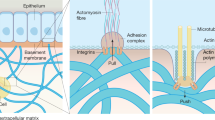
Abstract
Cells’ local mechanical environment can be as important in guiding cellular responses as many well-characterized biochemical cues. Hydrogels that mimic the native extracellular matrix can provide these mechanical cues to encapsulated cells, allowing for the study of their impact on cellular behaviours. Moreover, by harnessing cellular responses to mechanical cues, hydrogels can be used to create tissues in vitro for regenerative medicine applications and for disease modelling. This Primer outlines the importance and challenges of creating hydrogels that mimic the mechanical and biological properties of the native extracellular matrix. The design of hydrogels for mechanobiology studies is discussed, including the appropriate choice of cross-linking chemistry and strategies to tailor hydrogel mechanical cues. Techniques for characterizing hydrogels are explained, highlighting methods used to analyse cell behaviour. Example applications in regenerative medicine and for studying fundamental mechanobiological processes are provided, along with a discussion of the limitations of hydrogels as mimetics of the native extracellular matrix. The Primer ends with an outlook for the field, focusing on emerging technologies that will enable new insights into mechanobiology and its role in tissue homeostasis and disease.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
$99.00 per year
only $99.00 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Petzold, J. & Gentleman, E. Intrinsic mechanical cues and their impact on stem cells and embryogenesis. Front. Cell Dev. Biol. 9, 761871 (2021).
Carey, E. J. Direct observations on the transformation of the mesenchyme in the thigh of the pig embryo (Sus scrofa), with especial reference to the genesis of the thigh muscles, of the knee- and hip-joints, and of the primary bone of the femur. J. Morphol. 37, 1–77 (1922).
Panciera, T., Azzolin, L., Cordenonsi, M. & Piccolo, S. Mechanobiology of YAP and TAZ in physiology and disease. Nat. Rev. Mol. Cell Biol. 18, 758–770 (2017).
van Helvert, S., Storm, C. & Friedl, P. Mechanoreciprocity in cell migration. Nat. Cell Biol. 20, 8–20 (2018).
Vining, K. H. & Mooney, D. J. Mechanical forces direct stem cell behaviour in development and regeneration. Nat. Rev. Mol. Cell Biol. 18, 728–742 (2017).
Huse, M. Mechanical forces in the immune system. Nat. Rev. Immunol. 17, 679–690 (2017).
Lei, K. et al. Cancer-cell stiffening via cholesterol depletion enhances adoptive T-cell immunotherapy. Nat. Biomed. Eng. 5, 1411–1425 (2021).
Dupont, S. & Wickstrom, S. A. Mechanical regulation of chromatin and transcription. Nat. Rev. Genet. 23, 624–643 (2022).
Foyt, D. A., Norman, M. D. A., Yu, T. T. L. & Gentleman, E. Exploiting advanced hydrogel technologies to address key challenges in regenerative medicine. Adv. Healthc. Mater. 7, e1700939 (2018).
Walters, N. J. & Gentleman, E. Evolving insights in cell–matrix interactions: elucidating how non-soluble properties of the extracellular niche direct stem cell fate. Acta Biomater. 11, 3–16 (2015).
Engler, A. J., Sen, S., Sweeney, H. L. & Discher, D. E. Matrix elasticity directs stem cell lineage specification. Cell 126, 677–689 (2006). Seminal work that identified a role for 2D matrix stiffness in directing hMSC differentiation.
Gilbert, P. M. et al. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science 329, 1078–1081 (2010). This article reports that culturing muscle satellite cells on 2D PEG hydrogels that match the stiffness of native muscle preserves their capacity for self-renewal and to contribute to muscle regeneration.
Kim, S., Uroz, M., Bays, J. L. & Chen, C. S. Harnessing mechanobiology for tissue engineering. Dev. Cell 56, 180–191 (2021).
Khetan, S. et al. Degradation-mediated cellular traction directs stem cell fate in covalently crosslinked three-dimensional hydrogels. Nat. Mater. 12, 458–465 (2013). This article highlighted that in covalently cross-linked hydrogels, mechano-sensing in 3D was dependent on cells’ ability to degrade and thus apply traction on their surrounding matrix.
Huebsch, N. Translational mechanobiology: designing synthetic hydrogel matrices for improved in vitro models and cell-based therapies. Acta Biomater. 94, 97–111 (2019).
Li, L., Eyckmans, J. & Chen, C. S. Designer biomaterials for mechanobiology. Nat. Mater. 16, 1164–1168 (2017).
Lee, H. P., Gu, L., Mooney, D. J., Levenston, M. E. & Chaudhuri, O. Mechanical confinement regulates cartilage matrix formation by chondrocytes. Nat. Mater. 16, 1243–1251 (2017). This article uses slow and fast relaxing alginate hydrogels to identify how mechanical confinement, which restricts cell volume changes, acts as an adhesion-independent means of 3D mechano-sensing.
Below, C. R. et al. A microenvironment-inspired synthetic three-dimensional model for pancreatic ductal adenocarcinoma organoids. Nat. Mater. 21, 110–119 (2022).
Ruiter, F. A. A. et al. Soft, dynamic hydrogel confinement improves kidney organoid lumen morphology and reduces epithelial–mesenchymal transition in culture. Adv. Sci. 9, e2200543 (2022).
Jowett, G. M. et al. ILC1 drive intestinal epithelial and matrix remodelling. Nat. Mater. 20, 250–259 (2021). This article shows that immune cells co-cultured with human iPSC intestinal organoids within PEG hydrogels prompt mesenchymal cells to remodel their local microenvironment, changing its mechanical stiffness.
