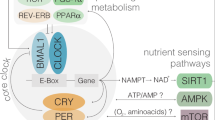
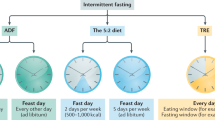
Abstract
Intermittent and periodic fasting (IF and PF, respectively) are emerging as safe strategies to affect longevity and healthspan by acting on cellular aging and disease risk factors, while causing no or minor side effects. IF lasting from 12 to 48 hours and repeated every 1 to 7 days and PF lasting 2 to 7 days and repeated once per month or less have the potential to prevent and treat disease, but their effect on cellular aging and the molecular mechanisms involved are only beginning to be unraveled. Here, we describe the different fasting methods and their effect on longevity in organisms ranging from yeast to humans, linking them to the major nutrient-sensing signaling pathways and focusing on the benefits of the fasting and the refeeding periods. We also discuss both the therapeutic potential and side effects of IF and PF with a focus on cancer, autoimmunity, neurodegeneration and metabolic and cardiovascular disease.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Fontana, L., Partridge, L. & Longo, V. D. Extending healthy life span—from yeast to humans. Science 328, 321–326 (2010).
McCay, C. M., Crowell, M. F. & Maynard, L. A. The effect of retarded growth upon the length of life span and upon the ultimate body size. 1935. Nutrition 5, 155–172 (1989).
Lin, S. J., Ford, E., Haigis, M., Liszt, G. & Guarente, L. Calorie restriction extends yeast life span by lowering the level of NADH. Genes Dev. 18, 12–16 (2004).
Hosono, R., Nishimoto, S. & Kuno, S. Alterations of life span in the nematode Caenorhabditis elegans under monoxenic culture conditions. Exp. Gerontol. 24, 251–264 (1989).
Bross, T. G., Rogina, B. & Helfand, S. L. Behavioral, physical, and demographic changes in Drosophila populations through dietary restriction. Aging Cell 4, 309–317 (2005).
Weindruch, R. & Walford, R. L. Dietary restriction in mice beginning at 1 year of age: effect on life-span and spontaneous cancer incidence. Science 215, 1415–1418 (1982).
Colman, R. J. et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science 325, 201–204 (2009).
Fontana, L. & Klein, S. Aging, adiposity, and calorie restriction. J. Am. Med. Assoc. 297, 986–994 (2007).
Abe, T. et al. Suppression of experimental autoimmune uveoretinitis by dietary calorie restriction. Jpn. J. Ophthalmol. 45, 46–52 (2001).
Jolly, C. A. & Fernandes, G. Diet modulates TH1 and TH2 cytokine production in the peripheral blood of lupus-prone mice. J. Clin. Immunol. 19, 172–178 (1999).
Kristan, D. M. Chronic calorie restriction increases susceptibility of laboratory mice (Mus musculus) to a primary intestinal parasite infection. Aging Cell 6, 817–825 (2007).
Gardner, E. M. Caloric restriction decreases survival of aged mice in response to primary influenza infection. J. Gerontol. A Biol. Sci. Med. Sci. 60, 688–694 (2005).
Mair, W., Piper, M. D. & Partridge, L. Calories do not explain extension of life span by dietary restriction in Drosophila. PLoS Biol. 3, e223 (2005).
Ross, M. H. Length of life and nutrition in the rat. J. Nutr. 75, 197–210 (1961).
McCay, C. M., Dilley, W. E. & Crowell, M. F. Growth rates of brook troutreared upon purified rations, upon dry skim milk diets, and upon feed combinations of cereal grains. J. Nutr. 1, 233–246 (1929).
Solon-Biet, S. M. et al. The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum-fed mice. Cell Metab. 19, 418–430 (2014).
Solon-Biet, S. M. et al. Dietary protein to carbohydrate ratio and caloric restriction: comparing metabolic outcomes in mice. Cell Rep. 11, 1529–1534 (2015).
Levine, M. E. et al. Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metab. 19, 407–417 (2014).
Brandhorst, S. et al. A periodic diet that mimics fasting promotes multi-system regeneration, enhanced cognitive performance, and healthspan. Cell Metab. 22, 86–99 (2015).
Cheng, C.-W. et al. Fasting-mimicking diet promotes Ngn3-driven β-cell regeneration to reverse diabetes. Cell 168, 775–788 (2017).
