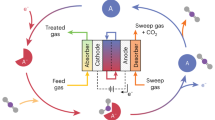
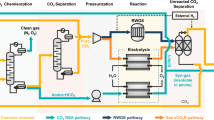
Abstract
The build-up of carbon dioxide in the atmosphere is one of the grand challenges facing society. Addressing this challenge by removing CO2 from the atmosphere or mitigating point source emissions through the separation and concentration of CO2 from these dilute sources requires reductions in energetic and monetary cost relative to traditional thermal and pressure swing methods. Electrochemical methods of CO2 separation have drawn increasing attention in recent years as potentially cheap, low-energy, scalable carbon capture technologies. In this Primer, we provide an overview of the experimentation and analysis needed for the study of electrochemical methods for CO2 separation, including a discussion of the considerations necessary for targeting the application of such techniques. This Primer focuses on ambient temperature techniques such as pH swing and direct redox processes, which utilize similar experimental set-ups. We include considerations on the choice of redox agent and an outlook on this growing body of research. Experimentation to address real-world conditions, particularly at practical oxygen concentrations, and novel system designs that overcome transport limitations or, potentially, couple capture and CO2 utilization are emerging areas in the field.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
$99.00 per year
only $99.00 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
IPCC. Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. Climate Change 2021: The Physical Science Basis (Cambridge Univ. Press, 2021).
Mac Dowell, N., Fennell, P. S., Shah, N. & Maitland, G. C. The role of CO2 capture and utilization in mitigating climate change. Nat. Clim. Chang. 7, 243–249 (2017).
National Academies of Sciences Engineering and Medicine. Negative emissions technologies and reliable sequestration. National Academies https://www.nap.edu/catalog/25259 (2019).
Halliday, C. & Hatton, T. A. Sorbents for the capture of CO2 and other acid gases: a review. Ind. Eng. Chem. Res. 60, 9313–9346 (2021).
Bui, M. et al. Carbon capture and storage (CCS): the way forward. Energy Environ. Sci. 11, 1062–1176 (2018).
Lackner, K. S. et al. The urgency of the development of CO2 capture from ambient air. Proc. Natl. Acad. Sci. USA 109, 13156–13162 (2012).
Pathak, M. et al. Technical Summary in Climate Change 2022: Mitigation of Climate Change. Contribution of Working Group III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change (eds Shukla, P. R. et al.) (Cambridge Univ. Press, 2022).
Gelles, T., Lawson, S., Rownaghi, A. A. & Rezaei, F. Recent advances in development of amine functionalized adsorbents for CO2 capture. Adsorption 26, 5–50 (2019).
Zheng, R. F. et al. A single-component water-lean post-combustion CO2 capture solvent with exceptionally low operational heat and total costs of capture — comprehensive experimental and theoretical evaluation. Energy Environ. Sci. 13, 4106–4113 (2020).
Rochelle, G. T. Thermal degradation of amines for CO2 capture. Curr. Opin. Chem. Eng. 1, 183–190 (2012).
Rochelle, G. T. in Absorption-based Post-combustion Capture of Carbon Dioxide (ed. Feron, P. M. H.) 35–67 (Elsevier, 2016).
Petra Nova Parish Holdings. W. A. Parish post-combustion CO2 capture and sequestration demonstration project final report. OSTI.gov https://www.osti.gov/servlets/purl/1608572 (2020).
Renfrew, S. E., Starr, D. E. & Strasser, P. Electrochemical approaches toward CO2 capture and concentration. ACS Catal. 10, 13058–13074 (2020).
Sharifian, R., Wagterveld, M., Digdaya, I. A., Xiang, C. & Vermaas, D. A. Electrochemical carbon dioxide capture to close the carbon cycle. Energy Environ. Sci. 10, 147–154 (2021).
Kang, J. S., Kim, S. & Hatton, T. A. Redox-responsive sorbents and mediators for electrochemically based CO2 capture. Curr. Opin. Green Sustain. Chem. 31, 100504 (2021).
Gurkan, B. et al. Perspective and challenges in electrochemical approaches for reactive CO2 separations. iScience 24, 103422 (2021).
Muroyama, A. P., Pătru, A. & Gubler, L. Review — CO2 separation and transport via electrochemical methods. J. Electrochem. Soc. 167, 133504 (2020).
