and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
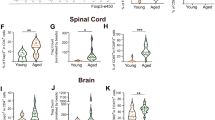
Extended Data Fig. 1 Developing and aged mice have different oligodendrocyte mRNA expression profiles.
a, Classification of the differentially expressed genes in O4-positive immature oligodendrocytes from developing (3-week-old) and aged (1.5-year-old) mouse brains. The top 100 differentially expressed genes were assigned to indicated gene ontology (GO) classes of biological processes. b, The number of oligodendrocytes after ML233 treatment. Cells were incubated with the indicated concentration of ML233 for 3 days. Relative cell number of the culture labeled with Olig2 (n = 4), P = 0.9. Each data point represents biologically independent experiments. Scale bar, 20 μm. Data are the mean ± s.e.m. and were assessed using one‐way ANOVA with post hoc Tukey’s multiple comparison test.
Extended Data Fig. 2 Changes in Myrf expression do not influence APJ-mediated oligodendrocyte differentiation.
a, Relative Myrf mRNA expression in cultured oligodendrocytes transfected with Myrf siRNA and control siRNA (n = 3). Each data point represents biologically independent experiments. P < 0.001. b, Relative Sox10 mRNA expression in cultured oligodendrocytes transfected with Myrf siRNA and control siRNA (n = 3). Each data point represents biologically independent experiments. P = 0.11. c, Relative Myrf mRNA expression in cultured oligodendrocytes treated with ML233 for 3 days and in control oligodendrocytes (n = 5). Each data point represents biologically independent experiments. P = 0.31. Data are the mean ± s.e.m. and were assessed using and two-tailed Welch’s t-test.
Extended Data Fig. 3 APJ signaling specifically stimulates oligodendrocyte differentiation.
a, Representative images of MBP expression in mouse oligodendrocyte culture. Cells were transfected with Cy5-labeled indicated siRNA and then cultured in the presence of ML233 for an additional 3 days. Quantitation of the MBP-positive area under the culture conditions indicated in the images (n = 3). Each data point represents biologically independent experiments. P = 0.007, 0.009 (left to right). Closed arrowheads, MBP-expressing cells transfected with siRNA; open arrowheads, MBP-negative cells transfected with siRNA. b, Immunoblot of Myrf expression in the nuclear and total fractions. Samples were prepared from 3-week-old mice and aged mice (n = 3), P = 0.01. A cropped image of the gel is presented (see Source Date 2 for full image). c, Immunoblot of Myrf expression in the nuclear and total fractions. Samples were prepared from wild-type mice and APJ KO mice (n = 3), P = 0.014. A cropped image of the gel is presented (see Source Date 2 for full image). d, Representative images of MBP expression in mouse oligodendrocyte culture. Cells were treated with the PKC inhibitor Gö6983 (10 μM) for 30 min and then cultured in the presence of ML233 for an additional 3 days. Quantitation of the MBP-positive area under the culture conditions indicated in the images (n = 4). Each data point represents biologically independent experiments. P = 0.004, 0.002 (left to right). e, Representative images of MBP expression in mouse oligodendrocyte culture. Cells were treated with the PI3K inhibitor LY294002 (10 μM) for 30 min and then cultured in the presence of ML233 for an additional 3 days. Quantitation of the MBP-positive area under the culture conditions indicated in the images (n = 4). Each data point represents biologically independent experiments. P < 0.001, 0.002 (left to right). f, Representative images of MBP expression in mouse oligodendrocyte culture. Cells were treated with the MEK inhibitor U0126 (10 μM) for 30 min and then cultured in the presence of ML233 for an additional 3 days. Quantitation of the MBP-positive area under the culture conditions indicated in the images (n = 4). Each data point represents biologically independent experiments. P = 0.004, 0.006 (left to right). g, Representative images of MBP expression in mouse oligodendrocyte culture. Cells were cultured in the neuronal supernatant for 3 days. Neuronal supernatant was collected from the cortical neuron culture with ML233 pretreatment. Quantitation of the MBP-positive area under the culture conditions indicated in the images (n = 4). Each data point represents biologically independent experiments. P = 0.31. Scale bars, 100 μm. Data are the mean ± s.e.m. and were assessed using one‐way ANOVA with post hoc Tukey’s multiple comparison test (a, d, e, and f) and two-tailed Welch’s t-test (b, c, and g).
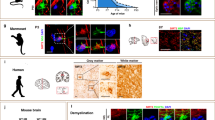
Extended Data Fig. 4 APJ expresses oligodendrocyte in the brain.
a, Representative images of brain sections prepared from 3-week-old wild-type mice with O4 (green) and Olig2 (magenta) labeling. n = 3 biologically independent animals. b, Representative images of brain sections prepared from 3-week-old wild-type mice with PDGFRα or APC (green) and APJ (magenta) labeling. n = 3 biologically independent animals. c, Representative images of brain sections prepared from 3-week-old wild-type mice with NeuN, lectin, or GFAP (magenta) labeling and APJ (green) labeling. n = 3 biologically independent animals. d, Representative images of brain sections prepared from 3-week-old wild-type mice labeled with MAG and PLP-specific antibodies. Quantitation of the MAG- and PLP-negative areas in the corpus callosum (n = 3), P < 0.001 (MAG), 0.049 (PLP). e, Representative images of brain sections prepared from 4-week-old cKO mice and control mice with O4 and APJ labeling. Quantitation of APJ expression in O4-labeled cells (n = 3), P = 0.004. Scale bars, 200 μm for d, 20 μm for others. Data are the mean ± s.e.m. and were assessed using two-tailed Welch’s t-test.