Giobbe, G. G. et al. Extracellular matrix hydrogel derived from decellularized tissues enables endodermal organoid culture. Nat. Commun. 10, 5658 (2019).
Kim, S. et al. Tissue extracellular matrix hydrogels as alternatives to Matrigel for culturing gastrointestinal organoids. Nat. Commun. 13, 1692 (2022).
Moura, B. S. et al. Advancing tissue decellularized hydrogels for engineering human organoids. Adv. Funct. Mater. https://doi.org/10.1002/adfm.202202825 (2022).
Hughes, C. S., Postovit, L. M. & Lajoie, G. A. Matrigel: a complex protein mixture required for optimal growth of cell culture. Proteomics 10, 1886–1890 (2010).
Nichol, J. W. et al. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials 31, 5536–5544 (2010).
Singh, R. K., Seliktar, D. & Putnam, A. J. Capillary morphogenesis in PEG–collagen hydrogels. Biomaterials 34, 9331–9340 (2013).
Baldwin, A. D. & Kiick, K. L. Polysaccharide-modified synthetic polymeric biomaterials. Biopolymers 94, 128–140 (2010).
Kloxin, A. M., Kasko, A. M., Salinas, C. N. & Anseth, K. S. Photodegradable hydrogels for dynamic tuning of physical and chemical properties. Science 324, 59–63 (2009).
Lutolf, M. P. et al. Synthetic matrix metalloproteinase-sensitive hydrogels for the conduction of tissue regeneration: engineering cell-invasion characteristics. Proc. Natl Acad. Sci. USA 100, 5413–5418 (2003).
Phelps, E. A. et al. Maleimide cross-linked bioactive PEG hydrogel exhibits improved reaction kinetics and cross-linking for cell encapsulation and in situ delivery. Adv. Mater. 24, 64–70 (2012).
Das, R. K., Gocheva, V., Hammink, R., Zouani, O. F. & Rowan, A. E. Stress-stiffening-mediated stem-cell commitment switch in soft responsive hydrogels. Nat. Mater. 15, 318–325 (2016). This article describes using fully synthetic polyisocyanopeptide hydrogels to identify a role for stress stiffening in directing hMSC fate in 3D.
Kouwer, P. H. et al. Responsive biomimetic networks from polyisocyanopeptide hydrogels. Nature 493, 651–655 (2013).
Zimoch, J. et al. Polyisocyanopeptide hydrogels: a novel thermo-responsive hydrogel supporting pre-vascularization and the development of organotypic structures. Acta Biomater. 70, 129–139 (2018).
Faroni, A., Workman, V. L., Saiani, A. & Reid, A. J. Self-assembling peptide hydrogel matrices improve the neurotrophic potential of human adipose-derived stem cells. Adv. Healthc. Mater. 8, e1900410 (2019).
Kyburz, K. A. & Anseth, K. S. Synthetic mimics of the extracellular matrix: how simple is complex enough? Ann. Biomed. Eng. 43, 489–500 (2015).
Huettner, N., Dargaville, T. R. & Forget, A. Discovering cell-adhesion peptides in tissue engineering: beyond RGD. Trends Biotechnol. 36, 372–383 (2018).
Reyes, C. D. & Garcia, A. J. Engineering integrin-specific surfaces with a triple-helical collagen-mimetic peptide. J. Biomed. Mater. Res. A 65, 511–523 (2003).
Nagase, H. & Fields, G. B. Human matrix metalloproteinase specificity studies using collagen sequence-based synthetic peptides. Biopolymers 40, 399–416 (1996).
Patterson, J. & Hubbell, J. A. SPARC-derived protease substrates to enhance the plasmin sensitivity of molecularly engineered PEG hydrogels. Biomaterials 32, 1301–1310 (2011).
Madl, C. M. et al. Maintenance of neural progenitor cell stemness in 3D hydrogels requires matrix remodelling. Nat. Mater. 16, 1233–1242 (2017).
Hennink, W. E. & van Nostrum, C. F. Novel crosslinking methods to design hydrogels. Adv. Drug Deliv. Rev. 54, 13–36 (2002).
Rizwan, M., Baker, A. E. G. & Shoichet, M. S. Designing hydrogels for 3D cell culture using dynamic covalent crosslinking. Adv. Healthc. Mater. 10, e2100234 (2021).
Wilems, T. S. et al. Effects of free radical initiators on polyethylene glycol dimethacrylate hydrogel properties and biocompatibility. J. Biomed. Mater. Res. A 105, 3059–3068 (2017).
Azagarsamy, M. A. & Anseth, K. S. Bioorthogonal click chemistry: an indispensable tool to create multifaceted cell culture scaffolds. ACS Macro Lett. 2, 5–9 (2013).
Hoyle, C. E. & Bowman, C. N. Thiol–ene click chemistry. Angew. Chem. Int. Ed. 49, 1540–1573 (2010).
Ehrbar, M. et al. Enzymatic formation of modular cell-instructive fibrin analogs for tissue engineering. Biomaterials 28, 3856–3866 (2007).
Hu, B. H. & Messersmith, P. B. Rational design of transglutaminase substrate peptides for rapid enzymatic formation of hydrogels. J. Am. Chem. Soc. 125, 14298–14299 (2003).
Tang, S. C., Richardson, B. M. & Anseth, K. S. Dynamic covalent hydrogels as biomaterials to mimic the viscoelasticity of soft tissues. Prog. Mater. Sci. 120, 100738 (2021).