Cheng, C.-W. et al. Prolonged fasting reduces IGF-1/PKA to promote hematopoietic-stem-cell-based regeneration and reverse immunosuppression. Cell Stem Cell 14, 810–823 (2014).
Choi, I. Y. et al. A diet mimicking fasting promotes regeneration and reduces autoimmunity and multiple sclerosis symptoms. Cell Rep. 15, 2136–2146 (2016).
Rangan, P. et al. Fasting-mimicking diet modulates microbiota and promotes intestinal regeneration to reduce inflammatory bowel disease pathology. Cell Rep. 26, 2704–2719 (2019).
Lazare, S. et al. Lifelong dietary intervention does not affect hematopoietic stem cell function. Exp. Hematol. 53, 26–30 (2017).
Longo, V. D. & Mattson, M. P. Fasting: molecular mechanisms and clinical applications. Cell Metab. 19, 181–192 (2014).
Anson, R. M. et al. Intermittent fasting dissociates beneficial effects of dietary restriction on glucose metabolism and neuronal resistance to injury from calorie intake. Proc. Natl Acad. Sci. USA 100, 6216–6220 (2003).
Trepanowski, J. F. et al. Effect of alternate-day fasting on weight loss, weight maintenance, and cardioprotection among metabolically healthy obese adults: a randomized clinical trial. JAMA Intern. Med. 177, 930–938 (2017).
Varady, K. A. et al. Effects of weight loss via high fat vs. low fat alternate day fasting diets on free fatty acid profiles. Sci. Rep. 5, 7561 (2015).
Johnson, J. B. et al. Alternate day calorie restriction improves clinical findings and reduces markers of oxidative stress and inflammation in overweight adults with moderate asthma. Free Radic. Biol. Med. 42, 665–674 (2007).
Harvie, M. N. et al. The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: a randomized trial in young overweight women. Int. J. Obes. (Lond.) 35, 714–727 (2011).
Mattson, M. P., Longo, V. D. & Harvie, M. Impact of intermittent fasting on health and disease processes. Ageing Res. Rev. 39, 46–58 (2017).
Chaix, A. et al. Time-restricted feeding prevents obesity and metabolic syndrome in mice lacking a circadian clock. Cell Metab. 29, 303–319 (2018).
Chaix, A., Zarrinpar, A., Miu, P. & Panda, S. Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab. 20, 991–1005 (2014).
Longo, V. D. & Panda, S. Fasting, circadian rhythms, and time-restricted feeding in healthy lifespan. Cell Metab. 23, 1048–1059 (2016).
Wan, R. et al. Cardioprotective effect of intermittent fasting is associated with an elevation of adiponectin levels in rats. J. Nutr. Biochem. 21, 413–417 (2010).
Cahill, G. F. Starvation in man. N. Engl. J. Med. 282, 668–675 (1970).
Browning, J. D., Baxter, J., Satapati, S. & Burgess, S. C. The effect of short-term fasting on liver and skeletal muscle lipid, glucose, and energy metabolism in healthy women and men. J. Lipid Res. 53, 577–586 (2012).
Foster, D. W. Studies in the ketosis of fasting. J. Clin. Invest. 46, 1283–1296 (1967).
Weir, H. J. et al. Dietary restriction and AMPK increase lifespan via mitochondrial network and peroxisome remodeling. Cell Metab. 26, 884–896 (2017).
Longo V. D. & Cortellino, S. Fasting, dietary restriction, and Immunosenescence. J. Allergy Clin. Immunol. 146, 1002–1004 (2020).
de Cabo, R. & Mattson, M. P. Effects of intermittent fasting on health, aging, and disease. N. Engl. J. Med. 381, 2541–2551 (2019).
Grandison, R. C., Wong, R., Bass, T. M., Partridge, L. & Piper, M. D. W. Effect of a standardised dietary restriction protocol on multiple laboratory strains of Drosophila melanogaster. PLoS ONE 4, e4067 (2009).
Le Bourg, E. & Minois, N. Failure to confirm increased longevity in Drosophila melanogaster submitted to a food restriction procedure. J. Gerontol. A. Biol. Sci. Med. Sci. 51, B280–B283 (1996).