Rheinhardt, J. H., Singh, P., Tarakeshwar, P. & Buttry, D. A. Electrochemical capture and release of carbon dioxide. ACS Energy Lett. 2, 454–461 (2017).
Ishida, H., Ohba, T., Yamaguchi, T. & Ohkubo, K. Interaction between CO2 and electrochemically reduced species of N-propyl-4,4′-bipyridinium cation. Chem. Lett. 23, 905–908 (1994).
Ranjan, R. et al. Reversible electrochemical trapping of carbon dioxide using 4,4′-bipyridine that does not require thermal activation. J. Phys. Chem. Lett. 6, 4943–4946 (2015).
Singh, P. et al. Electrochemical capture and release of carbon dioxide using a disulfide–thiocarbonate redox cycle. J. Am. Chem. Soc. 139, 1033–1036 (2017).
Dubois, D. L., Miedaner, A., Bell, W. & Smart, J. C. in Electrochemical and Electrocatalytic Reactions of Carbon Dioxide (ed. Sullivan, B. P.) iii (Elsevier, 1993).
Scovazzo, P., Poshusta, J., DuBois, D., Koval, C. & Noble, R. Electrochemical separation and concentration of <1% carbon dioxide from nitrogen. J. Electrochem. Soc. 150, D91 (2003).
Mizen, M. B. & Wrighton, M. S. Reductive addition of CO2 to 9,10-phenanthrenequinone. J. Electrochem. Soc. 136, 941 (1989).
Liu, Y., Ye, H., Diederichsen, K. M., Van Voorhis, T. & Hatton, T. A. Electrochemically mediated carbon dioxide separation with quinone chemistry in salt-concentrated aqueous media. Nat. Commun. 11, 2278 (2020).
Gurkan, B., Simeon, F. & Hatton, T. A. Quinone reduction in ionic liquids for electrochemical CO2 separation. ACS Sustain. Chem. Eng. 3, 1394–1405 (2015).
Voskian, S. & Hatton, T. A. Faradaic electro-swing reactive adsorption for CO2 capture. Energy Environ. Sci. 12, 3530–3547 (2019).
Appel, A. M., Newell, R., Dubois, D. L. & Dubois, M. R. Concentration of carbon dioxide by electrochemically modulated complexation with a binuclear copper complex. Inorg. Chem. 44, 3046–3056 (2005).
Stern, M. C., Simeon, F., Herzog, H. & Hatton, T. A. Post-combustion carbon dioxide capture using electrochemically mediated amine regeneration. Energy Environ. Sci. 6, 2505 (2013).
Jin, S., Wu, M., Gordon, R. G., Aziz, M. J. & Kwabi, D. G. pH swing cycle for CO2 capture electrochemically driven through proton-coupled electron transfer. Energy Environ. Sci. 13, 3706–3722 (2020).
Jin, S., Wu, M., Jing, Y., Gordon, R. G. & Aziz, M. J. Low energy carbon capture via electrochemically induced pH swing with electrochemical rebalancing. Nat. Commun. 13, 2140 (2022).
Xie, H. et al. Low-energy-consumption electrochemical CO2 capture driven by biomimetic phenazine derivatives redox medium. Appl. Energy 259, 114119 (2020).
Luo, L. et al. Regeneration of Na2Q in an electrochemical CO2 capture system. Energy Fuels 35, 12260–12269 (2021).
Stucki, S., Schuler, A. & Constantinescu, M. Coupled CO2 recovery from the atmosphere and water electrolysis: feasibility of a new process for hydrogen storage. Int. J. Hydrog. Energy 20, 653–663 (1995).
de Lannoy, C.-F. et al. Indirect ocean capture of atmospheric CO2: part I. Prototype of a negative emissions technology. Int. J. Greenh. Gas Control. 70, 243–253 (2018).
Digdaya, I. A. et al. A direct coupled electrochemical system for capture and conversion of CO2 from oceanwater. Nat. Commun. 11, 1–10 (2020).
Eisaman, M. D. et al. CO2 separation using bipolar membrane electrodialysis. Energy Environ. Sci. 4, 1319–1328 (2011).