Parhi, R. Cross-linked hydrogel for pharmaceutical applications: a review. Adv. Pharm. Bull. 7, 515–530 (2017).
Douglas, S. M. et al. Self-assembly of DNA into nanoscale three-dimensional shapes. Nature 459, 414–418 (2009).
Hartgerink, J. D., Beniash, E. & Stupp, S. I. Self-assembly and mineralization of peptide-amphiphile nanofibers. Science 294, 1684–1688 (2001).
Chrisnandy, A., Blondel, D., Rezakhani, S., Broguiere, N. & Lutolf, M. P. Synthetic dynamic hydrogels promote degradation-independent in vitro organogenesis. Nat. Mater. 21, 479–487 (2021). This original research article explores recent advances in using hybrid hydrogels (covalent and supramolecular) to create materials that exhibit stress relaxation and support the culture of crypt-bearing intestinal organoids.
Dankers, P. Y. et al. Hierarchical formation of supramolecular transient networks in water: a modular injectable delivery system. Adv. Mater. 24, 2703–2709 (2012).
Diba, M. et al. Engineering the dynamics of cell adhesion cues in supramolecular hydrogels for facile control over cell encapsulation and behavior. Adv. Mater. 33, e2008111 (2021). This article describes supramocular hydrogels based on the ureido-pyrimidinone motif with tunable mechanical, dynamic and bioactive properties for cell and spheroid encapsulation.
Vereroudakis, E. et al. Competitive supramolecular associations mediate the viscoelasticity of binary hydrogels. ACS Cent. Sci. 6, 1401–1411 (2020).
Hafeez, S. et al. Modular mixing of benzene-1,3,5-tricarboxamide supramolecular hydrogelators allows tunable biomimetic hydrogels for control of cell aggregation in 3D. Biomater. Sci. 10, 4740–4755 (2022).
Loebel, C. et al. Tailoring supramolecular guest–host hydrogel viscoelasticity with covalent fibrinogen double networks. J. Mater. Chem. B 7, 1753–1760 (2019).
Dhand, A. P. et al. Simultaneous one-pot interpenetrating network formation to expand 3D processing capabilities. Adv. Mater. 34, e2202261 (2022).
Evans, N. D. & Gentleman, E. The role of material structure and mechanical properties in cell–matrix interactions. J. Mater. Chem. B 2, 2345–2356 (2014).
Guimaraes, C. F., Gasperini, L., Marques, A. P. & Reis, R. L. The stiffness of living tissues and its implications for tissue engineering. Nat. Rev. Mater. 5, 351–370 (2020).
Mason, B. N. & Reinhart-King, C. A. Controlling the mechanical properties of three-dimensional matrices via non-enzymatic collagen glycation. Organogenesis 9, 70–75 (2013).
Lust, S. T. et al. Selectively cross-linked tetra-PEG hydrogels provide control over mechanical strength with minimal impact on diffusivity. ACS Biomater. Sci. Eng. 7, 4293–4304 (2021).
Chaudhuri, O. et al. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat. Mater. 15, 326–334 (2016).
Tang, S. et al. Adaptable fast relaxing boronate-based hydrogels for probing cell–matrix interactions. Adv. Sci. 5, 1800638 (2018).
Nam, S., Hu, K. H., Butte, M. J. & Chaudhuri, O. Strain-enhanced stress relaxation impacts nonlinear elasticity in collagen gels. Proc. Natl Acad. Sci. USA 113, 5492–5497 (2016).
Clark, A. G. et al. Self-generated gradients steer collective migration on viscoelastic collagen networks. Nat. Mater. 21, 1200–1210 (2022).
McKinnon, D. D., Domaille, D. W., Cha, J. N. & Anseth, K. S. Biophysically defined and cytocompatible covalently adaptable networks as viscoelastic 3D cell culture systems. Adv. Mater. 26, 865–872 (2014).
Alvarez, Z. et al. Bioactive scaffolds with enhanced supramolecular motion promote recovery from spinal cord injury. Science 374, 848–856 (2021).
Zheng, Y., Kim Liong Han, M., Jiang, Q., Feng, J. & del Campo, A. 4D hydrogel for dynamic cell culture with orthogonal, wavelength-dependent mechanical and biochemical cues. Mater. Horiz. 7, 111–116 (2020).
Kloxin, A. M., Tibbitt, M. W. & Anseth, K. S. Synthesis of photodegradable hydrogels as dynamically tunable cell culture platforms. Nat. Protoc. 5, 1867–1887 (2010).
DeForest, C. A. & Anseth, K. S. Photoreversible patterning of biomolecules within click-based hydrogels. Angew. Chem. Int. Ed. 51, 1816–1819 (2012).
Rosales, A. M., Vega, S. L., DelRio, F. W., Burdick, J. A. & Anseth, K. S. Hydrogels with reversible mechanics to probe dynamic cell microenvironments. Angew. Chem. Int. Ed. 56, 12132–12136 (2017). This articles describes hyaluronic acid-based hydrogels with reversible mechanical properties that rely on photodegradation and photopolymerization strategies.
Doyle, A. D., Carvajal, N., Jin, A., Matsumoto, K. & Yamada, K. M. Local 3D matrix microenvironment regulates cell migration through spatiotemporal dynamics of contractility-dependent adhesions. Nat. Commun. 6, 8720 (2015).