Catterson, J. H. et al. Short-term, intermittent fasting induces long-lasting gut health and TOR-independent lifespan extension. Curr. Biol. 28, 1714–1724 (2018).
Goodrick, C. L., Ingram, D. K., Reynolds, M. A., Freeman, J. R. & Cider, N. L. Effects of intermittent feeding upon growth and life span in rats. Gerontology 28, 233–241 (1982).
Talan, M. I. & Ingram, D. K. Effect of intermittent feeding on thermoregulatory abilities of young and aged C57BL/6J mice. Arch. Gerontol. Geriatr. 4, 251–259 (1985).
Goodrick, C. L., Ingram, D. K., Reynolds, M. A., Freeman, J. R. & Cider, N. Effects of intermittent feeding upon body weight and lifespan in inbred mice: interaction of genotype and age. Mech. Ageing Dev. 55, 69–87 (1990).
Xie, K. et al. Every-other-day feeding extends lifespan but fails to delay many symptoms of aging in mice. Nat. Commun. 8, 155 (2017).
Pettan-Brewer, C. & Treuting, P. M. Practical pathology of aging mice. Pathobiol. Aging Age Relat. Dis. https://doi.org/10.3402/pba.v1i0.7202 (2011).
Blackwell, B. N., Bucci, T. J., Hart, R. W. & Turturro, A. Longevity, body weight, and neoplasia in ad libitum-fed and diet-restricted C57BL6 mice fed NIH-31 open formula diet. Toxicol. Pathol. 23, 570–582 (1995).
Arum, O., Bonkowski, M. S., Rocha, J. S. & Bartke, A. The growth hormone receptor gene-disrupted mouse fails to respond to an intermittent fasting diet. Aging Cell 8, 756–760 (2009).
Liao, C. Y., Rikke, B. A., Johnson, T. E., Diaz, V. & Nelson, J. F. Genetic variation in the murine lifespan response to dietary restriction: from life extension to life shortening. Aging Cell. 9, 92–95 (2010).
Singh, R. et al. Late-onset intermittent fasting dietary restriction as a potential intervention to retard age-associated brain function impairments in male rats. Age 34, 917–933 (2012).
Lee, G. D. et al. Transient improvement in cognitive function and synaptic plasticity in rats following cancer chemotherapy. Clin. Cancer Res. 12, 198–205 (2006).
Mager, D. E. et al. Caloric restriction and intermittent fasting alter spectral measures of heart rate and blood pressure variability in rats. FASEB J. 20, 631–637 (2006).
Wan, R., Camandola, S. & Mattson, M. P. Intermittent fasting and dietary supplementation with 2-deoxy-D-glucose improve functional and metabolic cardiovascular risk factors in rats. FASEB J. 17, 1133–1134 (2003).
Hatori, M. et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 15, 848–860 (2012).
Gabel, K. et al. Effects of 8-hour time restricted feeding on body weight and metabolic disease risk factors in obese adults: a pilot study. Nutr. Healthy Aging 4, 345–353 (2018).
Gabel, K. et al. Differential effects of alternate-day fasting versus daily calorie restriction on insulin resistance. Obesity (Silver Spring) 27, 1443–1450 (2019).
Stekovic, S. et al. Alternate day fasting improves physiological and molecular markers of aging in healthy, non-obese humans. Cell Metab. 30, 462–476 (2019).
Melkani, G. C. & Panda, S. Time-restricted feeding for prevention and treatment of cardiometabolic disorders. J. Physiol. 595, 3691–3700 (2017).
Wilkinson, M. J. et al. Ten-hour time-restricted eating reduces weight, blood pressure, and atherogenic lipids in patients with metabolic syndrome. Cell Metab. 31, 92–104 (2020).
Cienfuegos, S., Gabel, K. & Kalam, F. et al. Effects of 4- and 6-h time-restricted feeding on weight and cardiometabolic health: a randomized controlled trial in adults with obesity. Cell Metab. 32, 366–378 (2020).
Heilbronn, L. K., Smith, S. R., Martin, C. K., Anton, S. D. & Ravussin, E. Alternate-day fasting in nonobese subjects: effects on body weight, body composition, and energy metabolism. Am. J. Clin. Nutr. 81, 69–73 (2005).
Harvie, M. et al. The effect of intermittent energy and carbohydrate restriction v. daily energy restriction on weight loss and metabolic disease risk markers in overweight women. Br. J. Nutr. 110, 1534–1547 (2013).