Legrand, L., Schaetzle, O., de Kler, R. C. F. & Hamelers, H. V. M. Solvent-free CO2 capture using membrane capacitive deionization. Environ. Sci. Technol. 52, 9478–9485 (2018).
Rahimi, M., Catalini, G., Puccini, M. & Hatton, T. A. Bench-scale demonstration of CO2 capture with an electrochemically driven proton concentration process. RSC Adv. 10, 16832–16843 (2020).
Eisaman, M. D. et al. Energy-efficient electrochemical CO2 capture from the atmosphere. Tech. Proc. 2009 NSTI Nanotechnol. Conf. Expo NSTI-Nanotech 2009 3, 78–81 (2009).
Winnick, J., Marshall, R. D. & Schubert, F. H. An electrochemical device for carbon dioxide concentration. I. System design and performance. Ind. Eng. Chem. Process. Des. Dev. 13, 59–63 (1974).
Bove, D. et al. Process analysis of molten carbonate fuel cells in carbon capture applications. Int. J. Hydrog. Energy 46, 15032–15045 (2021).
Campanari, S., Chiesa, P., Manzolini, G. & Bedogni, S. Economic analysis of CO2 capture from natural gas combined cycles using molten carbonate fuel cells. Appl. Energy 130, 562–573 (2014).
Duan, L., Xia, K., Feng, T., Jia, S. & Bian, J. Study on coal-fired power plant with CO2 capture by integrating molten carbonate fuel cell system. Energy 117, 578–589 (2016).
Rosen, J. et al. Molten carbonate fuel cell performance for CO2 capture from natural gas combined cycle flue gas. J. Electrochem. Soc. 167, 064505 (2020).
Sullivan, I. et al. Coupling electrochemical CO2 conversion with CO2 capture. Nat. Catal. 4, 952–958 (2021).
Khurram, A., He, M. & Gallant, B. M. Tailoring the discharge reaction in Li-CO2 batteries through Incorporation of CO2 capture chemistry. Joule 2, 2649–2666 (2018).
Wang, M., Shaw, R., Gencer, E. & Hatton, T. A. Technoeconomic analysis of the electrochemically mediated amine regeneration CO2 capture process. Ind. Eng. Chem. Res. 59, 14085–14095 (2020).
Sabatino, F. et al. Evaluation of a direct air capture process combining wet scrubbing and bipolar membrane electrodialysis. Ind. Eng. Chem. Res. 59, 7007–7020 (2020).
Dinh, T.-V., Choi, I.-Y., Son, Y.-S. & Kim, J.-C. A review on non-dispersive infrared gas sensors: improvement of sensor detection limit and interference correction. Sens. Actuators B Chem. 231, 529–538 (2016).
Yasuda, T., Yonemura, S. & Tani, A. Comparison of the characteristics of small commercial NDIR CO2 sensor models and development of a portable CO2 measurement device. Sensors 12, 3641–3655 (2012).
Harris, D. & Lucy, C. Quantitative Chemical Analysis (W.H. Freeman, 2016).
de Matos, M. A. A. & da Silva Ferreira, V. Gas mass-flow meters: principles and applications. Flow. Meas. Instrum. 21, 143–149 (2010).
Chen, J., Zhang, K., Wang, L. & Yang, M. Design of a high precision ultrasonic gas flowmeter. Sensors 20, 4804 (2020).
Zhao, Y., Hu, H., Bi, D. & Yang, Y. Research on the optical fiber gas flowmeters based on intermodal interference. Opt. Lasers Eng. 82, 122–126 (2016).
Butler, J. N. Carbon Dioxide Equilibria and Their Applications (Routledge, 2019).
Dickson, A. G., Sabine, C. L. & Christian, J. R. Guide to Best Practices for Ocean CO2 Measurements (North Pacific Marine Science Organization, 2007).
Zeebe, R. E. & Wolf-Gladrow, D. CO2 in Seawater: Equilibrium, Kinetics, Isotopes (Elsevier, 2001).
Severinghaus, J. W. & Bradley, A. F. Electrodes for blood pO2 and pCO2 determination. J. Appl. Physiol. 13, 515–520 (1958).
Mitchell, M. J., Jensen, O. E., Cliffe, K. A. & Maroto-Valer, M. M. A model of carbon dioxide dissolution and mineral carbonation kinetics. Proc. R. Soc. A Math. Phys. Eng. Sci. 466, 1265–1290 (2010).