Aston, R., Sewell, K., Klein, T., Lawrie, G. & Grondahl, L. Evaluation of the impact of freezing preparation techniques on the characterisation of alginate hydrogels by cryo-SEM. E Polym. J. 82, 1–15 (2016).
Moyle, L. A. et al. Three-dimensional niche stiffness synergizes with Wnt7a to modulate the extent of satellite cell symmetric self-renewal divisions. Mol. Biol. Cell 31, 1703–1713 (2020).
Pluen, A., Netti, P. A., Jain, R. K. & Berk, D. A. Diffusion of macromolecules in agarose gels: comparison of linear and globular configurations. Biophys. J. 77, 542–552 (1999).
Cai, L., Dewi, R. E. & Heilshorn, S. C. Injectable hydrogels with in situ double network formation enhance retention of transplanted stem cells. Adv. Funct. Mater. 25, 1344–1351 (2015).
Kang, M., Day, C. A., Kenworthy, A. K. & DiBenedetto, E. Simplified equation to extract diffusion coefficients from confocal FRAP data. Traffic 13, 1589–1600 (2012).
Rehmann, M. S. et al. Tuning and predicting mesh size and protein release from step growth hydrogels. Biomacromolecules 18, 3131–3142 (2017).
Wisdom, K. M. et al. Matrix mechanical plasticity regulates cancer cell migration through confining microenvironments. Nat. Commun. 9, 4144 (2018).
Nam, S., Lee, J., Brownfield, D. G. & Chaudhuri, O. Viscoplasticity enables mechanical remodeling of matrix by cells. Biophys. J. 111, 2296–2308 (2016).
Oyen, M. L. Mechanical characterisation of hydrogel materials. Int. Mater. Rev. 59, 44–59 (2014).
Chaudhuri, O., Cooper-White, J., Janmey, P. A., Mooney, D. J. & Shenoy, V. B. Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature 584, 535–546 (2020). This article provides a detailed review of tissue and extracellular matrix viscoelasticity and its impact on cells.
Chaudhuri, O. et al. Extracellular matrix stiffness and composition jointly regulate the induction of malignant phenotypes in mammary epithelium. Nat. Mater. 13, 970–978 (2014).
Norman, M. D. A., Ferreira, S. A., Jowett, G. M., Bozec, L. & Gentleman, E. Measuring the elastic modulus of soft culture surfaces and three-dimensional hydrogels using atomic force microscopy. Nat. Protoc. 16, 2418–2449 (2021). This protocol describes how to use atomic force microscopy to measure the elastic modulus of soft hydrogels.
Buxboim, A., Rajagopal, K., Brown, A. E. X. & Discher, D. E. How deeply cells feel: methods for thin gels. J. Phys. Condens. Matter 22, 194116 (2010).
Tusan, C. G. et al. Collective cell behavior in mechanosensing of substrate thickness. Biophys. J. 114, 2743–2755 (2018).
Chen, F., Tillberg, P. W. & Boyden, E. S. Optical imaging. Expansion microscopy. Science 347, 543–548 (2015).
Blatchley, M. R. et al. In situ super-resolution imaging of organoids and extracellular matrix interactions via phototransfer by allyl sulfide exchange-expansion microscopy (PhASE-ExM). Adv. Mater. 34, e2109252 (2022).
Chen, B. C. et al. Lattice light-sheet microscopy: imaging molecules to embryos at high spatiotemporal resolution. Science 346, 1257998 (2014).
Gut, G., Herrmann, M. D. & Pelkmans, L. Multiplexed protein maps link subcellular organization to cellular states. Science 361, eaar7042 (2018).
Hickey, J. W. et al. Spatial mapping of protein composition and tissue organization: a primer for multiplexed antibody-based imaging. Nat. Methods 19, 284–295 (2022).
Ruan, J. L. et al. An improved cryosection method for polyethylene glycol hydrogels used in tissue engineering. Tissue Eng. Part C Methods 19, 794–801 (2013).
Valdez, J. et al. On-demand dissolution of modular, synthetic extracellular matrix reveals local epithelial–stromal communication networks. Biomaterials 130, 90–103 (2017).
Darnell, M. et al. Material microenvironmental properties couple to induce distinct transcriptional programs in mammalian stem cells. Proc. Natl Acad. Sci. USA 115, E8368–E8377 (2018).
Stowers, R. S. et al. Matrix stiffness induces a tumorigenic phenotype in mammary epithelium through changes in chromatin accessibility. Nat. Biomed. Eng. 3, 1009–1019 (2019).
Madl, C. M., LeSavage, B. L., Khariton, M. & Heilshorn, S. C. Neural progenitor cells alter chromatin organization and neurotrophin expression in response to 3D matrix degradability. Adv. Healthc. Mater. 9, e2000754 (2020).
Qazi, T. H., Mooney, D. J., Duda, G. N. & Geissler, S. Biomaterials that promote cell–cell interactions enhance the paracrine function of MSCs. Biomaterials 140, 103–114 (2017).
Drzeniek, N. M. et al. Bio-instructive hydrogel expands the paracrine potency of mesenchymal stem cells. Biofabrication 13, 045002 (2021).
Ferreira, S. A. et al. Neighboring cells override 3D hydrogel matrix cues to drive human MSC quiescence. Biomaterials 176, 13–23 (2018).
Sawicki, L. A., Choe, L. H., Wiley, K. L., Lee, K. H. & Kloxin, A. M. Isolation and identification of proteins secreted by cells cultured within synthetic hydrogel-based matrices. ACS Biomater. Sci. Eng. 4, 836–845 (2018).