Sichieri, R., Everhart, J. E. & Roth, H. A prospective study of hospitalization with gallstone disease among women: role of dietary factors, fasting period, and dieting. Am. J. Public Health 81, 880–884 (1991).
Rong, S. et al. Association of skipping breakfast with cardiovascular and all-cause mortality. J. Am. Coll. Cardiol. 73, 2025–2032 (2019).
Safdie, F. M. et al. Fasting and cancer treatment in humans: a case series report. Aging 1, 988–1007 (2009).
Raffaghello, L. et al. Starvation-dependent differential stress resistance protects normal but not cancer cells against high-dose chemotherapy. Proc. Natl Acad. Sci. USA 105, 8215–8220 (2008).
Goldhamer, A., Lisle, D., Parpia, B., Anderson, S. V. & Campbell, T. C. Medically supervised water-only fasting in the treatment of hypertension. J. Manipulative Physiol. Ther. 24, 335–339 (2001).
Goldhamer, A. C. et al. Medically supervised water-only fasting in the treatment of borderline hypertension. J. Altern. Complement. Med. 8, 643–650 (2002).
Wei, M. et al. Fasting-mimicking diet and markers/risk factors for aging, diabetes, cancer, and cardiovascular disease. Sci. Transl. Med. 9, eaai8700 (2017).
Mitchell, S. J. et al. Effects of sex, strain, and energy intake on hallmarks of aging in mice. Cell Metab. 23, 1093–1112 (2016).
Mirzaei, H., Raynes, R. & Longo, V. D. The conserved role of protein restriction in aging and disease. Curr. Opin. Clin. Nutr. Metab. Care 19, 74–79 (2016).
Mitchell, S. J. et al. Daily fasting improves health and survival in male mice independent of diet composition and calories. Cell Metab. 29, 221–228 (2018).
Varady, K. A., Roohk, D. J., Bruss, M. & Hellerstein, M. K. Alternate-day fasting reduces global cell proliferation rates independently of dietary fat content in mice. Nutr. Burbank Los Angel. Cty. Calif 25, 486–491 (2009).
Hahn, O., Drews, L. F. & Nguyen, A. et al. A nutritional memory effect counteracts benefits of dietary restriction in old mice. Nat. Metab. 1, 1059–1073 (2019).
Drinda, S. et al. Effects of periodic fasting on fatty liver index—a prospective observational study. Nutrients 11, 2601 (2019).
Wilhelmi de Toledo, F., Grundler, F., Bergouignan, A., Drinda, S. & Michalsen, A. Safety, health improvement and well-being during a 4 to 21-day fasting period in an observational study including 1422 subjects. PLoS ONE 14, e0209353 (2019).
Redman, L. M. et al. Metabolic slowing and reduced oxidative damage with sustained caloric restriction support the rate of living and oxidative damage theories of aging. Cell Metab. 27, 805–815 (2018).
Oh, T. J. et al. Body-weight fluctuation and incident diabetes mellitus, cardiovascular disease and mortality: a 16-year prospective cohort study. J. Clin. Endocrinol. Metab. 104, 639–646 (2019).
Longo, V. D. Programmed longevity, youthspan, and juventology. Aging Cell 18, e12843 (2018).
Harrison, D. E. et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460, 392–395 (2009).
Selman, C. et al. Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science 326, 140–144 (2009).
Ikeno, Y. et al. Reduced incidence and delayed occurrence of fatal neoplastic diseases in growth hormone receptor/binding protein knockout mice. J. Gerontol. A. Biol. Sci. Med. Sci. 64, 522–529 (2009).
Junnila, R. K., List, E. O., Berryman, D. E., Murrey, J. W. & Kopchick, J. J. The GH/IGF-1 axis in ageing and longevity. Nat. Rev. Endocrinol. 9, 366–376 (2013).
Bitto, A. et al. Transient rapamycin treatment can increase lifespan and healthspan in middle-aged mice. eLife 5, e16351 (2016).
Chen, C., Liu, Y., Liu, Y. & Zheng, P. mTOR regulation and therapeutic rejuvenation of aging hematopoietic stem cells. Sci. Signal. 2, ra75 (2009).
