Abstract
Dietary restriction (DR) promotes healthy aging in diverse species. Essential amino acids play a key role, but the molecular mechanisms are unknown. The evolutionarily conserved Sestrin protein, an inhibitor of activity of the target of rapamycin complex 1 (TORC1), has recently been discovered as a sensor of amino acids in vitro. Here, we show that Sestrin null mutant flies have a blunted response of lifespan to DR. A mutant Sestrin fly line, with blocked amino acid binding and TORC1 activation, showed delayed development, reduced fecundity, extended lifespan and protection against lifespan-shortening, high-protein diets. Sestrin mediated reduced intestinal stem cell activity and gut cell turnover from DR, and stem cell proliferation in response to dietary amino acids, by regulating the TOR pathway and autophagy. Sestrin expression in intestinal stem cells was sufficient to maintain gut homeostasis and extend lifespan. Sestrin is thus a molecular link between dietary amino acids, stem cell function and longevity.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The structure of human Sestrin2 (Uniprot: P58004) used to model the structure of the fly Sestrin protein (Uniprot: Q9W1K5) is PDB 5DJ4 (https://doi.org/10.2210/pdb5DJ4/pdb). All data that support the findings of this study are available from the corresponding authors upon request. Source data are provided with this paper.
References
Fontana, L., Partridge, L. & Longo, V. D. Extending healthy life span—from yeast to humans. Science 328, 321–326 (2010).
Kenyon, C. J. The genetics of ageing. Nature 464, 504–512 (2010).
Weindruch, R., Walford, R. L., Fligiel, S. & Guthrie, D. The retardation of aging in mice by dietary restriction: longevity, cancer, immunity and lifetime energy intake. J. Nutr. 116, 641–654 (1986).
Colman, R. J. et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science 325, 201–204 (2009).
Mattison, J. A. et al. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature 489, 318–321 (2012).
Most, J., Tosti, V., Redman, L. M. & Fontana, L. Calorie restriction in humans: an update. Ageing Res. Rev. 39, 36–45 (2017).
Mair, W., Piper, M. D. & Partridge, L. Calories do not explain extension of life span by dietary restriction in Drosophila. PLoS Biol. 3, e223 (2005).
Solon-Biet, S. M. et al. The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum-fed mice. Cell Metab. 19, 418–430 (2014).
Green, C. L. & Lamming, D. W. Regulation of metabolic health by essential dietary amino acids. Mech. Ageing Dev. 177, 186–200 (2019).
Simpson, S. J. et al. Dietary protein, aging and nutritional geometry. Ageing Res. Rev. 39, 78–86 (2017).
Grandison, R. C., Piper, M. D. & Partridge, L. Amino-acid imbalance explains extension of lifespan by dietary restriction in Drosophila. Nature 462, 1061–1064 (2009).
Miller, R. A. et al. Methionine-deficient diet extends mouse lifespan, slows immune and lens aging, alters glucose, T4, IGF-I and insulin levels, and increases hepatocyte MIF levels and stress resistance. Aging Cell 4, 119–125 (2005).
Yu, D. et al. Short-term methionine deprivation improves metabolic health via sexually dimorphic, mTORC1-independent mechanisms. FASEB J. 32, 3471–3482 (2018).
Juricic, P., Gronke, S. & Partridge, L. Branched-chain amino acids have equivalent effects to other essential amino acids on lifespan and ageing-related traits in Drosophila. J. Gerontol. A 75, 24–31 (2019).
Fontana, L. et al. Decreased consumption of branched-chain amino acids improves metabolic health. Cell Rep. 16, 520–530 (2016).
Solon-Biet, S. M. et al. Branched-chain amino acids impact health and lifespan indirectly via amino acid balance and appetite control. Nat. Metab. 1, 532–545 (2019).
Lee, J., Seroogy, K. B. & Mattson, M. P. Dietary restriction enhances neurotrophin expression and neurogenesis in the hippocampus of adult mice. J. Neurochem. 80, 539–547 (2002).
Cerletti, M., Jang, Y. C., Finley, L. W., Haigis, M. C. & Wagers, A. J. Short-term calorie restriction enhances skeletal muscle stem cell function. Cell Stem Cell 10, 515–519 (2012).
Fan, P., Liu, P., Song, P., Chen, X. & Ma, X. Moderate dietary protein restriction alters the composition of gut microbiota and improves ileal barrier function in adult pig model. Sci. Rep. 7, 43412 (2017).
Regan, J. C. et al. Sex difference in pathology of the ageing gut mediates the greater response of female lifespan to dietary restriction. eLife 5, e10956 (2016).