Legant, W. R. et al. Measurement of mechanical tractions exerted by cells in three-dimensional matrices. Nat. Methods 7, 969–971 (2010). This study demonstrated the technique of 3D traction force microscopy to study mechanical interactions between cells and hydrogels in 3D.
Toyjanova, J. et al. 3D viscoelastic traction force microscopy. Soft Matter 10, 8095–8106 (2014).
Grashoff, C. et al. Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature 466, 263–266 (2010).
Stabley, D. R., Jurchenko, C., Marshall, S. S. & Salaita, K. S. Visualizing mechanical tension across membrane receptors with a fluorescent sensor. Nat. Methods 9, 64–67 (2011).
Shin, D. S. et al. Synthesis of microgel sensors for spatial and temporal monitoring of protease activity. ACS Biomater. Sci. Eng. 4, 378–387 (2018).
Blache, U. et al. Notch-inducing hydrogels reveal a perivascular switch of mesenchymal stem cell fate. EMBO Rep. 19, e45964 (2018).
Ferreira, S. A. et al. Bi-directional cell–pericellular matrix interactions direct stem cell fate. Nat. Commun. 9, 4049 (2018).
Loebel, C., Mauck, R. L. & Burdick, J. A. Local nascent protein deposition and remodelling guide mesenchymal stromal cell mechanosensing and fate in three-dimensional hydrogels. Nat. Mater. 18, 883–891 (2019).
Horton, E. R. et al. Extracellular matrix production by mesenchymal stromal cells in hydrogels facilitates cell spreading and is inhibited by FGF-2. Adv. Healthc. Mater. 9, e1901669 (2020).
Schultz, K. M., Kyburz, K. A. & Anseth, K. S. Measuring dynamic cell–material interactions and remodeling during 3D human mesenchymal stem cell migration in hydrogels. Proc. Natl Acad. Sci. USA 112, E3757–E3764 (2015).
Guo, M. et al. Probing the stochastic, motor-driven properties of the cytoplasm using force spectrum microscopy. Cell 158, 822–832 (2014).
Han, Y. L. et al. Cell swelling, softening and invasion in a three-dimensional breast cancer model. Nat. Phys. 16, 101–108 (2020).
Mizuno, D., Tardin, C., Schmidt, C. F. & Mackintosh, F. C. Nonequilibrium mechanics of active cytoskeletal networks. Science 315, 370–373 (2007).
Pokki, J., Zisi, I., Schulman, E., Indana, D. & Chaudhuri, O. Magnetic probe-based microrheology reveals local softening and stiffening of 3D collagen matrices by fibroblasts. Biomed. Microdevices 23, 27 (2021).
Swift, J. et al. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science 341, 1240104 (2013).
Iskratsch, T., Wolfenson, H. & Sheetz, M. P. Appreciating force and shape-the rise of mechanotransduction in cell biology. Nat. Rev. Mol. Cell Biol. 15, 825–833 (2014). This perspectives article explores the emergence of the study of mechanobiology and the basics of mechanotransduction, from the timescales associated with mechanotransduction to the formation of focal adhesions and mature stress fibers.
Dupont, S. et al. Role of YAP/TAZ in mechanotransduction. Nature 474, 179–183 (2011). This original research article explores how YAP/TAZ act as sensors during mechanotransduction and mediate cell response to various stimuli including matrix stiffness, cell geometry, and cytoskeletal tension.
Zhao, B., Tumaneng, K. & Guan, K. L. The Hippo pathway in organ size control, tissue regeneration and stem cell self-renewal. Nat. Cell Biol. 13, 877–883 (2011).
Guvendiren, M. & Burdick, J. A. Stiffening hydrogels to probe short- and long-term cellular responses to dynamic mechanics. Nat. Commun. 3, 792 (2012).
Huebsch, N. et al. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat. Mater. 9, 518–526 (2010). This article was one of the first that identified a role for 3D matrix stiffness in hMSC differentiation, which was driven by integrin binding and reorganoisation of adhesive ligands.
Blache, U., Stevens, M. M. & Gentleman, E. Harnessing the secreted extracellular matrix to engineer tissues. Nat. Biomed. Eng. 4, 357–363 (2020).
Sharma, B. et al. Human cartilage repair with a photoreactive adhesive–hydrogel composite. Sci. Transl. Med. 5, 167ra166 (2013).
Aisenbrey, E. A. et al. A stereolithography-based 3D printed hybrid scaffold for in situ cartilage defect repair. Macromol. Biosci. 18, 201700267 (2018).
Coluccino, L. et al. Porous poly(vinyl alcohol)-based hydrogel for knee meniscus functional repair. ACS Biomater. Sci. Eng. 4, 1518–1527 (2018).
Fujii, M. & Sato, T. Somatic cell-derived organoids as prototypes of human epithelial tissues and diseases. Nat. Mater. 20, 156–169 (2021).
Spence, J. R. et al. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature 470, 105–109 (2011).
Lancaster, M. A. et al. Cerebral organoids model human brain development and microcephaly. Nature 501, 373–379 (2013).
Miller, A. J. et al. Generation of lung organoids from human pluripotent stem cells in vitro. Nat. Protoc. 14, 518–540 (2019).
Pagliuca, F. W. et al. Generation of functional human pancreatic β cells in vitro. Cell 159, 428–439 (2014).
Takasato, M. et al. Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature 536, 238 (2016).