Fang, Y. et al. Effects of rapamycin on growth hormone receptor knockout mice. Proc. Natl Acad. Sci. USA 115, E1495–E1503 (2018).
Lamming, D. W. et al. Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science 335, 1638–1643 (2012).
Lamming, D. W. et al. Depletion of Rictor, an essential protein component of mTORC2, decreases male lifespan. Aging Cell 13, 911–917 (2014).
Yu, D. et al. Calorie-restriction-induced insulin sensitivity is mediated by adipose mTORC2 and not required for lifespan extension. Cell Rep. 29, 236–248 (2019).
Campbell, R. G., Johnson, R. J., King, R. H., Taverner, M. R. & Meisinger, D. J. Interaction of dietary protein content and exogenous porcine growth hormone administration on protein and lipid accretion rates in growing pigs. J. Anim. Sci. 68, 3217–3225 (1990).
Pedrosa, R. G., Donato, J., Pires, I. S. & Tirapegui, J. Leucine supplementation increases serum insulin-like growth factor 1 concentration and liver protein/RNA ratio in rats after a period of nutritional recovery. Appl. Physiol. Nutr. Metab. 38, 694–697 (2013).
Wolfson, R. L. et al. Sestrin2 is a leucine sensor for the mTORC1 pathway. Science 351, 43–48 (2016).
Chantranupong, L. et al. The CASTOR proteins are arginine sensors for the mTORC1 pathway. Cell 165, 153–164 (2016).
Solon-Biet, S. M. et al. Branched chain amino acids impact health and lifespan indirectly via amino acid balance and appetite control. Nat. Metab. 1, 532–545 (2019).
Di Biase, S. et al. Fasting regulates EGR1 and protects from glucose- and dexamethasone-dependent sensitization to chemotherapy. PLoS Biol. 15, e2001951 (2017).
Bartke, A., Sun, L. Y. & Longo, V. Somatotropic signaling: trade-offs between growth, reproductive development, and longevity. Physiol. Rev. 93, 571–598 (2013).
Fontana, L., Weiss, E. P., Villareal, D. T., Klein, S. & Holloszy, J. O. Long-term effects of calorie or protein restriction on serum IGF-1 and IGFBP-3 concentration in humans. Aging Cell 7, 681–687 (2008).
Moro, T. et al. Effects of eight weeks of time-restricted feeding (16/8) on basal metabolism, maximal strength, body composition, inflammation, and cardiovascular risk factors in resistance-trained males. J. Transl. Med. 14, 290 (2016).
Sorochynska, O. M. et al. Every-other-day feeding decreases glycolytic and mitochondrial energy-producing potentials in the brain and liver of young mice. Front. Physiol. 10, 1432 (2019).
Mihaylova, M. M. et al. Fasting activates fatty acid oxidation to enhance intestinal stem cell function during homeostasis and aging. Cell Stem Cell 22, 769–778 (2018).
Yilmaz, Ö. H. et al. mTORC1 in the Paneth cell niche couples intestinal stem-cell function to calorie intake. Nature 486, 490–495 (2012).
Lee, J., Duan, W., Long, J. M., Ingram, D. K. & Mattson, M. P. Dietary restriction increases the number of newly generated neural cells, and induces BDNF expression, in the dentate gyrus of rats. J. Mol. Neurosci. 15, 99–108 (2000).
Fusco, S. et al. A CREB–Sirt1–Hes1 circuitry mediates neural stem cell response to glucose availability. Cell Rep. 14, 1195–1205 (2016).
Horvath, S. et al. Obesity accelerates epigenetic aging of human liver. Proc. Natl Acad. Sci. USA 111, 15538–15543 (2014).
Jiang, N. et al. Dietary and genetic effects on age-related loss of gene silencing reveal epigenetic plasticity of chromatin repression during aging. Aging 5, 813–824 (2013).
Schultz, M. B. & Sinclair, D. A. When stem cells grow old: phenotypes and mechanisms of stem cell aging. Development 143, 3–14 (2016).
Zammit, P. S. et al. Pax7 and myogenic progression in skeletal muscle satellite cells. J. Cell Sci. 119, 1824–1832 (2006).
Madeo, F., Eisenberg, T., Pietrocola, F. & Kroemer, G. Spermidine in health and disease. Science 359, eaan2788 (2018).
