Abstract
Organs-on-chips (OoCs) are systems containing engineered or natural miniature tissues grown inside microfluidic chips. To better mimic human physiology, the chips are designed to control cell microenvironments and maintain tissue-specific functions. Combining advances in tissue engineering and microfabrication, OoCs have gained interest as a next-generation experimental platform to investigate human pathophysiology and the effect of therapeutics in the body. There are as many examples of OoCs as there are applications, making it difficult for new researchers to understand what makes one OoC more suited to an application than another. This Primer is intended to give an introduction to the aspects of OoC that need to be considered when developing an application-specific OoC. The Primer covers guiding principles and considerations to design, fabricate and operate an OoC, as well as subsequent assaying techniques to extract biological information from OoC devices. Alongside this is a discussion of current and future applications of OoC technology, to inform design and operational decisions during the implementation of OoC systems.
Similar content being viewed by others
Introduction
The organ-on-a-chip (OoC) is an intriguing scientific and technological development in which biology is coupled with microtechnology1,2 to mimic key aspects of human physiology. The chip takes the form of a microfluidic device containing networks of hair-fine microchannels for guiding and manipulating minute volumes (picolitres up to millilitres) of solution3,4,5. The organ is a more relatable term that refers to the miniature tissues grown and residing in the microfluidic chips, which can recapitulate one or more tissue-specific functions. Although they are much simpler than native tissues and organs, scientists have discovered that these systems can often serve as effective mimics of human physiology and disease. OoCs comprise advanced in vitro technology that enables experimentation with biological cells and tissues outside the body. This is achieved by containing them inside vessels conditioned to sustain a reasonable semblance of the in vivo environment, from a biochemical and physical point of view. Working on the microscale lends a unique opportunity to attain a higher level of control over the microenvironment that ensures tissue life support, as well as a means to directly observe cell and tissue behaviour.
The OoC is a relatively recent addition to the toolbox of model biological systems available to life science researchers to probe aspects of human pathophysiology and disease. These systems cover a spectrum of physiological relevance, with 2D cell cultures the least relevant, followed in increasing order by 3D cell cultures, organoids and OoCs. Unsurprisingly, the use of model organisms such as mice and Drosophila physiologically exceeds engineered tissue approaches6,7. While biological complexity increases with physiological relevance in model organisms, this unfortunately leads to increased experimental difficulty. In vivo physiological processes are, in many ways, the least accessible to direct investigation in mice, humans and other mammals, despite significant advances in in vivo imaging. However, 2D and 3D cell cultures, such as spheroids and stem cell-derived organoids, sacrifice some aspects of in vivo relevance to facilitate experimentation. The OoC may be regarded as a bridging technology, offering the ability to work with complex cell cultures, while providing better engineered microenvironments to maximize the model.
Following on from early concepts, including animal-on-a-chip8, body-on-a-chip9 and breathing lung-on-a-chip10, research in the OoC and microphysiological systems fields has grown exponentially; evidenced by numerous excellent reviews published recently1,2,11. Recognition of OoC technology now extends far beyond university laboratories, driven by a need to better understand the human physiology underlying health and disease, and to find new approaches to improve the human condition. The World Economic Forum, for instance, selected the OoC as one of the top ten emerging technologies in 2016 (ref.12). This indicates that there is a strong need for human-like testing systems in the pharmaceutical industry and also a maturity of the OoC technologies to build them. Similarly, cosmetic, food and chemical industries stand to benefit enormously from OoC technologies for both production and testing, as society seeks humanized in vitro alternatives to animal testing.
OoC technology has benefited from converging advances in tissue engineering and microfabrication. Cell and tissue engineering have progressed from primitive 2D monocultures to complex 3D co-culture systems. Much emphasis has been placed on cellular microenvironment and geometrical arrangement, which enables cell manipulation to achieve cell polarization13,14,15, direct cell–cell interaction16,17 and propagation of chemical and electrical signalling18,19,20. Besides more advanced cell lines21, handling of primary cell sources and integrating them into artificial structures to promote organ-like functions has become more robust and reliable22,23. The emergence of induced pluripotent stem cell (iPSC) technology promises personalization of OoCs, as patient-specific cells can be differentiated from iPSCs obtained from individual donors and incorporated into the OoCs24,25,26. This allows study of disease phenotypes and drug responses in a patient-specific manner27.
The second driving force behind OoC success has been microsystems technology, the umbrella term for fabrication processes borrowed from integrated circuit industry. This approach uses lithographic pattern transfer to create structures in the nanometre and micrometre range28,29. Milestones in the development of OoCs are coupled with key technological developments in microsystems technology (Box 1). First used in analytical chemistry to engineer laboratory-on-a-chip devices3,30,31,32, microsystems technology has driven the development of both microfluidic and miniaturized actuator and sensing capabilities of OoCs. This has resulted in a shift to how in vitro bioreactor and cell biological systems are designed33,34, operated35,36,37,38,39 and monitored38,40,41. Gone are the typical flat polystyrene (PS) surfaces of the well plate or Petri dish. In vitro organ function can now be observed in chips individualized for the organ of interest. The chips are tailored to replicate cellular and extracellular features of the organ that can respond to biochemical and physical cues to maintain and simulate organ function. Crucially, OoC systems enable multi-parametric read-outs of organ function, providing a window into the integrated biology of humans and animals.
OoC technologies have advanced and matured substantially and it is predicted that interest will continue to grow in the coming years. However, for those new to the field, it may seem as if there are at least as many examples of OoCs in the literature as there are applications. Deciding where to start may be daunting. This Primer is designed to introduce the aspects of OoCs that need to be considered when developing an experiment. Important inventions over the past two decades are summarized, with the goal of illustrating how these have advanced the field. The Primer covers guiding principles and considerations to design, fabricate and operate an OoC as well as subsequent assaying techniques to extract biological information. Also included is a discussion of applications for OoC technology.