Poznansky, M. C. et al. Efficient generation of human T cells from a tissue-engineered thymic organoid. Nat. Biotechnol. 18, 729–734 (2000).
Kratochvil, M. J. et al. Engineered materials for organoid systems. Nat. Rev. Mater. 4, 606–622 (2019).
Gjorevski, N. et al. Designer matrices for intestinal stem cell and organoid culture. Nature 539, 560–564 (2016). This article was the first to report using synthetic hydrogels to support murine intestinal organoids and identified roles for 3D matrix stiffness and degradability in maintaining their culture.
Gjorevski, N. et al. Tissue geometry drives deterministic organoid patterning. Science 375, eaaw9021 (2022).
Ranga, A. et al. Neural tube morphogenesis in synthetic 3D microenvironments. Proc. Natl Acad. Sci. USA 113, E6831–E6839 (2016).
Indana, D., Agarwal, P., Bhutani, N. & Chaudhuri, O. Viscoelasticity and adhesion signaling in biomaterials control human pluripotent stem cell morphogenesis in 3D culture. Adv. Mater. 33, e2101966 (2021).
Decembrini, S., Hoehnel, S., Brandenberg, N., Arsenijevic, Y. & Lutolf, M. P. Hydrogel-based milliwell arrays for standardized and scalable retinal organoid cultures. Sci. Rep. 10, 10275 (2020).
Brandenberg, N. et al. High-throughput automated organoid culture via stem-cell aggregation in microcavity arrays. Nat. Biomed. Eng. 4, 863–874 (2020).
Luciano, M. et al. Cell monolayers sense curvature by exploiting active mechanics and nuclear mechanoadaptation. Nat. Phys. 17, 1382 (2021).
Pahapale, G. J. et al. Directing multicellular organization by varying the aspect ratio of soft hydrogel microwells. Adv. Sci. 9, e2104649 (2022).
Cruz-Acuna, R. et al. Synthetic hydrogels for human intestinal organoid generation and colonic wound repair. Nat. Cell Biol. 19, 1326–1335 (2017).
Sorrentino, G. et al. Mechano-modulatory synthetic niches for liver organoid derivation. Nat. Commun. 11, 3416 (2020).
Yoder, B. K. Role of primary cilia in the pathogenesis of polycystic kidney disease. J. Am. Soc. Nephrol. 18, 1381–1388 (2007).
Bernink, J. H. et al. Human type 1 innate lymphoid cells accumulate in inflamed mucosal tissues. Nat. Immunol. 14, 221–229 (2013).
Davidson, M. D., Burdick, J. A. & Wells, R. G. Engineered biomaterial platforms to study fibrosis. Adv. Healthc. Mater. 9, e1901682 (2020).
Batan, D. et al. Hydrogel cultures reveal transient receptor potential vanilloid 4 regulation of myofibroblast activation and proliferation in valvular interstitial cells. FASEB J. 36, e22306 (2022).
Sadeghi, A. H. et al. Engineered 3D cardiac fibrotic tissue to study fibrotic remodeling. Adv. Healthc. Mater. 6, 201601434 (2017).
Spill, F., Reynolds, D. S., Kamm, R. D. & Zaman, M. H. Impact of the physical microenvironment on tumor progression and metastasis. Curr. Opin. Biotechnol. 40, 41–48 (2016).
Zaman, M. H. The role of engineering approaches in analysing cancer invasion and metastasis. Nat. Rev. Cancer 13, 596–603 (2013).
Zhang, X. et al. Unraveling the mechanobiology of immune cells. Curr. Opin. Biotechnol. 66, 236–245 (2020).
Judokusumo, E., Tabdanov, E., Kumari, S., Dustin, M. L. & Kam, L. C. Mechanosensing in T lymphocyte activation. Biophys. J. 102, L5–L7 (2012).
Harrison, D. L., Fang, Y. & Huang, J. T-cell mechanobiology: force sensation, potentiation, and translation. Front. Phys. 7, 45 (2019).
Chin, M. H. W., Norman, M. D. A., Gentleman, E., Coppens, M. O. & Day, R. M. A Hydrogel-integrated culture device to interrogate T cell activation with physicochemical cues. ACS Appl. Mater. Interfaces 12, 47355–47367 (2020).
O’Connor, R. S. et al. Substrate rigidity regulates human T cell activation and proliferation. J. Immunol. 189, 1330–1339 (2012).
Alatoom, A. et al. Artificial biosystem for modulation of interactions between antigen-presenting cells and T cells. Adv. Biosyst. 4, e2000039 (2020).
Zhang, J. et al. Micropatterned soft hydrogels to study the interplay of receptors and forces in T cell activation. Acta Biomater. 119, 234–246 (2021).
Hickey, J. W. et al. Engineering an artificial T-cell stimulating matrix for immunotherapy. Adv. Mater. 31, e1807359 (2019). This article shows that the biophysical properties of engineered HA hydrogels potentiate T cell stimulation, which can be harnessed for adoptive immunotherapy.
Majedi, F. S. et al. T-cell activation is modulated by the 3D mechanical microenvironment. Biomaterials 252, 120058 (2020).
Chin, M. H. W., Gentleman, E., Coppens, M. O. & Day, R. M. Rethinking cancer immunotherapy by embracing and engineering complexity. Trends Biotechnol. 38, 1054–1065 (2020).
Maude, S. L. et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N. Engl. J. Med. 378, 439–448 (2018).
Neelapu, S. S. et al. Chimeric antigen receptor T-cell therapy — assessment and management of toxicities. Nat. Rev. Clin. Oncol. 15, 47–62 (2018).