García-Prat, L. et al. Autophagy maintains stemness by preventing senescence. Nature 529, 37–42 (2016).
Wei, S. et al. Intermittent administration of a fasting-mimicking diet intervenes in diabetes progression, restores β cells and reconstructs gut microbiota in mice. Nutr. Metab. 15, 80 (2018).
Holmes, A. J., Chew, Y. V. & Colakoglu, F. et al. Diet–microbiome interactions in health are controlled by intestinal nitrogen source constraints. Cell Metab. 25, 140–151 (2017).
Camandola, S. & Mattson, M. P. Brain metabolism in health, aging, and neurodegeneration. EMBO J. 36, 1474–1492 (2017).
Cignarella, F. et al. Intermittent fasting confers protection in CNS autoimmunity by altering the gut microbiota. Cell Metab. 27, 1222–1235 (2018).
Fulop, T., Witkowski, J. M., Olivieri, F. & Larbi, A. The integration of inflammaging in age-related diseases. Semin. Immunol. 40, 17–35 (2018).
Mirzaei, H., Di Biase, S. & Longo, V. D. Dietary interventions, cardiovascular aging, and disease: animal models and human studies. Circ. Res. 118, 1612–1625 (2016).
Mattson, M. P. Energy intake and exercise as determinants of brain health and vulnerability to injury and disease. Cell Metab. 16, 706–722 (2012).
Halagappa, V. K. M. et al. Intermittent fasting and caloric restriction ameliorate age-related behavioral deficits in the triple-transgenic mouse model of Alzheimer’s disease. Neurobiol. Dis. 26, 212–220 (2007).
Parrella, E. et al. Protein restriction cycles reduce IGF-1 and phosphorylated Tau, and improve behavioral performance in an Alzheimer’s disease mouse model. Aging Cell 12, 257–268 (2013).
Arumugam, T. V. et al. Age and energy intake interact to modify cell stress pathways and stroke outcome. Ann. Neurol. 67, 41–52 (2010).
Cheng, A. et al. Involvement of PGC-1α in the formation and maintenance of neuronal dendritic spines. Nat. Commun. 3, 1250 (2012).
Liu, Y. et al. SIRT3 mediates hippocampal synaptic adaptations to intermittent fasting and ameliorates deficits in APP mutant mice. Nat. Commun. 10, 1886 (2019).
Cheng, A. et al. SIRT3 haploinsufficiency aggravates loss of GABAergic interneurons and neuronal network hyperexcitability in an Alzheimer’s disease model. J. Neurosci. 40, 694–709 (2019).
Crabtree, D. M. & Zhang, J. Genetically engineered mouse models of Parkinson’s disease. Brain Res. Bull. 88, 13–32 (2012).
Griffioen, K. J. et al. Dietary energy intake modifies brainstem autonomic dysfunction caused by mutant α-synuclein. Neurobiol. Aging 34, 928–935 (2013).
Bai, X. et al. Rapamycin improves motor function, reduces 4-hydroxynonenal adducted protein in brain, and attenuates synaptic injury in a mouse model of synucleinopathy. Pathobiol. Aging Age Relat. Dis. 5, 28743 (2015).
Denkinger, M. D., Leins, H., Schirmbeck, R., Florian, M. C. & Geiger, H. HSC aging and senescent immune remodeling. Trends Immunol. 36, 815–824 (2015).
de Haan, G. & Lazare, S. S. Aging of hematopoietic stem cells. Blood 131, 479–487 (2018).
Ostroukhova, M. et al. The role of low-level lactate production in airway inflammation in asthma. Am. J. Physiol. Lung Cell. Mol. Physiol. 302, L300–307 (2012).
Choi, I. Y., Lee, C. & Longo, V. D. Nutrition and fasting mimicking diets in the prevention and treatment of autoimmune diseases and immunosenescence. Mol. Cell Endocrinol. 455, 4–12 (2017).
Tang, D. et al. Dietary restriction improves repopulation but impairs lymphoid differentiation capacity of hematopoietic stem cells in early aging. J. Exp. Med. 213, 535–553 (2016).
Piccio, L., Stark, J. L. & Cross, A. H. Chronic calorie restriction attenuates experimental autoimmune encephalomyelitis. J. Leukoc. Biol. 84, 940–948 (2008).