Experimentation
OoCs are designed to provide a suitably in vivo-like environment to guide a collection of cells to assemble into a 3D tissue capable of replicating one or more organ-level functions or to culture organotypic tissue to retain function. This section gives an overview of choices and considerations when designing an OoC tailored to a particular in vitro tissue experiment. OoC development should be guided by its context of use, which is related to the application area it will be used for, and defines the expected data42,43. The considerations, strategies and equipment needed for any general OoC experiment are reviewed in the order they present themselves when setting up a new experiment (Fig. 1). Finally, several case studies of single-organ and multi-organ OoCs provide insight into the development of these systems for specific applications in drug research.
a | Design and conceptualization of the organ-on-a-chip (OoC) system need to factor in number of organ models, how they are communicating and culture configuration of each organ model. b | Materials and fabrication technique need to be selected for construction of the OoC device, depending on scale, read-out functionality and feature resolution required. Sterilization and surface modification are tied to material choices. c | Choice of cell sources (primary, immortalized, stem cell-derived) will need to balance between biological functionality and practical operability of device. d | Finally, OoC system will be operated with peripheral equipment including pumps, incubators, sensors and microscopes to properly maintain, stimulate and monitor cells inside the OoC system. ECM, extracellular matrix; TEER, transepithelial electrical resistance.
Conceptualization and design
Single-organ systems often achieve a high degree of biological authenticity, allowing evaluation of the response of a specific organ to a compound or mixture of compounds. Multi-organ systems provide a framework to examine the potential interaction of one organ with at least one other, principally through the exchange of metabolites or soluble signalling molecules. Both single-OoC and multi-OoC systems are often referred to as microphysiological systems, as they are designed to model features of human or animal biology within a microscale culture44. Multi-OoC systems, which model the physiological systemic response in the body, are commonly referred to as body-on-a-chip35. The choice of a single-organ or multi-organ system depends on the desired functionalities needed for the system to be a good model of the physiological processes. The degree of complexity should be kept to the minimum required to represent the biological application without introducing unnecessary factors that make the system difficult to use and analyse. Multi-organ systems tend to involve more complex engineering design than single-organ systems. This allows control of the transport and distribution of culture media between the individual organs. Consequently, there is a trend in the OoC systems that have been developed, where single-OoCs are more biologically detailed models of an organ whereas multi-OoCs use less detailed organ models and focus on the systemic interactions between organs35.
The next design consideration is to decide the approach for forming functional tissues within the OoC. In a top-down or organotypic approach, a primary tissue — for example, an organ slice from a biopsy — or engineered tissue — such as a preformed organoid — is incorporated into the OoC system. In a bottom-up approach, isolated cells from primary, immortalized lines or stem cell-derived sources are cultured inside an a priori empty microfluidic environment, which supports the remodelling of the cells into a functional neo-tissue. The selected strategy informs the design of the OoC architectures. This serves a dual purpose of organizing and supporting cells within the OoC in a specific cell culture configuration, as well as routing fluids, such as the culture medium, to connect tissue components in a manner that reflects their connectivity in vivo. Although there are many variations of OoC device architectures, they can generally be divided into two classes, based on the organ system they create. The first comprises solid organ chips, where cells are cultured as 3D tissue masses that can interact with one another and the culture medium in a defined manner. Examples of this architecture type include micro-pillar and microwell arrays that are often used in liver (Fig. 1), tumour, cardiac and adipose OoCs11,45. The second class contains barrier tissue chips, where the device architecture supports the cells to form a natural barrier between fluid compartments. This allows selective transport processes across the barrier to be studied. These architectures are typically found in gut, lung and skin OoCs (Fig. 1). The choices for either architecture and the culture strategy depend on the final functionality of the OoC46.
Material selection and fabrication
The choice of material depends on numerous factors, including the functionality of the final device, microfabrication strategy, read-outs and biocompatibility. A typical OoC device consists of various material combinations to build the final device. Amongst the most commonly used materials are silicone rubber, such as poly(dimethylsiloxane) (PDMS); glass; and thermoplastics such as PS, poly(methyl methacrylate) (PMMA), polycarbonate (PC) or cyclic olefin copolymer (COC). However, there is no perfect standard material, as different materials have their advantages and disadvantages (Table 1). Decisions regarding material choice are often a compromise between desired functionality, access to fabrication facilities and development stage of the product (Supplementary Fig. 1). Glass is robust and inert, but expensive and requires advanced processing facilities. Silicon allows fabrication of intricate nanostructures to form on-chip sensors or barrier gratings, although this is expensive and laborious due to the need for clean-room facilities. Moreover, it is not transparent, presenting compatibility issues with conventional inverted microscopes. Thermoplastics ensure transparency and are easy to mass-produce, but are challenging when creating complex designs at the prototyping phase. Currently, PDMS is the most widely used material for development of OoC devices because devices with high-resolution microstructures and nanostructures can be easily produced by replica moulding in PDMS from microfabricated templates. PDMS is ideal for biological applications due to its biocompatibility, optical transparency and gas permeability. Furthermore, the elasticity of PDMS has been exploited to apply mechanical stimulation to cells10. Recently, breakthrough technology has been proposed that enables high-speed and high-quality PDMS 3D processing through laser pyrolysis47. However, this material is known to adsorb and absorb a wide range of (bio)chemicals, which may influence experimental results, especially for drug testing applications48,49.
PS is one of the alternatives often seen in standard cell culture tools, such as tissue culture flasks and multiwell plates, as well as newly developed OoCs50,51. What is noteworthy is that production yield, which is one of the obstacles of the existing OoC fabrication method, can be solved by mass production through injection moulding, whereas single prototypes are made by micro-milling. This productivity can provide a route to efficient OoC-based high-throughput screening that allows automated testing of a large number of drug compounds for a specific biological target. Injection moulding is not suitable for OoC models having complex designs and functional features such as stretching. The fabrication method should thus be adopted considering the experimental purpose.