Soli, A. L. & Byrne, R. H. CO2 system hydration and dehydration kinetics and the equilibrium CO2/H2CO3 ratio in aqueous NaCl solution. Mar. Chem. 78, 65–73 (2002).
Wang, X., Conway, W., Burns, R., McCann, N. & Maeder, M. Comprehensive study of the hydration and dehydration reactions of carbon dioxide in aqueous solution. J. Phys. Chem. A 114, 1734–1740 (2010).
Millero, F. J. The marine inorganic carbon cycle. Chem. Rev. 107, 308–341 (2007).
Millero, F. J. Chemical Oceanography (CRC, 2013).
Mojica Prieto, F. J. & Millero, F. J. The values of pK1 + pK2 for the dissociation of carbonic acid in seawater. Geochim. Cosmochim. Acta 66, 2529–2540 (2002).
Middelburg, J. J. in Marine Carbon Biogeochemistry 77–105 (Springer, 2019).
Vorholz, J., Harismiadis, V. I., Panagiotopoulos, A. Z., Rumpf, B. & Maurer, G. Molecular simulation of the solubility of carbon dioxide in aqueous solutions of sodium chloride. Fluid Phase Equilib. 226, 237–250 (2004).
Wanderley, R. R., Evjen, S., Pinto, D. D. D. & Knuutila, H. K. The salting-out effect in some physical absorbents for CO2 capture. Chem. Eng. Trans. 69, 97–102 (2018).
Weiss, R. F. Carbon dioxide in water and seawater: the solubility of a non-ideal gas. Mar. Chem. 2, 203–215 (1974).
Gilbert, K., Bennett, P. C., Wolfe, W., Zhang, T. & Romanak, K. D. CO2 solubility in aqueous solutions containing Na+, Ca2+, Cl−, SO42− and HCO3–: the effects of electrostricted water and ion hydration thermodynamics. Appl. Geochem. 67, 59–67 (2016).
Schulz, K. G., Barcelos e Ramos, J., Zeebe, R. E. & Riebesell, U. CO2 perturbation experiments: similarities and differences between dissolved inorganic carbon and total alkalinity manipulations. Biogeosciences 6, 2145–2153 (2009).
Głąb, S. & Hulanicki, A. in Encyclopedia of Analytical Science (eds Worsfold, P., Townshend, A. & Poole, C.) 72–78 (Elsevier, 2005).
Rahimi, M. et al. Carbon dioxide capture using an electrochemically driven proton concentration process. Cell Rep. Phys. Sci. 1, 100033 (2020).
Blommaert, M. A., Vermaas, D. A., Izelaar, B., in’t Veen, B. & Smith, W. A. Electrochemical impedance spectroscopy as a performance indicator of water dissociation in bipolar membranes. J. Mater. Chem. A 7, 19060–19069 (2019).
Bard, A. J. & Faulkner, L. R. Electrochemical Methods: Fundamentals and Applications (Wiley, 2000).
Diederichsen, K. M., Liu, Y., Ozbek, N., Seo, H. & Hatton, T. A. Toward solvent-free continuous-flow electrochemically mediated carbon capture with high-concentration liquid quinone chemistry. Joule 6, 221–239 (2022).
Elgrishi, N. et al. A practical beginner’s guide to cyclic voltammetry. J. Chem. Educ. 95, 197–206 (2018).
Seo, H., Rahimi, M. & Hatton, T. A. Electrochemical carbon dioxide capture and release with a redox-active amine. J. Am. Chem. Soc. 144, 2164–2170 (2022).
Watkins, J. D. et al. Redox-mediated separation of carbon dioxide from flue gas. Energy Fuels 29, 7508–7515 (2015).
Hemmatifar, A., Kang, J. S., Ozbek, N., Tan, K.-J. & Hatton, T. A. Electrochemically mediated direct CO2 capture by a stackable bipolar cell. ChemSusChem https://doi.org/10.1002/cssc.202102533 (2022).
Liu, Y. et al. Electrochemically mediated gating membrane with dynamically controllable gas transport. Sci. Adv. 6, eabc1741 (2020).
Wang, M. et al. Flue gas CO2 capture via electrochemically mediated amine regeneration: system design and performance. Appl. Energy 255, 113879 (2019).