Schuster, S. J. et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N. Engl. J. Med. 380, 45–56 (2019).
Blache, U., Popp, G., Dünkel, A., Koehl, U. & Fricke, S. Potential solutions for manufacture of CAR T cells in cancer immunotherapy. Nat. Commun. 13, 5225 (2022).
Finck, A. V., Blanchard, T., Roselle, C. P., Golinelli, G. & June, C. H. Engineered cellular immunotherapies in cancer and beyond. Nat. Med. 28, 678–689 (2022).
Hu, Q. et al. Inhibition of post-surgery tumour recurrence via a hydrogel releasing CAR-T cells and anti-PDL1-conjugated platelets. Nat. Biomed. Eng. 5, 1038–1047 (2021).
Wang, K. et al. GD2-specific CAR T cells encapsulated in an injectable hydrogel control retinoblastoma and preserve vision. Nat. Cancer 1, 990–997 (2020).
Barrett, T. et al. NCBI GEO: archive for functional genomics data sets — update. Nucleic Acids Res. 41, D991–D995 (2013).
Blache, U. et al. Mesenchymal stromal cell activation by breast cancer secretomes in bioengineered 3D microenvironments. Life Sci. Alliance 2, e201900304 (2019).
Vizcaino, J. A. et al. ProteomeXchange provides globally coordinated proteomics data submission and dissemination. Nat. Biotechnol. 32, 223–226 (2014).
Trappmann, B. et al. Extracellular-matrix tethering regulates stem-cell fate. Nat. Mater. 11, 642–649 (2012). This manuscript shows that varying nanoscale anchoring distance of cell adhesive ligands on soft hydrogels potentiates stiffness sensing to guide cell fate.
Wen, J. H. et al. Interplay of matrix stiffness and protein tethering in stem cell differentiation. Nat. Mater. 13, 979–987 (2014).
Cossu, G. et al. Lancet commission: stem cells and regenerative medicine. Lancet 391, 883–910 (2018).
Sun, J. Y. et al. Highly stretchable and tough hydrogels. Nature 489, 133–136 (2012).
Xu, L. et al. Conjoined-network rendered stiff and tough hydrogels from biogenic molecules. Sci. Adv. 5, eaau3442 (2019).
Rodrigo-Navarro, A., Sankaran, S., Dalby, M. J., del Campo, A. & Salmeron-Sanchez, M. Engineered living biomaterials. Nat. Rev. Mater. 6, 1175–1190 (2021).
Neves, S. C., Moroni, L., Barrias, C. C. & Granja, P. L. Leveling up hydrogels: hybrid systems in tissue engineering. Trends Biotechnol. 38, 292–315 (2020).
Champeau, M. et al. 4D printing of hydrogels: a review. Adv. Funct. Mater. 30, 1910606 (2020).
Weiss, F., Lauffenburger, D. & Friedl, P. Towards targeting of shared mechanisms of cancer metastasis and therapy resistance. Nat. Rev. Cancer 22, 157–173 (2022).
Shellard, A. & Mayor, R. Collective durotaxis along a self-generated stiffness gradient in vivo. Nature 600, 690–694 (2021). This article demonstrates that embryonic cell populations self-generate a stiffness gradient to collectively direct their migration by durotaxis.
Davidson, M. D. et al. Programmable and contractile materials through cell encapsulation in fibrous hydrogel assemblies. Sci. Adv. 7, eabi8157 (2021).
Mok, S. et al. Mapping cellular-scale internal mechanics in 3D tissues with thermally responsive hydrogel probes. Nat. Commun. 11, 4757 (2020).
Daly, A. C., Riley, L., Segura, T. & Burdick, J. A. Hydrogel microparticles for biomedical applications. Nat. Rev. Mater. 5, 20–43 (2020).
Shadish, J. A., Benuska, G. M. & DeForest, C. A. Bioactive site-specifically modified proteins for 4D patterning of gel biomaterials. Nat. Mater. 18, 1005–1014 (2019).
Baneyx, G., Baugh, L. & Vogel, V. Coexisting conformations of fibronectin in cell culture imaged using fluorescence resonance energy transfer. Proc. Natl Acad. Sci. USA 98, 14464–14468 (2001).
Legant, W. R. et al. High-density three-dimensional localization microscopy across large volumes. Nat. Methods 13, 359–365 (2016).
Razafiarison, T. et al. Biomaterial surface energy-driven ligand assembly strongly regulates stem cell mechanosensitivity and fate on very soft substrates. Proc. Natl Acad. Sci. USA 115, 4631–4636 (2018). This study shows that cell mechanotransduction and fate depend heavily on hydrogel hydrophilicity and the conformation of secreted matrix proteins.
Davidson, M. D. et al. Mechanochemical adhesion and plasticity in multifiber hydrogel networks. Adv. Mater. 32, e1905719 (2020).
Friedl, P. & Alexander, S. Cancer invasion and the microenvironment: plasticity and reciprocity. Cell 147, 992–1009 (2011).
Weigelin, B., Bakker, G. J. & Friedl, P. Intravital third harmonic generation microscopy of collective melanoma cell invasion: principles of interface guidance and microvesicle dynamics. Intravital 1, 32–43 (2012).
Charelli, L. E., Ferreira, J. P. D., Naveira-Cotta, C. P. & Balbino, T. A. Engineering mechanobiology through organoids-on-chip: a strategy to boost therapeutics. J. Tissue Eng. Regen. Med. 15, 883–899 (2021).