Micchelli, C. A. & Perrimon, N. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature 439, 475–479 (2006).
Ohlstein, B. & Spradling, A. The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature 439, 470–474 (2006).
Biteau, B. et al. Lifespan extension by preserving proliferative homeostasis in Drosophila. PLoS Genet. 6, e1001159 (2010).
Guo, L., Karpac, J., Tran, S. L. & Jasper, H. PGRP-SC2 promotes gut immune homeostasis to limit commensal dysbiosis and extend lifespan. Cell 156, 109–122 (2014).
Fan, X. et al. Rapamycin preserves gut homeostasis during Drosophila aging. Oncotarget 6, 35274–35283 (2015).
Gronke, S., Clarke, D. F., Broughton, S., Andrews, T. D. & Partridge, L. Molecular evolution and functional characterization of Drosophila insulin-like peptides. PLoS Genet. 6, e1000857 (2010).
Bjedov, I. et al. Mechanisms of life span extension by rapamycin in the fruit fly Drosophila melanogaster. Cell Metab. 11, 35–46 (2010).
Zoncu, R., Efeyan, A. & Sabatini, D. M. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat. Rev. Mol. Cell Biol. 12, 21–35 (2011).
Shimobayashi, M. & Hall, M. N. Making new contacts: the mTOR network in metabolism and signalling crosstalk. Nat. Rev. Mol. Cell Biol. 15, 155–162 (2014).
Chantranupong, L. et al. The Sestrins interact with GATOR2 to negatively regulate the amino-acid-sensing pathway upstream of mTORC1. Cell Rep. 9, 1–8 (2014).
Lee, J. H., Budanov, A. V. & Karin, M. Sestrins orchestrate cellular metabolism to attenuate aging. Cell Metab. 18, 792–801 (2013).
Budanov, A. V. & Karin, M. p53 target genes Sestrin1 and Sestrin2 connect genotoxic stress and mTOR signaling. Cell 134, 451–460 (2008).
Wolfson, R. L. et al. Sestrin2 is a leucine sensor for the mTORC1 pathway. Science 351, 43–48 (2016).
Saxton, R. A. et al. Structural basis for leucine sensing by the Sestrin2-mTORC1 pathway. Science 351, 53–58 (2016).
Lee, J. H., Cho, U. S. & Karin, M. Sestrin regulation of TORC1: is Sestrin a leucine sensor? Sci. Signal. 9, re5 (2016).
Lee, J. H. et al. Sestrin as a feedback inhibitor of TOR that prevents age-related pathologies. Science 327, 1223–1228 (2010).
Arrese, E. L. & Soulages, J. L. Insect fat body: energy, metabolism, and regulation. Annu. Rev. Entomol. 55, 207–225 (2010).
Scott, R. C., Schuldiner, O. & Neufeld, T. P. Role and regulation of starvation-induced autophagy in the Drosophila fat body. Dev. Cell 7, 167–178 (2004).
Luis, N. M. et al. Intestinal IRE1 is required for increased triglyceride metabolism and longer lifespan under dietary restriction. Cell Rep. 17, 1207–1216 (2016).
Biteau, B., Hochmuth, C. E. & Jasper, H. JNK activity in somatic stem cells causes loss of tissue homeostasis in the aging Drosophila gut. Cell Stem Cell 3, 442–455 (2008).
Rera, M., Clark, R. I. & Walker, D. W. Intestinal barrier dysfunction links metabolic and inflammatory markers of aging to death in Drosophila. Proc. Natl Acad. Sci. USA 109, 21528–21533 (2012).
Wang, X. & Proud, C. G. Nutrient control of TORC1, a cell-cycle regulator. Trends Cell Biol. 19, 260–267 (2009).
Kamada, Y. et al. Tor directly controls the Atg1 kinase complex to regulate autophagy. Mol. Cell. Biol. 30, 1049–1058 (2010).
Nagy, P., Varga, A., Kovacs, A. L., Takats, S. & Juhasz, G. How and why to study autophagy in Drosophila: it’s more than just a garbage chute. Methods 75, 151–161 (2015).
Nezis, I. P. et al. Autophagic degradation of dBruce controls DNA fragmentation in nurse cells during late Drosophila melanogaster oogenesis. J. Cell Biol. 190, 523–531 (2010).
Williams, R. A., Smith, T. K., Cull, B., Mottram, J. C. & Coombs, G. H. ATG5 is essential for ATG8-dependent autophagy and mitochondrial homeostasis in Leishmania major. PLoS Pathog. 8, e1002695 (2012).