Wang, M. & Hatton, T. A. Flue gas CO2 capture via electrochemically mediated amine regeneration: desorption unit design and analysis. Ind. Eng. Chem. Res. 59, 10120–10129 (2020).
Ke, X., Prahl, J. M., Alexander, J. I. D. & Savinell, R. F. Redox flow batteries with serpentine flow fields: distributions of electrolyte flow reactant penetration into the porous carbon electrodes and effects on performance. J. Power Sources 384, 295–302 (2018).
Xie, H. et al. Low-energy electrochemical carbon dioxide capture based on a biological redox proton carrier. Cell Rep. Phys. Sci. 1, 100046 (2020).
Stern, M. C., Hatton, T. A. & Alan Hatton, T. Bench-scale demonstration of CO2 capture with electrochemically-mediated amine regeneration. RSC Adv. 4, 5906 (2014).
Eisaman, M. D., Alvarado, L., Larner, D., Wang, P. & Littau, K. A. CO2 desorption using high-pressure bipolar membrane electrodialysis. Energy Environ. Sci. 4, 4031 (2011).
Seader, J. D., Henley, E. J. & Roper, D. K. Separation Process Principles (Wiley, 2011).
Stern, M. C. et al. Electrochemically mediated separation for carbon capture. Energy Procedia 4, 860–867 (2011).
Clarke, L. E., Leonard, M. E., Hatton, T. A. & Brushett, F. R. Thermodynamic modeling of CO2 separation systems with soluble, redox-active capture species. Ind. Eng. Chem. Res. https://doi.org/10.1021/acs.iecr.1c04185 (2022).
Pärnamäe, R. et al. Bipolar membranes: a review on principles, latest developments, and applications. J. Memb. Sci. 617, 118538 (2021).
Al-Dhubhani, E. et al. Entanglement-enhanced water dissociation in bipolar membranes with 3D electrospun junction and polymeric catalyst. ACS Appl. Energy Mater. 4, 3724–3736 (2021).
Leonard, M. E., Clarke, L. E., Forner-Cuenca, A., Brown, S. M. & Brushett, F. R. Investigating electrode flooding in a flowing electrolyte, gas-fed carbon dioxide electrolyzer. ChemSusChem 13, 400–411 (2020).
Rueda-García, D., Dubal, D. P., Hugenin, F. & Gómez-Romero, P. Hurdles to organic quinone flow cells. Electrode passivation by quinone reduction in acetonitrile Li electrolytes. J. Power Sources 350, 9–17 (2017).
Greco, K. V., Forner-Cuenca, A., Mularczyk, A., Eller, J. & Brushett, F. R. Elucidating the nuanced effects of thermal pretreatment on carbon paper electrodes for vanadium redox flow batteries. ACS Appl. Mater. Interfaces 10, 44430–44442 (2018).
Yi, Y. et al. Electrochemical corrosion of a glassy carbon electrode. Catal. Today 295, 32–40 (2017).
Sharifian, R., Boer, L., Wagterveld, R. M. & Vermaas, D. A. Oceanic carbon capture through electrochemically induced in situ carbonate mineralization using bipolar membrane. Chem. Eng. J. 438, 135326 (2022).
Wielend, D., Apaydin, D. H. & Sariciftci, N. S. Anthraquinone thin-film electrodes for reversible CO2 capture and release. J. Mater. Chem. A 6, 15095–15101 (2018).
Rahimi, M. et al. An electrochemically mediated amine regeneration process with a mixed absorbent for postcombustion CO2 capture. Environ. Sci. Technol. 54, 8999–9007 (2020).
Simeon, F. et al. Electrochemical and molecular assessment of quinones as CO2 -binding redox molecules for carbon capture. J. Phys. Chem. C. 126, 1389–1399 (2022).
Bui, A. T., Hartley, N. A., Thom, A. J. W. & Forse, A. C. Trade-off between redox potential and strength of electrochemical CO2 capture in quinones. Preprint at https://doi.org/10.26434/chemrxiv-2022-8zt6r-v2 (2022).
Barlow, J. M. & Yang, J. Y. Oxygen-stable electrochemical CO2 capture and concentration with quinones using alcohol additives. J. Am. Chem. Soc. https://doi.org/10.1021/jacs.2c04044 (2022).