Zhang, Y. S. et al. 3D extrusion bioprinting. Nat. Rev. Methods Primers 1, 75 (2021).
Liu, L. et al. Cyclic stiffness modulation of cell-laden protein–polymer hydrogels in response to user-specified stimuli including light. Adv. Biosyst. 2, 201800240 (2018).
Grim, J. C. et al. A reversible and repeatable thiol-ene bioconjugation for dynamic patterning of signaling proteins in hydrogels. ACS Cent. Sci. 4, 909–916 (2018).
Arkenberg, M. R., Moore, D. M. & Lin, C. C. Dynamic control of hydrogel crosslinking via sortase-mediated reversible transpeptidation. Acta Biomater. 83, 83–95 (2019).
Broguiere, N., Formica, F. A., Barreto, G. & Zenobi-Wong, M. Sortase A as a cross-linking enzyme in tissue engineering. Acta Biomater. 77, 182–190 (2018).
Corral-Acero, J. et al. The ‘Digital Twin’ to enable the vision of precision cardiology. Eur. Heart J. 41, 4556–4564 (2020).
Garcia, R. Nanomechanical mapping of soft materials with the atomic force microscope: methods, theory and applications. Chem. Soc. Rev. 49, 5850–5884 (2020).
Seliktar, D. Designing cell-compatible hydrogels for biomedical applications. Science 336, 1124–1128 (2012).
Acknowledgements
U.B. acknowledges funding from the Fraunhofer Cluster of Immune Mediated Diseases (CIMD). L.R. and P.Y.W.D. gratefully acknowledge the Gravitation Program ‘Materials Driven Regeneration’, funded by the Netherlands Organization for Scientific Research (024.003.013). O.C. acknowledges support from a National Institutes of Health (NIH) National Cancer Institute grant (R37 CA214136). B.H. acknowledges support from a US National Science Foundation (NSF) Postdoctoral Fellowship in Biology grant (2209411). E.G. acknowledges support from the UK Regenerative Medicine Platform ‘Acellular/Smart Materials — 3D Architecture’ (MR/R015651/1) and the Rosetrees Trust. E.M.F. and A.M.K. acknowledge support from the NIH Director’s New Innovator Award (DP2 HL152424-01) and from the NSF through the University of Delaware Materials Research Science and Engineering Center (DMR-2011824). The authors thank B. Simona for providing helpful suggestions during manuscript revision.
Author information
Authors and Affiliations
Contributions
Introduction (E.G. and U.B.); Experimentation (E.G., U.B., L.R., O.C., P.Y.W.D. and J.G.S.); Results (E.G., U.B., O.C. and B.H.); Applications (E.G., U.B., E.M.F. and A.M.K.); Reproducibility and data deposition (E.G., U.B., E.M.F. and A.M.K.); Limitations and optimizations (E.G., U.B. and J.G.S.); Outlook (E.G. and U.B.); Overview of the Primer (E.G.).
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Methods Primers thanks Sara Baratchi, Sylvain Gabriele and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Anoikis
-
Cell death in anchorage-dependent cells driven by a lack of cell–extracellular matrix interactions.
- Creep
-
The process by which a viscoelastic material undergoes gradually increasing and irreversible deformation in response to a constant applied force.
- Disease modelling
-
The modelling of a diseased tissue using animal models or cells/organoids cultured in vitro to study disease progression and possible treatment.
- Elastic modulus
-
The tangent of a material’s stress–strain curve, which is an intrinsic property of the material. The modulus is a measure of stiffness that is independent of the material’s size and geometry.
- Matrix metalloproteinase
-
(MMP). A class of cellular proteases that biochemically degrade extracellular matrix proteins.
- Mechanosensing
-
The ability of a cell to probe or sense mechanical cues in its extracellular environment, including force, stress, strain, confinement and substrate topology.
- Mechanotransduction
-
The cellular conversion of mechanical stimuli into biochemical signalling, such as via activation of receptors, signalling pathways, transcriptional activity or protein activation.
- Mesh size
-
The distance between polymers or cross-link points in a polymer network. Mesh size can impact solute diffusivity.
- Micro-rheology
-
Microscale measurements of the mechanical properties of a material.
- Organoids
-
3D stem cell-derived tissue cultures that exhibit aspects or properties of the parent organ.
- Stiffness
-
A material’s ability to resist deformation in response to an applied force.
- Stress relaxation
-
The process by which a viscoelastic material, which initially resists an applied strain, reduces its resistance to the deformation over time. In hydrogels this is often related to fluid movements within the solid material backbone, or in weakly cross-linked hydrogels to the unbinding of weak cross links followed by polymer flow.
- Stress stiffening
-
The process by which materials stiffen due to an applied strain, sometimes as a result of fibre alignment.
- Tissue engineering
-
The combination of biologically active materials platforms with cells to develop systems that may be used to restore or treat damaged tissues.
- Viscoelasticity
-
A mechanical response on a material that combines characteristics of viscous liquids and elastic solids.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Blache, U., Ford, E.M., Ha, B. et al. Engineered hydrogels for mechanobiology. Nat Rev Methods Primers 2, 98 (2022). https://doi.org/10.1038/s43586-022-00179-7
Accepted:
Published:
DOI: https://doi.org/10.1038/s43586-022-00179-7
This article is cited by
-
Model-based modular hydrogel design
Nature Reviews Bioengineering (2024)
-
Synthetic living materials in cancer biology
Nature Reviews Bioengineering (2023)