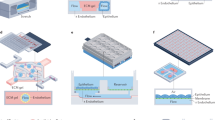
Meanwhile, as 3D printing technology advances, some groups have reported OoC models generated by 3D printing (or additive manufacturing)52. This method quickly and accurately creates complex 3D structures that have been difficult to create with the other methods mentioned so far. The use of 3D-printed microfluidic devices for OoC applications is currently limited by inadequate optical transparency, as resin formulations and post-processing steps are not yet optimized for this property. In addition, the biocompatibility of 3D-printed resins also needs to be verified. Nonetheless, OoC technology has reached a point where effective fabrication methods can be adopted through material selection based on experimental purposes (Fig. 2). Various options have been presented, including etched microchannel configuration via PDMS-based soft lithography, production of complex structures via 3D printing and plastic-based OoC mass production via injection moulding.
a,b | With biological compatibility and tunable mechanical properties, poly(dimethylsiloxane) (PDMS) is fabricated using soft lithography to develop an elaborate microstructure323 (part a) or 3D rapid prototyping by laser processing47 (part b). c | Integrated device fabrication with gold- or platinum-deposited substrate such as an electrode array324. d | For large-scale experiments such as high-throughput screening, plastic-based injection moulding could be selected50,51. e,f | For rapid prototyping with a high degree of design freedom, resin-based (part e) or hydrogel-based (part f) 3D printing is available325,326. HCS, high-content screening.
Sterilization
OoC devices differ from conventional cell culture platforms (such as multiwell plates) due to their 3D architectures and the materials from which they are fabricated, as highlighted by a previous review53. However, performing cell culture in OoC devices shares similar requirements with conventional platforms. Sterility of the OoC devices must therefore be ensured to avoid microbial contamination, including in the various microfluidic components that will be used to set up the entire OoC system. The variation in materials used in OoC devices and microfluidic components requires additional precaution in choosing the appropriate sterilization methods to prevent component damage. Inappropriate use of sterilization methods may cause damage to the OoC devices and microfluidic components, resulting in undesirable leaks during system assembly. Many common plastics such as PMMA and PC may not be suitable for conventional autoclave sterilization due to their low thermal resistance. Other methods of sterilization, such as UV or ethanol treatment, are often employed in the laboratory setting, although these methods should be used with caution. UV penetration may be limited by the material’s opacity and ability to absorb the germicidal UV rays. Ethanol soaking is not compatible with some materials, such as PMMA, as they may be partially dissolved. Ethanol can also be absorbed into PDMS devices if soaked for extended periods of time (longer than overnight), which can adversely affect cells when it is subsequently leached out. Gamma irradiation and ethylene oxide treatment should be the preferred sterilization options in industrial or clinical settings.
Surface treatment
Treatment of device surfaces that come into contact with cells may be necessary to ensure biocompatibility or enhance cell adhesion. In 3D spheroid or organoid cultures, treatment with pluronic acid is often adopted to passivate the chip surface to prevent undesirable spheroid or organoid dissociation via potential cell attachment. This is crucial, as loss of the 3D tissue architecture may cause a concomitant loss in physiological organ function54. On the other hand, protein and extracellular matrix (ECM) coatings may be used if we wish to promote cell attachment to the chip substrate. These coatings are a must for the formation of attached, confluent monolayers of cells needed to emulate the intestinal epithelium in the gut14,55, for instance, or the endothelium in blood–brain barrier (BBB) OoCs56,57,58. To create higher-fidelity (patho)physiological models, tissue-specific and disease-specific matrices may be used, for example to induce crypt-like structures in gut-on-a-chip devices59,60. These can include complex biomatrices such as Matrigel, or simpler biomatrices derived from fibrin or collagen. Cells can be mixed with the liquid precursor of these biomatrices, which can be subsequently loaded into the OoC device and allowed to polymerize into a gel. Some organs such as the liver and skin require a 3D microenvironment in order to attain physiological function, and hence biomatrices can serve as a useful scaffold for cells to remodel into a 3D structure. Biomatrices may also be essential for maintenance or differentiation of certain cell types. For example, Matrigel has been found to promote neuronal maintenance and differentiation in neural stem cells61, whereas culturing of myoblasts within a suspended overhanging fibrin-based gel promoted myogenic differentiation62.
Selection of biological elements
Here, we focus the discussion mainly on the selection of isolated cells because they are more commonly employed in OoCs. The integration of tissue slices into microfluidic devices has been previously reviewed63. There are several criteria when selecting an appropriate cell source that should be considered in the context of the application of the OoC.
Patient specificity
If the OoC is intended to model physiological variations in individuals or specific patient (for example, diseased versus healthy) populations, primary and stem cell-derived cells are both viable options. However, primary cells are often less accessible due to a lack of both availability of donor tissues and well-established protocols for isolating the cells from the tissues with high purity. With the advent of iPSC technology64, patient-derived stem cells become more accessible with minimal invasiveness. iPSCs can be differentiated into various cell lineages, and are particularly useful when one is trying to generate human leukocyte antigen (HLA) or patient-matched tissues in a multi-OoC system65.
Intrinsic functionality of the cells
OoCs often need to recapitulate tissue-specific physiological functions that are essential for their intended application, such as xenobiotic metabolism in the liver, barrier functions for the intestine and skin, and contractility of cardiac and skeletal muscles. Many immortalized cell lines as well as human pluripotent stem cell-derived cells often express a limited subset or fraction of the functional capacity as compared with primary cells66. Although primary cells may possess the full in vivo complement of cellular functions, they often lose their tissue-specific functions rapidly when maintained in vitro67, which may limit the useful time window of the OoC, especially for long-term studies. Primary cells can have inter-donor variability, which may pose a challenge for data reproducibility when not looking at patient-specific responses. Therefore, it is helpful to prioritize the different cellular functions based on the intended application of the OoC when considering different cell sources.