Kafami, L. et al. Intermittent feeding attenuates clinical course of experimental autoimmune encephalomyelitis in C57BL/6 mice. Avicienna J. Med. Biotechnol. 2, 47–52 (2010).
Collins, N. et al. The bone marrow protects and optimizes immunological memory during dietary restriction. Cell 178, 1088–1101 (2019).
Jordan, S. et al. Dietary intake regulates the circulating inflammatory monocyte pool. Cell 178, 1102–1114 (2019).
Nagai, M. et al. Fasting–refeeding impacts immune cell dynamics and mucosal immune responses. Cell 178, 1072–1087 (2019).
Lee, C. et al. Fasting cycles retard growth of tumors and sensitize a range of cancer cell types to chemotherapy. Sci. Transl. Med. 4, 124ra27 (2012).
Buono, R. & Longo, V. D. Starvation, stress resistance, and cancer. Trends Endocrinol. Metab. 29, 271–280 (2018).
Wei, T., Ye, P., Peng, X., Wu, L.-L. & Yu, G.-Y. Circulating adiponectin levels in various malignancies: an updated meta-analysis of 107 studies. Oncotarget 7, 48671–48691 (2016).
Elgendy, M. et al. Combination of hypoglycemia and metformin impairs tumor metabolic plasticity and growth by modulating the PP2A–GSK3β–MCL-1 axis. Cancer Cell 35, 798–815 (2019).
Di Biase, S. et al. Fasting-mimicking diet reduces HO-1 to promote T cell-mediated tumor cytotoxicity. Cancer Cell 30, 136–146 (2016).
Brandhorst, S. & Longo, V. D. Fasting and caloric restriction in cancer prevention and treatment. Recent Results Cancer Res. 207, 241–266 (2016).
Di Tano, M. et al. Synergistic effect of fasting-mimicking diet and vitamin C against KRAS mutated cancers. Nat. Commun. 11, 2332 (2020).
Caffa, I. et al. Fasting-mimicking diet and hormone therapy induce breast cancer regression. Nature 583, 620–624 (2020).
Pietrocola, F. et al. Caloric restriction mimetics enhance anticancer Immunosurveillance. Cancer Cell 30, 147–160 (2016).
Salazar, A. M. et al. Intestinal snakeskin limits microbial dysbiosis during aging and promotes longevity. iScience 9, 229–243 (2018).
Varady, K. A. et al. Alternate day fasting for weight loss in normal weight and overweight subjects: a randomized controlled trial. Nutr. J. 12, 146 (2013).
Tinsley, G. M. et al. Time-restricted feeding in young men performing resistance training: a randomized controlled trial. Eur. J. Sport Sci. 17, 200–207 (2017).
Acknowledgements
We would like to thank for their support the Associazione Italiana per la Ricerca sul Cancro (AIRC, IG nos. 17605 and 21820 to V.D.L.), the BC161452 grant of the Breast Cancer Research Program (US Department of Defense; to V.D.L.), and the NIA/NIH grants AG034906 and AG20642 to V.D.L.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
V.D.L. declares the following competing interests: V.D.L. has equity interest in L-Nutra, a company that develops medical food. The University of Southern California has licensed intellectual property to L-Nutra. As part of this license agreement, the University has the potential to receive royalty payments from L-Nutra.
Additional information
Peer review information Nature Aging thanks Rozalyn Anderson, Stephen Simpson and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Longo, V.D., Di Tano, M., Mattson, M.P. et al. Intermittent and periodic fasting, longevity and disease. Nat Aging 1, 47–59 (2021). https://doi.org/10.1038/s43587-020-00013-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s43587-020-00013-3
This article is cited by
-
Short-term fasting of a single amino acid extends lifespan
GeroScience (2024)
-
The impact of a fasting mimicking diet on the metabolic health of a prospective cohort of patients with prostate cancer: a pilot implementation study
Prostate Cancer and Prostatic Diseases (2023)
-
Refeeding-associated AMPKγ1 complex activity is a hallmark of health and longevity
Nature Aging (2023)
-
Fasting for 48 h induced similar glucose intolerance in both sexes despite greater perceived stress and decreased estradiol levels in females
European Journal of Applied Physiology (2023)
-
Could dietary restrictions affect periodontal disease? A systematic review
Clinical Oral Investigations (2023)