Rahimi, M., Zucchelli, F., Puccini, M. & Alan Hatton, T. Improved CO2 capture performance of electrochemically mediated amine regeneration processes with ionic surfactant additives. ACS Appl. Energy Mater. 3, 10823–10830 (2020).
Prajapati, A. et al. Migration-assisted, moisture gradient process for ultrafast, continuous CO2 capture from dilute sources at ambient conditions. Energy Environ. Sci. 15, 680–692 (2022).
Koytsoumpa, E. I., Bergins, C. & Kakaras, E. The CO2 economy: review of CO2 capture and reuse technologies. J. Supercrit. Fluids 132, 3–16 (2018).
Reiter, G. & Lindorfer, J. Evaluating CO2 sources for power-to-gas applications — a case study for Austria. J. CO2 Util. 10, 40–49 (2015).
Zhang, J., Xiao, P., Li, G. & Webley, P. A. Effect of flue gas impurities on CO2 capture performance from flue gas at coal-fired power stations by vacuum swing adsorption. Energy Procedia 1, 1115–1122 (2009).
Meuleman, E., Cottrell, A. & Ghayur, A. in Absorption-based Post-combustion Capture of Carbon Dioxide (ed. Feron, P. H. M.) 519–551 (Elsevier, 2016).
IEA. About CCUS. IEA https://www.iea.org/reports/about-ccus (2021).
IEA. Net Zero by 2050. IEA https://www.iea.org/reports/net-zero-by-2050 (2021).
Eisaman, M. D. Negative emissions technologies: the tradeoffs of air-capture economics. Joule 4, 516–520 (2020).
Matuszewski, M., Ciferno, J., Marano, J. & Chen, S. (S.). Research and development goals for CO2 capture technology. OSTI.gov https://www.osti.gov/servlets/purl/1597091/ (2011).
Wang, M., Hariharan, S., Shaw, R. A. & Hatton, T. A. Energetics of electrochemically mediated amine regeneration process for flue gas CO2 capture. Int. J. Greenh. Gas. Control. 82, 48–58 (2019).
De Luna, P. et al. What would it take for renewably powered electrosynthesis to displace petrochemical processes? Science 364, eaav3506 (2019).
Min, K., Choi, W., Kim, C. & Choi, M. Oxidation-stable amine-containing adsorbents for carbon dioxide capture. Nat. Commun. 9, 726 (2018).
Newman, J. & Thomas-Alyea, K. E. Electrochemical Systems (Wiley, 2004).
Aghaie, M., Rezaei, N. & Zendehboudi, S. A systematic review on CO2 capture with ionic liquids: current status and future prospects. Renew. Sustain. Energy Rev. 96, 502–525 (2018).
Keith, D. W., Holmes, G., St. Angelo, D. & Heidel, K. A process for capturing CO2 from the atmosphere. Joule 2, 1573–1594 (2018).
Voskian, S. Electrochemically Mediated Separations and Catalysis. PhD thesis, Massachusetts Institute of Technology (2019).
Birdja, Y. Y. et al. Advances and challenges in understanding the electrocatalytic conversion of carbon dioxide to fuels. Nat. Energy 4, 732–745 (2019).
Gao, X., Omosebi, A., Perrone, R. & Liu, K. Promoting CO2 release from CO32−-containing solvents during water electrolysis for direct air capture. J. Electrochem. Soc. 169, 044527 (2022).
Huang, C. et al. CO2 capture from flue gas using an electrochemically reversible hydroquinone/quinone solution. Energy Fuels 33, 3380–3389 (2019).
Winter, T. et al. Redox-responsive 2-aminoanthraquinone core–shell particles for structural colors and carbon capture. ACS Appl. Polym. Mater. 3, 4651–4660 (2021).
Bhattacharya, M., Sebghati, S., VanderLinden, R. T. & Saouma, C. T. Toward combined carbon capture and recycling: addition of an amine alters product selectivity from CO to formic acid in manganese catalyzed reduction of CO2. J. Am. Chem. Soc. 142, 17589–17597 (2020).
Yang, Z.-Z., Zhao, Y.-N. & He, L.-N. CO2 chemistry: task-specific ionic liquids for CO2 capture/activation and subsequent conversion. RSC Adv. 1, 545 (2011).