Expansion capacity
Expansion capacity refers to the ability of the cells to undergo proliferation in vitro and directly relates to the number of cells that can be practically generated for seeding into an OoC device. Although microfluidic devices house a minute number of cells (typically in the order of 103–105 cells), their design can influence how many cells are practically needed during the seeding process. This may be limiting for primary cells, which have no or limited doubling capacity before they undergo senescence. Although human iPSCs can be expanded indefinitely, the differentiation and cell harvesting process can result in significant cell loss, capping the number of cells that one can produce in a single batch. On the other hand, when using immortalized cell lines which have high proliferation rates, one needs to consider the minute cell culture volumes present in OoC devices to prevent nutrient depletion as well as occlusion of the fluidic channels by the growing tissue mass.
Supporting cell types
The stromal environment of tissues is composed of supporting cells (such as fibroblasts, pericytes and vasculature) that often contribute to the functionality of the engineered tissue, and play major roles in disease progression. The incorporation of stromal cells into engineered tissues has been shown to modulate cell signalling and structural support via modification and deposition of the ECM, and influence whether a drug treatment will succeed or fail. The stroma is dramatically changed in the context of disease, particularly with respect to systemic diseases such as fibrosis and cancer. Thus, the inclusion of stromal cells and their proper microenvironment can be critical in recapitulating specific tissue functionality and physiological responses that are representative of the in vivo situation.
Functional time window
iPSCs need to be differentiated for 2–3 weeks to mature into specific cell lineages. Primary cells forming barrier tissues, such as the skin, BBB and intestinal epithelium, often require an extended culture period to remodel into cell sheets with well-established cell–cell contacts to have physiological barrier functions68. The device design and operation should be sufficiently robust to cater to long-term cultures (typically a few weeks) if on-chip stem cell differentiation and tissue maturation are considered.
Additional cell considerations for systemic studies
For systemic studies, the presence of a functional circulatory system (blood or surrogate with different cell types) and the immune system may be required. Both are challenging to work with and both elements have been underdeveloped up until now.
Supporting life inside the device
Selection of cell culture medium
For a single-OoC involving only a single cell type, culture medium that was originally formulated for conventional cultures can similarly be adopted. An added complexity for media selection is introduced when generating single/multi-OoCs that involve multiple cell types, each having different nutrient requirements. The optimized co-culture medium must be able to maintain the viability and functional phenotype of each distinct cell population. Once this criterion is satisfied, we can then consider the suitability of the medium in supporting downstream assays without undesired interference. In the optimization of a suitable co-culture medium for OoC applications, many groups have adopted various mixtures of the original culture medium used for each individual cell type with relatively good outcomes. These include multicellular OoC models mimicking adipose tissue69, and also multi-organ OoC models recapitulating liver–kidney interactions70 and liver–adipose–skin–lung interactions71. However, with an increasing number of different cell types being co-cultured, the optimization of a suitable co-culture medium becomes more challenging. Compartmentalization of cells into individual compartments via permeable membranes can circumvent the need for a common co-culture medium, thus allowing for cell-specific media to be supplied to each compartment while still permitting paracrine interactions between the compartments, as shown in OoC devices mimicking the liver72 and skin73. If possible, serum-free media should be considered as sera constitute a large source of variation and may interfere with assays74. Media should be optimized in experiments with the OoC, starting with the most commonly used cell culture medium for non-OoC work with the same cells, such as minimal essential medium (MEM)-based medium for epithelial cell cultures or endothelial cell growth medium (ECGM) for endothelial cells.
Creation of perfusion circuits
Media perfusion is a hallmark of OoC devices, serving as a circulatory system mimic that maintains a concentration gradient for nutrient and waste convective transport. To drive media perfusion through the OoC device, various pumps have been adapted for OoC applications (Supplementary Fig. 2). These include conventional syringe pumps75,76, microvalve-driven actuator pumps77,78 and peristaltic pumps38,79. Alternatively, various groups have also adopted hydrostatic pressure-based pump-free systems to drive perfusion80,81,82. The choice of pumps will greatly depend on whether the culture runs on a one-pass type of perfusion flow or a recirculatory flow configuration. Conventional syringe pumps and pump-free gravity-driven flows typically support one-pass perfusion flows, whereas other pump variants such as peristaltic pumps are amenable to driving recirculatory flow. Recently, however, we have seen the emergence of OoC devices with gravity-driven recirculatory flows, which removes the need for physical pumps, hence minimizing overall system complexity while still mimicking soluble factor crosstalk between multiple organ compartments82,83,84,85,86. The choice for either type of flow and for the flow rate (volume per time) depends on the needs of the organ model and the in vivo situation, including the appropriate level of shear stress induced by flow over a tissue surface.
One-pass flow ensures a stable supply of fresh nutrients, but inter-tissue communication is only possible in one direction (namely downstream). A recirculating flow has the advantage of recirculating signalling molecules, which allows chemical communication between the different elements of a multi-organ device. Recirculation, however, does not allow for continuous replenishment of nutrients, and will lead to an accumulation of waste products. Although OoC systems can be operated for several days without medium exchange, periodic removal of a portion of medium from the OoC with replacement by fresh medium is necessary. This removal has the useful consequence that material is provided for offline analysis. The fraction removed and frequency of removal can be variable24,87. Fluid volume in an OoC is also very limited, particularly in a design that emulates physiologically realistic values of the ratio of blood/interstitial fluid to body mass85. The amount of sample that can be withdrawn is thus small (<100 µl medium per day, even if full replacement is used). Partial fluid replacement is preferred to sustain more physiological conditions, so samples of only 25–50 µl per day may be available for offline analysis in systems emulating realistic fluid to cell ratios.