Extended Data Fig. 5 Peripheral apelin promotes myelination.
a, Representative images of brain sections from 4-week-old KO and control mice labeled with Black Gold. The graphs show the quantitation of myelination in the corpus callosum under the indicated conditions (n = 4). Each data point represents biologically independent animals. P = 0.006. b, Representative images of brain sections from 3-month-old cKO mice and control mice labeled with Black Gold. The graphs show the quantitation of myelination in the corpus callosum under the indicated conditions (n = 4). Each data point represents biologically independent animals. P = 0.007. c, Representative images of brain sections from 3-week-old mice treated with neutralizing apelin antibodies and control IgG. Sections were labeled with Black Gold. The graphs show the quantitation of myelination in the corpus callosum under the indicated conditions (n = 4). Each data point represents biologically independent animals. P = 0.003. d, Representative images of brain sections prepared from cKO mice and control mice, stained with hematoxylin and eosin (n = 4). Each data point represents biologically independent animals. P = 0.823. Scale bars, 200 μm. Data are the mean ± s.e.m. and were assessed using two-tailed Welch’s t-test.
Extended Data Fig. 6 APJ-mediated remyelination is independent of inflammation.
a, The number of splenocytes obtained from mice after EAE induction. Mice were treated with ML233 15 days after EAE induction. Samples were prepared 28 days after EAE induction (n = 3). Each data point represents biologically independent animals. P = 0.571. b, Relative BrdU incorporation into the cultured splenocyte. Mice were treated with ML233 15 days after EAE induction. Samples were prepared 28 days after EAE induction (n = 4 for control, 3 for ML233). Each data point represents biologically independent animals. P = 0.121. c, Representative images of spinal cord sections stained with hematoxylin and eosin. Mice were treated with ML233 15 days after EAE induction. Samples were prepared 28 days after EAE induction (n = 3). Each data point represents biologically independent animals. P = 0.393. d, Representative flow cytometric contour plots show IFN-γ and IL-17A expression in CD45+ CD4+ T cells in the spinal cord. Immune cells were gated according to side scatter (SSC) and forward scatter (FSC) level (red line), and CD45+ CD4+ double-positive T cells were further gated (red rectangle). Samples were prepared 28 days after EAE induction. Right panels show the percentage of the IFN-γ (upper panel) and IL-17A-positive cells (lower panel) in the spinal cord (n = 4 for control, 3 for ML233), P = 0.96 (IFN-γ), 0.952 (IL-17). e, Relative expression of indicated cytokine in the supernatant of splenocyte culture. Splenocyte were obtained from EAE mice 2 weeks after ML233 treatment (n = 4). Each data point represents biologically independent experiments. Scale bar, 200 μm. Data are the mean ± s.e.m. and were assessed using two-tailed Welch’s t-test.
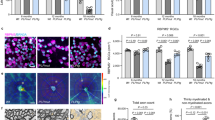
Extended Data Fig. 7 Pyr-apelin-13 stimulates remyelination.
a, Representative images of spinal cords labeled with Black Gold. Mice were treated with Pyr-apelin-13 15 days after EAE induction, and spinal cords were collected 30 days after EAE induction. Quantitation of the Black Gold-negative area in the spinal cord (n = 4). Each data point represents biologically independent animals. b, Change in the EAE score of mice treated with Pyr-apelin13 over time (n = 8). P = 0.011, 0.02 (left to right). Scale bars, 200 μm. Data are the mean ± s.e.m. and were assessed using two-tailed Welch’s t-test (a) and Mann-Whitney’s U test (b).
Extended Data Fig. 8 APJ-mediated remyelination is independent of neuronal functions.
a, Representative images of spinal cord section stained with c-fos (green) and NeuN (magenta). Quantitation of the c-fos labeled neuron in the spinal cord (n = 4). Each data point represents biologically independent animals. P = 0.483. b, Relative expression of indicated mRNA expression in the spinal cord neurons obtained from EAE mice with ML233 treatment (n = 4). Each data point represents biologically independent animals. P = 0.802 (IGF-1), 0.722 (Sama6A), 0.202 (BMP5), 0.186 (BMP6), 0.575 (BMP7), 0.661 (Lingo-1). Scale bars, 200 μm. Data are the mean ± s.e.m. and were assessed using two-tailed Welch’s t-test.
Supplementary information
Source data
Source Data Fig. 2
Unprocessed western blots.
Source Data Extended Data Fig. 3
Unprocessed western blots.
Rights and permissions
About this article
Cite this article
Ito, M., Muramatsu, R., Kato, Y. et al. Age-dependent decline in remyelination capacity is mediated by apelin–APJ signaling. Nat Aging 1, 284–294 (2021). https://doi.org/10.1038/s43587-021-00041-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s43587-021-00041-7
This article is cited by
-
Myeloid cell-associated aromatic amino acid metabolism facilitates CNS myelin regeneration
npj Regenerative Medicine (2024)
-
M2 macrophage-derived cathepsin S promotes peripheral nerve regeneration via fibroblast–Schwann cell-signaling relay
Journal of Neuroinflammation (2023)
-
Central nervous system regeneration: the roles of glial cells in the potential molecular mechanism underlying remyelination
Inflammation and Regeneration (2022)
-
Microglial ASD-related genes are involved in oligodendrocyte differentiation
Scientific Reports (2021)