Li, T. et al. Electrolytic conversion of bicarbonate into CO in a flow cell. Joule 3, 1487–1497 (2019).
Oloye, O. & O’Mullane, A. P. Electrochemical capture and storage of CO2 as calcium carbonate. ChemSusChem 13, 1767–1775 (2021).
Wang, M., Herzog, H. J. & Hatton, T. A. CO2 capture using electrochemically mediated amine regeneration. Ind. Eng. Chem. Res. 59, 7087–7096 (2020).
Acknowledgements
K.M.D. acknowledges support from an appointment to the Intelligence Community Postdoctoral Research Fellowship Program at the Massachusetts Institute of Technology (MIT), administered by the Oak Ridge Institute for Science and Education (ORISE) through an interagency agreement between the US Department of Energy (DOE) and the Office of the Director of National Intelligence (ODNI).
Author information
Authors and Affiliations
Contributions
All authors researched data for the article. All authors contributed substantially to discussion of the content. All authors contributed towards writing the article. K.M.D. and T.A.H. reviewed and edited the manuscript before submission. Introduction (K.M.D., R.S. and D.V.); Experimentation (R.S., K.M.D., Y.L., J.S.K. and S.K.); Results (Y.L. and K.M.D.); Applications (R.S. and B.M.G.); Reproducibility and data deposition (K.M.D.); Limitations and optimizations (K.M.D.); Outlook (T.A.H. and D.V.); Overview of the Primer (K.M.D. and T.A.H.).
Corresponding author
Ethics declarations
Competing interests
T.A.H. is a co-founder and Scientific Advisory Board member of Verdox, Inc. D.V and R.S. co-founded SeaO2 and are involved as advisor and CTO, respectively. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Methods Primers thanks Eileen Yu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
IEA Emissions Factors 2021: https://www.iea.org/data-and-statistics/data-product/emissions-factors-2021
Glossary
- Amine scrubbing
-
A carbon separation technique that reacts CO2 with aqueous solutions of amine-based molecules in an absorption column, followed by release of CO2 and regeneration of the amine at elevated temperature in a stripping column.
- Calcium looping
-
A carbon separation process that uses high temperatures (>600 °C) to oxidize calcium carbonate to release pure CO2 and calcium oxide, followed by reaction of calcium oxide with CO2 (in a dilute stream) to form additional calcium carbonate.
- Temperature swing processes
-
Processes that capture CO2 from dilute sources at low temperature and release it at elevated temperature to regenerate the sorbent.
- Organic redox
-
An electrochemical process that employs organic molecules that change oxidation state under applied potentials.
- Electrophile displacement
-
A process in which a bound species is replaced by another species with a stronger binding constant.
- Bipolar membrane
-
(BPM). Cation exchange membranes and anion exchange membranes laminated or electro-spun together with a water dissociation catalyst in between.
- pH swing
-
A process that changes the pH of a liquid to induce a change in CO2 solubility.
- Ohmic resistance
-
Resistance to the flow of electrons that follows Ohm’s law, typically related to current flow through a metal or the ionic conductivity of an electrolyte.
- Column flooding
-
Flooding that occurs when liquid covers the full cross section of the column, leading to gas bubbling through the liquid with reduced overall mass transfer and increased pressure drop.
- Baffling
-
Liquid flow directing devices within channels.
- Nyquist plot
-
A representation of an electrochemical impedance spectroscopy experiment in which the negative of the imaginary component of the impedance is plotted against the real component of the impedance over a range of frequencies.
- Direct air capture
-
(DAC). The separation of CO2 from air.
- Direct ocean capture
-
(DOC). The separation of CO2 directly from ocean water to indirectly capture CO2 from the atmosphere.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Diederichsen, K.M., Sharifian, R., Kang, J.S. et al. Electrochemical methods for carbon dioxide separations. Nat Rev Methods Primers 2, 68 (2022). https://doi.org/10.1038/s43586-022-00148-0
Accepted:
Published:
DOI: https://doi.org/10.1038/s43586-022-00148-0
This article is cited by
-
Redox-tunable isoindigos for electrochemically mediated carbon capture
Nature Communications (2024)
-
Water management and heat integration in direct air capture systems
Nature Chemical Engineering (2024)