Besides the use of pump/gravity-driven perfusion flows to drive organ OoC interactions, another method adopted is the transfer of conditioned medium from one single-OoC (such as the liver) to another single-OoC (such as the kidney) in a sequential manner88,89. This is known as functional coupling of OoCs88, whereas the perfusion methods described above with direct interactions between OoC modules are known as physical coupling of OoCs. Functional coupling does away with the technical challenge of incorporating pumps or means of creating gravity-driven flow, and the need for interconnections between different OoC modules. However, it should be noted that functional coupling may not be useful when monitoring real-time crosstalk interactions between OoC modules, such as the gut–liver axis. Furthermore, certain cell-secreted factors may need to be continuously concentrated due to their highly degradable nature90. As such, the transfer of conditioned medium from one OoC to another may result in the loss of an effective amount of these cell-secreted factors, with remaining numbers of factors insufficient to elicit a response in the target OoC. Physical coupling would be more appropriate in the scenarios mentioned above.
Control of the cell microenvironment
Cells interact with soluble factors, the ECM and neighbouring cells in a 3D milieu with specific physico-chemical properties. This is collectively known as the cell microenvironment, which must be carefully controlled in the OoC system for cells to function properly. As compared with conventional bulk cell culture systems, OoC systems offer more precise control over the cell microenvironment because the geometries of the culture chamber and the associated physical and chemical phenomena of fluids are defined with microscale resolution (in the order of 101–102 µm).
The unique physics of microfluidics results in fundamental differences between microfluidic-based and conventional static cell cultures. Common phenomena observed in conventional macroscale cell cultures, such as bubble formation, evaporation and nutrient depletion, are exacerbated by the microscale volumes and material choices of OoCs, and can dramatically alter osmolality, pH and nutrient availability. It is critical to have a working knowledge of how various physical forces and mass transport phenomena at the microscale influence the presentation of various biochemical and mechanical cues, such as cytokines and shear stress, respectively. Dimensionless numbers expressing the relative dominance of competing physical phenomena that are relevant to microenvironmental control include the Reynolds number (Re; inertial/viscous forces), Péclet number (Pe; convective/diffusive transport) and Damköhler number (Da; diffusion/reaction timescales). Their links to various environmental factors, such as shear stress and soluble factor signalling, have been previously explained91. For instance, the Da of critical biochemical factors, such as growth factors and glucose, in cell cultures can be used to estimate the effective culture time in an OoC device. Owing to the smaller dimensions of OoCs compared with conventional well plates, the Da of biochemical factors is much smaller, implying that their reaction time dominates over diffusion time. Consequently, in a static diffusion-dominated OoC, the time interval between medium change is often shorter than that of the macroscale culture system, and can be scaled based on the relative change in channel height, h, and the known effective culture time of the macroscale culture system91. Perfusing the OoC at flow rates such that the Pe to Da ratio is high (≫1) ensures sufficient convective delivery of these biochemical factors without limiting cell uptake92,93.
Microfluidic control of environmental factors is often coupled. For example, the fluid shear stress and mass transport regime of soluble factors are both functions of flow velocity, which means changing the perfusion flow rate of an OoC can alter both shear stress magnitude and soluble signalling at the same time. Dimensionless numbers can be used to guide device dimensions and operating parameters to selectively keep one environmental factor constant (at least from the biological perspective that there are minimal effects on cells) while varying another. For example, when operating a multiplexed device to examine effects of different shear stress magnitudes on cells, the Pe can be used to ensure that all of the cells are in the convective mass transport zone (Pe > 1). This would help decouple effects of shear stress from autocrine soluble signalling as secreted factors would be washed away. Conversely, when trying to study the effects of secreted autocrine or paracrine factors, it may be preferable to operate at flow rates where all cells are subjected to shear stresses below a set threshold. This is particularly important to prevent detrimental effects on shear-sensitive cells, such as embryonic stem cells (<10–3 dynes cm–2)92, primary neurons (<10–3 dynes cm–2)94 and hepatocytes (<10–1 dynes cm–2)95,96. On the other hand, cells that experience shear stress physiologically have been reported to exhibit enhanced differentiated cell phenotypes and functions over a certain optimal range of shear stress magnitudes, as exemplified by intestinal epithelial cells (~10–2 dynes cm–2)97,98, airway epithelial cells (5 × 10–1 dynes cm–2)99,100, bone cells (10–1–101 dynes cm–2)100 and cardiovascular cells (100–101 dynes cm–2)100,101.
Care must be taken to ensure proper equilibration of culture medium with the gaseous environment. The gaseous composition of the air is important for maintaining proper oxygen concentration and physiological pH (7.0–7.4). Depending on the needs of the OoC, oxygen may be kept at atmospheric levels (21%), lowered or removed to recreate hypoxic (<10%)102 to anaerobic systems, or increased to improve oxygenation of tissue sections. Most conventional cell culture media (such as DMEM or RPMI 1640) are formulated with bicarbonate buffers, which yield a physiological pH of 7.0–7.4 only when equilibrated with 5% carbon dioxide. Hence, 5% carbon dioxide is typically used in conventional cell culture incubators. If the OoC is operated outside a conventional incubator, such as on a heating block or transparent indium–tin oxide heaters under atmospheric conditions, the medium pH can be maintained by the addition of HEPES buffer103 or pre-equilibrating the medium in a 5% carbon dioxide environment, first, before delivery into the OoC device34. Cells grown in OoCs may have a different baseline function from those grown in conventional cell culture grown on tissue culture plastics. Therefore, one needs to be careful when attributing differences observed between OoCs and conventional cell culture to the specific environmental cue being applied through the microfluidic device.