Barnard, N. D. et al. A low-fat vegan diet and a conventional diabetes diet in the treatment of type 2 diabetes: a randomized, controlled, 74-wk clinical trial. Am. J. Clin. Nutr. 89, 1588S–1596S (2009).
Lee, J. H. et al. Maintenance of metabolic homeostasis by Sestrin2 and Sestrin3. Cell Metab. 16, 311–321 (2012).
Kim, M. et al. Sestrins are evolutionarily conserved mediators of exercise benefits. Nat. Commun. 11, 190 (2020).
Segales, J. et al. Sestrin prevents atrophy of disused and aging muscles by integrating anabolic and catabolic signals. Nat. Commun. 11, 189 (2020).
Kapahi, P. et al. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr. Biol. 14, 885–890 (2004).
Hahn, O. et al. Hepatic gene body hypermethylation is a shared epigenetic signature of murine longevity. PLoS Genet. 14, e1007766 (2018).
Fok, W. C. et al. Combined treatment of rapamycin and dietary restriction has a larger effect on the transcriptome and metabolome of liver. Aging Cell 13, 311–319 (2014).
Dobson, A. J. et al. Tissue-specific transcriptome profiling of Drosophila reveals roles for GATA transcription factors in longevity by dietary restriction. npj Aging Mech. Dis. 4, 5 (2018).
Xi, J. et al. The TORC1 inhibitor Nprl2 protects age-related digestive function in Drosophila. Aging 11, 9811–9828 (2019).
Haller, S. et al. mTORC1 activation during repeated regeneration impairs somatic stem cell maintenance. Cell Stem Cell 21, 806–818 (2017).
Demetriades, C., Doumpas, N. & Teleman, A. A. Regulation of TORC1 in response to amino acid starvation via lysosomal recruitment of TSC2. Cell 156, 786–799 (2014).
Amcheslavsky, A., Ito, N., Jiang, J. & Ip, Y. T. Tuberous sclerosis complex and Myc coordinate the growth and division of Drosophila intestinal stem cells. J. Cell Biol. 193, 695–710 (2011).
Kapuria, S., Karpac, J., Biteau, B., Hwangbo, D. & Jasper, H. Notch-mediated suppression of TSC2 expression regulates cell differentiation in the Drosophila intestinal stem cell lineage. PLoS Genet. 8, e1003045 (2012).
Jasper, H. & Jones, D. L. Metabolic regulation of stem cell behavior and implications for aging. Cell Metab. 12, 561–565 (2010).
Rodgers, J. T. et al. mTORC1 controls the adaptive transition of quiescent stem cells from G0 to GAlert. Nature 510, 393–396 (2014).
Ertl, R. P., Chen, J., Astle, C. M., Duffy, T. M. & Harrison, D. E. Effects of dietary restriction on hematopoietic stem-cell aging are genetically regulated. Blood 111, 1709–1716 (2008).
Rafalski, V. A. & Brunet, A. Energy metabolism in adult neural stem cell fate. Prog. Neurobiol. 93, 182–203 (2011).
Yousefi, M. et al. Calorie restriction governs intestinal epithelial regeneration through cell-autonomous regulation of mTORC1 in reserve stem cells. Stem Cell Rep. 10, 703–711 (2018).
Nagy, P., Sandor, G. O. & Juhasz, G. Autophagy maintains stem cells and intestinal homeostasis in Drosophila. Sci. Rep. 8, 4644 (2018).
Asano, J. et al. Intrinsic autophagy is required for the maintenance of intestinal stem cells and for irradiation-induced intestinal regeneration. Cell Rep. 20, 1050–1060 (2017).
Pan, H. Z., Cai, N., Li, M., Liu, G. H. & Belmonte, J. C. I. Autophagic control of cell ‘stemness’. EMBO Mol. Med. 5, 327–331 (2013).
Filer, D. et al. RNA polymerase III limits longevity downstream of TORC1. Nature 552, 263–267 (2017).
Theodosiou, N. A. & Xu, T. Use of FLP/FRT system to study Drosophila development. Methods 14, 355–365 (1998).
Jiang, H., Grenley, M. O., Bravo, M. J., Blumhagen, R. Z. & Edgar, B. A. EGFR/Ras/MAPK signaling mediates adult midgut epithelial homeostasis and regeneration in Drosophila. Cell Stem Cell 8, 84–95 (2011).
Zielke, N. et al. Fly-FUCCI: a versatile tool for studying cell proliferation in complex tissues. Cell Rep. 7, 588–598 (2014).
Roman, G., Endo, K., Zong, L. & Davis, R. L. P{Switch}, a system for spatial and temporal control of gene expression in Drosophila melanogaster. Proc. Natl Acad. Sci. USA 98, 12602–12607 (2001).