Monitoring OoC status
Different read-out options and controls are necessary to monitor the status of the OoC during culturing and experimentation. The most common control is visual microscopic inspection of the OoC to regularly check whether the cells or tissue sections are normal in appearance (size, number, shape and so on) and there are no abnormalities (such as air bubbles or microbial contaminations) that will lead to device failure. Air bubbles may arise during device operation, such as upon connecting a new syringe with medium. This is undesirable as moving air bubbles can be destructive to cells in the microchannels by the interfacial force they exert. Ideally, air bubble formation should therefore be avoided by carefully connecting the tubing and forcing the air out before cells are introduced. In the presence of an air bubble, if the bubble is not obstructing the medium perfusion flow and cells are protected from direct bubble interaction (such as when cells are trapped in hydrogel/micro-pillar arrays), it is better to leave the air bubbles and allow them to dissolve into the medium or diffuse through the chip if the chip is gas permeable. However, if there is a high likelihood that the air bubbles will come into direct contact with the cells or block medium perfusion flow, flushing out the bubble may be essential to preserve the experimental set-up. When attempting to flush out air bubbles, extra care has to be taken to not dislodge the cells or break the chip’s seal with the large forces required to extract the air bubbles. Engineering controls such as the implementation of an on-chip or external bubble trap ahead of the cell culture chamber can also greatly eliminate the risk of air bubbles directly interacting with the cells. Other flow obstructions may be caused by fibres or accumulated debris, which can generally not be solved when cells are already present; the experiment can then continue if the obstruction is not in an essential place, or aborted if it is blocking an essential flow. Microbial contaminations are indicated by the presence of bacteria or yeasts inside the microchannel and always result in the termination of the experiment.
Case studies
Here, we provide insight into the development of single-organ and multi-organ OoC systems for specific applications, with a special emphasis on OoCs for drug research including absorption, distribution, metabolism, excretion and toxicology (ADME-Tox). We discuss conceptualization and design, material selection and fabrication, selection of biological elements and supporting life inside the device. The examples considered in this section exhibit evolution in their designs that are commensurate with advances in both microfabrication and biological models over the past decade. Once one or multiple research questions have been formulated, these can be translated into a list of functional requirements and technical specifications that guide the construction and implementation of the OoC system.
The desired functionalities of the system should be leading in its design. The first decision is between a single-organ or multi-organ system. Single-organ systems tend to be more suited to study specific tissue and organ functions, without having the complexity of other organ systems involved. Multi-organ systems are more suited to study interactions between different organ compartments, such as when the functions of one organ affect the functioning of another organ. We use drug absorption through the intestinal epithelium as an example of a single-organ system, and highlight different multi-organ systems investigating the metabolism of a drug in the liver and its subsequent therapeutic or toxic effects in other target organs.
Single-organ OoC systems
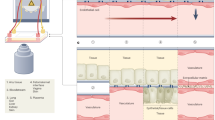
Intestinal absorption of orally administered drugs is commonly evaluated in Transwell assays, where a layer of intestinal epithelial cells is cultured on a permeable insert placed in one of the wells of a cell culture plate. These intestinal cells act as the barrier between the top compartment (mimicking the gut lumen) and the bottom compartment (mimicking the bloodstream); drug absorption can be measured over time by analysing samples taken from both compartments. This intestinal absorption process has been translated into a microfluidic format104 (Fig. 3A).
A | Gut-on-a-chip device for drug absorption studies104: Transwell cell culture insert that was the inspiration for this gut-on-a-chip (part Aa); and flowing microsystem, with uptake of drug molecules from apical channel and transport into basolateral channel (part Ab). B | Reconfigurable multi-organ-on-a-chip (OoC): includes four, seven or ten different organ models that can interact with one another via microchannels107 (part Ba); and various layers of device, including all connections for cell culture media and pneumatic channels to actuate integrated micropumps (part Bb). C | Multi-OoC developed consisting of several OoC compartments together in one device108: all microchannels and chambers filled with green dye for visualization (part Ca); and elements (1, liver chamber; 2 and 4, heart chamber with cantilevers and microelectrode arrays; 3, vulva cancer chamber; 5, breast cancer chamber) contained in device, as well as medium access ports and integrated read-out structures (part Cb). IV, intravenous; MPS, microphysiological system. Parts Aa and Ab adapted from ref.104, Springer Nature Limited. Parts Ba and Bb adapted from ref.107, CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/). Parts Ca and Cb adapted with permission from ref.108, AAAS.
The barrier consisting of intestinal cells grown on a porous membrane was retained, but replaced the static top and bottom compartments of the Transwell with microchannels containing a dynamic flow of cell medium (conceptualization and design). PDMS was used because it is a transparent, biocompatible and mouldable silicone rubber, with moulds produced by photolithography, and the membrane was pretreated with a collagen surface coating to improve cell adhesion (material selection and fabrication). Caco-2 cells, the gold-standard cell line for drug absorption studies, were cultured inside the microchannel; these cells lack many other functions that intestinal epithelial cells have, but they are very suitable for absorption studies (selection of biological elements). The intestinal OoCs were kept in an incubator, with the cell medium provided by two syringe pumps without recirculation (supporting life inside the device). The device was inspected by optical microscopy, and the permeability of model drugs was monitored using high-performance liquid chromatography and fluorescence-based assays with samples of the OoC effluent.
A more complex intestinal single-organ OoC was developed to address whether enteric coronavirus infections could be modelled in an OoC format, including the testing of antiviral drugs105. The process of viral infection is far more complex, and requires multiple different organ functions, including immune components, to be present. A similar two-channel OoC as above was used, based on a porous membrane, but with the inclusion of more biological functionalities such as the barrier function of both the intestinal epithelium and the blood vessel wall, as well as an immune component (conceptualization and design). The OoC consisted of stretchable silicone rubber with two channels and a permeable membrane, pretreated with collagen and Matrigel. The stretchability of the membrane was exploited to actuate peristalsis-like motions of the tissue (material selection and fabrication). Intestinal organoids were cultured from patient biopsy samples. Endothelial cells were cultured on the other side of the membrane, to include the functions of intestinal blood vessels. Peripheral blood mononuclear cells were isolated from patient blood and inserted into the bottom microchannel of the device to act as blood vessels (selection of biological elements). All incubation conditions, including flows and cyclic stretching, were controlled by a commercially available apparatus (supporting life inside the device). Various different read-outs, including on-chip fluorescent staining and offline gene analysis, were used to assess enteric viral infections in a single-OoC format.