Pei, J., Kim, B. H. & Grishin, N. V. PROMALS3D: a tool for multiple protein sequence and structure alignments. Nucleic Acids Res. 36, 2295–2300 (2008).
Pettersen, E. F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Bischof, J., Maeda, R. K., Hediger, M., Karch, F. & Basler, K. An optimized transgenesis system for Drosophila using germ-line-specific phiC31 integrases. Proc. Natl Acad. Sci. USA 104, 3312–3317 (2007).
Markstein, M., Pitsouli, C., Villalta, C., Celniker, S. E. & Perrimon, N. Exploiting position effects and the gypsy retrovirus insulator to engineer precisely expressed transgenes. Nat. Genet. 40, 476–483 (2008).
Port, F., Chen, H. M., Lee, T. & Bullock, S. L. Optimized CRISPR/Cas tools for efficient germline and somatic genome engineering in Drosophila. Proc. Natl Acad. Sci. USA 111, E2967–E2976 (2014).
Rubin, G. M. et al. A Drosophila complementary DNA resource. Science 287, 2222–2224 (2000).
Acknowledgements
We thank J. H. Lee, B. Edgar, G. Juhasz, the Bloomington Stock Center and the VDRC Stock Center for fly strains and reagents. We are also grateful to all members of the Partridge Lab for helpful insights, and to C. Demetriades for critical comments. Imaging was performed in the FACS and Imaging Core Facility, and amino acid concentrations were determined in the Metabolomics Core Facility at the Max Planck Institute for Biology of Ageing. The work was supported by a Swiss National Science Foundation (SNSF) postdoc fellowship (P2BEP3_162093) to J.L. and by funding from the Max Planck Society to L.P. The research leading to these results has received funding from the European Research Council under the European Union’s Seventh Framework Programme (FP7/2007-2013)/ERC grant agreement no. 268739.
Author information
Authors and Affiliations
Contributions
J.L., S.G. and L.P. conceived and designed the study. J.L. conducted most experiments, U.T. and A.M.-H. provided assistance. J.E. contributed to the generation of transgenic flies. J.L. and S.G. analysed the data. J.L., S.G. and L.P. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended Data
Extended Data Fig. 1 Characterization of a Sestrin loss-of-function allele and its effects on fly development.
a, Genomic locus of the Sestrin gene. In Sesn3F6 mutant flies the first two non-coding exons of the Sestrin gene are deleted. b, qRT-PCR analysis showed strongly reduced Sestrin mRNA levels in Sesn3F6 mutant flies. Data are presented as mean ± SEM. N = 3 biologically independent samples. Unpaired two-tailed t-test. c, No Sestrin protein was detected in Sesn3F6 mutant flies by immunoblotting analysis. An additional control sample (Sesn8A11 null mutant) was loaded in between. d, Sesn3F6 mutant flies showed a decreased developmental time compared with wDah wild type control flies (N = 10 vials, 40 embryos each). Permutation test (R, statmod package) showed that Sesn3F6 mutants eclosed significantly earlier than wild type flies. P value was adjusted for multiple testing. e, Sesn3F6 mutant flies had increased body weight. Median, 25th and 75th percentiles, and Tukey whiskers are indicated in box-and-whisker plots. N = 20 pairs of flies, unpaired two-tailed t-test.
Extended Data Fig. 2 Sestrin mRNA and protein levels are not affected by the SesnR407A mutation.
a-c, qRT-PCR (a) and immunoblotting (b,c) analyses confirmed that there was no change in Sestrin mRNA or protein levels in SesnR407A mutant flies compared with Sesnwt control flies. The Myc tag was used to detect Sestrin proteins in immunoblotting. Data are presented as mean ± SEM. N = 3 biologically independent samples, unpaired two-tailed t-test.
Extended Data Fig. 3 SesnR407A mutation reduces cell growth and decreases fecundity.