Multi-organ OoC systems
A first multiple-organ system on a chip was designed to mimic systemic distribution of, and toxicity response to, the compound naphthalene106. The device contained a combination of four compartments representing the lung, liver, fat and other tissues. The dimensions of the tissue compartments and microchannels connecting them were specifically designed to mimic systemic blood flow distribution and residence times in the respective organs; after passing through the lung, fluid flow was volumetrically distributed to the fat (9%), liver (25%) and other tissue (66%) compartments. The study was focused on mimicking the toxicity of naphthalene metabolites, generated by the liver, on lung cells. The different compartments, therefore, had essential interconnections in the form of microchannels that allowed the distribution and metabolism of these compounds (conceptualization and design). The device was assembled from etched silicon and machined PMMA, which yielded very well-defined channels. These materials allowed for optical inspection of the compartments on the chip (material selection and fabrication). The lung and the liver compartments contained rat cell lines, whereas the fat and other tissue compartments remained cell-free, thereby not participating in the biological response but serving to better emulate the proportion of drugs distributed to the biologically active liver and lung compartments (selection of biological elements). Peristaltic pumps were used to recirculate media through the different organ compartments (supporting life inside the device). The distribution, metabolism and toxicology of compounds could be studied with this device. The introduction of a flow component into conventionally static cell culture models allowed the study of inter-organ interactions, which would not have been possible in a non-OoC format. However, extrapolation from this miniaturized rat-based system to humans remained difficult. Today, it has become possible to use human organoids or spheroids, or even simpler cell lines of human origin, in this type of experiment to eliminate the need to take species differences into account.
A different approach for a multi-OoC for drug metabolism was developed later, aimed at developing a physiologically relevant multi-organ system for drug discovery107 (Fig. 3B). This system included four, seven or ten organ models, which were coupled with integrated fluidic circuitry to study organ interactions (conceptualization and design). This integrated device was fabricated with easier to process polysulfone and polyurethane, with integrated air pressure-controlled valves as built-in pumps to recirculate media in a more controllable fashion (material selection and fabrication). The organs modelled were of human origin (primary cells, from cell lines and iPSC-derived cells) and included the kidney, muscle, liver and gut, amongst others (selection of biological elements). The pumping system was incorporated into the final device, requiring only external air pressure to regulate the valves by software (supporting life inside the device). The drug diclofenac was administered to the gut section of this system, where it was absorbed and, subsequently, distributed among the other organ models. The complexity that this system allowed was necessary to answer a more complex scientific question, but was met with an increase in the complexity of the peripheral system, with various pieces of supporting equipment required (including a 36-channel pressure/vacuum controller). This added complexity leads to additional costs for setting up the system, and a large laboratory footprint for a miniaturized system.
Another, different, approach for a multi-OoC was reported for the efficacy and toxicity testing of various anticancer agents108 (Fig. 3C). This system contained a reconfigurable design with multiple organs, including solid tumour-derived cells and leukaemia to study effects of the anticancer agents on their target as well as toxic effects on other organs (conceptualization and design). The system was composed of PDMS, glass and plastics, and so was easily fabricated (material selection and fabrication). Cancer cell lines modelling various malignant conditions were cultured in the OoC, similar to other systems (selection of biological elements). The device was pumpless, and hence more user-friendly as it required fewer supporting machines (supporting life inside the device). The entire device was placed on a rocking shaker for gravity-induced recirculating flows, thereby greatly reducing its laboratory footprint. A system such as this is more suited for biological applications, with less emphasis on the exact manipulations of individual microchannels in the device.
In general, the use of single-OoCs has two phases. In the first — preparatory — phase, biological elements such as cells or organoids are introduced in the microchannels of the device. These living elements need time in order to settle properly in the device. They may need time to proliferate to confluency in the microchannels (such as in the case of a barrier model), or for cell differentiation into the cell types that occur in a particular organ in vivo. In the second — experimentation — phase, the device has matured enough in terms of physiological relevance and functionality to be used in experimentation. Multi-OoC systems have the same two operational phases. The different single-OoC compartments are usually cultured separately in the first phase. When each of the single-OoCs has reached the required level of maturation (which is dictated by the desired functionality), the single-OoCs can be combined into an interconnected multi-OoC with a common cell culture medium. After combining all of the required elements in one multi-OoC device in a sustainable fashion, the multi-OoC system may be used for experimentation.
Results
OoC systems are complex and entail significant effort, often with long experimental times (up to 28 days). Being able to collect maximal information is important and requires a combination of offline and in situ analysis (Fig. 4). When evaluating read-outs, it is important to consider the timescale of measurement versus the physiological timescale (which can be seconds to days), the required spatial resolution on a system level and organ level, and whether the measurement is quantitative or qualitative. Measurements that are on the same timescale as the physiological response are critical. For instance, a change in cardiac conduction velocity can be measured in seconds, whereas changes in cell viability, if they occur at all, may take many hours. For example, resolving the propagation of action potentials in neural networks not only requires sub-millisecond recording but also a dense array of sensing points. Ideally, the read-outs can be related to a relevant biological or clinical end point. In vitro to in vivo extrapolation is key to understanding the mechanism of action of a drug or chemical on the body. Also, multi-organ systems monitored simultaneously with various organ-specific and local read-outs can lead directly to mechanistic insights of the whole-body response and organ resolution. In particular for drug development, it allows the measurement of not only the response of the target organ (the efficacy) but also side effects on other organs (the toxicity)35.
A | In situ measurements during operation of a recirculating organ-on-a-chip (OoC) system allow for near real-time assessment of cell states and functionality: integrated electrodes and cantilevers in OoC devices can be configured to measure various electromechanical signals indicative of transepithelial electrical resistance (TEER)127, electrophysiological signals using microelectrode arrays (MEAs)133 and cell/tissue contractility using cantilevers327 (part Aa); integrated biochemical sensors in the OoC device can provide online measurement of various soluble analytes, such as secreted proteins via immunosensors328 and dissolved oxygen329 (part Ab); sampling of recirculating medium can be used for offline measurement of soluble biomarkers using assay kits or liquid chromatography/mass spectrometry (LC/MS) (part Ac); and device can be coupled to an optical set-up for live-cell imaging to track cell functions such as migration330 (part Ad). B | End point measurements are often used to interrogate cell states at transcriptome and protein levels: expression of specific biomarkers can be imaged by immunohistochemical and/or cytochemical techniques (part Ba); and cells or tissues in the OoC device can be lysed and further processed for transcriptional and protein expression analysis (part Bb). NGS, next-generation sequencing. Part Aa adapted with permission from ref.127, RSC, ref.133, Elsevier, and ref.327, Elsevier. Part Ad adapted with permission from ref.330, PNAS.