a,b, SesnR407A mutation did not regulate pAMPKα activity. Fat body tissues from 3rd instar larvae grown under standard conditions were subjected to immunoblotting analysis. pAMPKα was normalized to Tubulin. Data are presented as mean ± SEM. N = 3 biologically independent samples, unpaired two-tailed t-test. c,d, SesnR407A-dependent reduction of cell size was cell autonomous. Confocal microscopy images of SesnR407A mutant clones in larval fat bodies (c). DNA (DAPI, blue), Actin (Phalloidin, red), and GFP (green). GFP-negative cells, outlined with white dashed lines, indicate Sestrin homozygous clones. Size of homozygous cells was normalized to wild type twin clones (marked by bright GFP). Scale bar: 25 μm. (d) Quantification of cell size from confocal images. N = 13 (Sesnwt) and N = 14 (SesnR407A) independent fat body samples, unpaired two-tailed t-test. e, SesnR407A mutant flies showed reduced cumulative egg laying. N = 10 vials with 20 flies each, two-tailed, Mann–Whitney test. Median, 25th and 75th percentiles, and Tukey whiskers are indicated in box-and-whisker plots (d, e). Outliers are shown as open circles. f, Reduced fecundity was restricted to early-life fecundity. Data are presented as mean ± SEM. N = 10 vials, two-tailed, Mann–Whitney test.
Extended Data Fig. 4 Sestrin is required for better maintenance of gut homeostasis by DR.
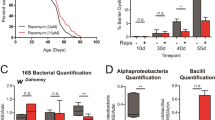
a-d, Sestrin function was required for preventing gut dysplasia from a high-protein diet. Gut dysplasia of wild type and Sesn mutant flies on fully fed (2.0x) and DR (1.0x) food was measured. (a,c) Representative gut images from 45 days old flies. DNA (DAPI) was blue. Epithelial layers are indicated by dashed lines. Scale bar represents 20 μm. (b, d) Quantification of gut dysplasia in Sesn3F6 (b) and in SesnR407A (d) mutant females under DR. N = 13 guts (b), N = 11 guts (d). Interaction between diet and genotypes was significant: two-way ANOVA, P = 0.024 (b), P = 0.011 (d). e,f, Sestrin was also required for maintenance of gut epithelial barrier function under DR. Smurf phenotypes of wild type and Sesn mutant flies on fully fed (2.0x) and DR (1.0x) food were scored at the age of 50 days. Proportion of Smurf flies in Sesn3F6 (e) and in SesnR407A (f) mutant females. N = 15 vials (e, f). Interaction between diet and genotypes was significant: two-way ANOVA, P = 0.04 (e), P = 0.02 (f). Median, 25th and 75th percentiles, and Tukey whiskers are indicated in box-and-whisker plots ((b, d-f)). Outliers are shown as open circles. Statistics in (b,d-f): two-way ANOVA followed by Bonferroni’s post-hoc test, P values were adjusted for multiple comparisons.
Extended Data Fig. 5 Sestrin over-expression induces autophagic flux in gut stem cells.
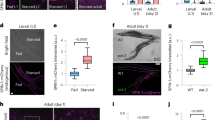
a,b, Sestrin mRNA expression levels in RNAi-mediated knockdown and over-expression conditions. The ubiquitous, constitutive da-Gal4 driver was used to drive expression of each construct. (a) RNAi-mediated Sestrin knockdown significantly reduced Sestrin mRNA level, whereas (b) Sestrin over-expression significantly increased Sestrin mRNA level. Data are presented as mean ± SEM. N = 3 biologically independent samples, unpaired two-tailed t-test. c, Autophagic flux in ISCs upon Sestrin over-expression. Sestrin and a GFP::mCherry::Atg8a reporter were co-expressed in ISCs using the esg-Gal4 driver. GFP in green, mCherry in red, and DAPI (DNA) in blue. A representative gut image from 6 guts was shown. A strong mCherry signal was detected when Sestrin was over-expressed. The lower panel shows the magnification of the inset in the upper panel. Scale bars: 20 μm.
Supplementary information
Supplementary Information
Supplementary Tables 1–7.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 2
Uncropped western blots.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 3
Uncropped western blots.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 7
Statistical source data.
Source Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 1
Uncropped western blots.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 2
Uncropped western blots.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 3
Uncropped western blots.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Rights and permissions
About this article
Cite this article
Lu, J., Temp, U., Müller-Hartmann, A. et al. Sestrin is a key regulator of stem cell function and lifespan in response to dietary amino acids. Nat Aging 1, 60–72 (2021). https://doi.org/10.1038/s43587-020-00001-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s43587-020-00001-7
This article is cited by
-
Inhibition of S6K lowers age-related inflammation and increases lifespan through the endolysosomal system
Nature Aging (2024)
-
Machine learning-based predictions of dietary restriction associations across ageing-related genes
BMC Bioinformatics (2022)
-
The functions and roles of sestrins in regulating human diseases
Cellular & Molecular Biology Letters (2022)
-
Dietary regulation in health and disease
Signal Transduction and Targeted Therapy (2022)
-
Sensing of the non-essential amino acid tyrosine governs the response to protein restriction in Drosophila
Nature Metabolism (2022)