Offline, online and in situ analysis
To profile OoC homeostasis and response to externally applied chemical or physical stimuli, the intermittent measurement of multiple compounds simultaneously is highly desirable. Offline high-performance liquid chromatography/mass spectrometry (HPLC/MS) or multiplexed (bead-based) protein-binding/DNA-binding assays often remain the most practical techniques in this regard, as both allow measurement of a wide range of different chemicals at the same time, of which many are responsive biomarkers. Both offline approaches can be performed in microlitre volumes of sample, which are compatible with the flow rates commonly used in one-pass perfusion, or removed periodically from recirculating medium circuits. Various commercial assay kits can be used as an alternative for selected biomarkers (such as albumin, lactate dehydrogenase, alanine aminotransferase, aspartate aminotransferase and urea, for liver systems), although possible interference due to medium components (particularly serum) must be carefully considered. In very low-volume systems, the number of measurements that can be made with such kits is quite limited, although new microarray techniques allow use of very low volumes (<5 µl) and parallelization of measurements (cell health assays).
Online or in situ measurements of the supernatant can offer measurements of nutrients and metabolites with minimal time delays. Small sensors are typically used, based on optical detection exploiting dyes109. In situ measurement of dissolved oxygen in OoCs has been reviewed in detail110,111,112. Dissolved oxygen is a key parameter in metabolism, especially in terms of CYP450 activity in the liver113,114,115. Dissolved oxygen and pH can both be measured using microprobes or pH-sensitive patches9. Optical detection requires the use of microscopy or optical fibres; automated techniques to reduce time and labour have been suggested116,117. Some analytes such as glucose and lactate can be measured by using electrochemical enzyme-based biosensors41,118. These online, near continuous measurements are desirable as periodic measurements may miss important fluctuations in response. If sensors or patches are integrated as arrays at close distance or in direct contact with tissues structures, analyte concentrations can be spatially resolved.
Measuring cell phenotype and function
Although biomarkers provide important biological insights into the body’s response to drugs, cosmetics, food ingredients and environmental exposure to chemicals, it is often the functional tissue response that may be the most informative. Ideally, the functional measurements are non-destructive and readily adapted to in situ analysis, particularly in systems with continuous recirculation of medium over extended periods (such as 28 days). These measurements should also provide rapid insights into responses — often within minutes — in order to achieve relatively high temporal resolution with respect to the timescale of the functions being monitored.
End point analyses
Although highly informative when it comes to cell status, gene and protein analyses in OoCs are difficult to do online, and may require that the device be dismantled to perform end point histology, lytic assays, transcriptomics and even cell viability assays119,120. A chip design concept that allows easy removal of tissues with no detrimental effect on tissue structure and morphology becomes essential in order to obtain representative results as well as maintain spatial organization of the cells in the tissue. Analysis of circulating cells such as immune cells and tumour cells can be done using medium samples taken from OoC devices121. However, many microfluidic devices can destroy circulating cells during experiments, so it is essential that the fluid dynamics be considered during device design. In other cases, cells have to be removed from the tissues in the device. Techniques to remove cells without damage do exist, but can be difficult to implement, requiring expertise to perform. For effective quantification, removal of all of the cells may be important so as not to bias the results.
Microscopy and high-content imaging
Microscopy and high-content imaging are among the most widespread analytical methods in cell biology, and thus also the most common measurement of cell and tissue function is visual inspection in OoC devices. Most OoC devices are constructed from optically transparent materials, such as glass, PDMS and thermoplastics, and can be constructed to fall within the imaging depth of conventional epifluorescence or confocal microscopes. It is thus possible to perform in situ staining of the cells and tissues in-chip with fluorescent stains or antibodies to assess cell viability or expression of specific biomarkers, by using the microfluidic function of the OoC to deliver the necessary reagents122.
In recent years, microscopy techniques including confocal microscopy and light-sheet microscopy, as well as imaging sample preparation protocols (clearing, staining and so on) and analysis, have been significantly advanced and adapted to deal with complex, 3D multicellular constructs. High-content imaging has the strength to provide both spatial and temporal information of cells and tissue morphology to help researchers untangle disease pathophysiology and assess novel therapies more effectively. Microscopy in OoC systems requires an optical window to the cells, along with a design specification that allows placement and alignment of the OoC on a microscope stage. Successful microscopy requires optimized matching of the tissue model, preparation protocols (fixing, staining, clearing) and microscopy method with the protocol for image and data analysis123. Machine learning algorithms can serve as a powerful tool to aid image and data analysis. An example would be the use of deep learning convolutional neural networks to track invasiveness of tumour spheroids124.
Transepithelial electrical resistance
Transepithelial electrical resistance (TEER) is a commonly used technique to measure the integrity and permeability of any barrier tissue (such as the gastrointestinal tract, the kidney and the BBB). Native barrier tissues in the body have TEER values associated with their function. The tightest barrier is the BBB with values of 1,500–8,000 Ω.cm2 (ref.125), whereas the proximal tube in the kidney has a value of about 70 Ω.cm2 (ref.126). Organ and tissue models should show physiologically accurate values. In some cases, tissue models can give values far greater than physiological values, whereas others fall short of realistic values. The completeness of the model is a determining factor in these situations, especially where the physiological system consists of several cell types and the model uses only a subset of these.
The measurement of TEER values in microscale systems can be done using the Ohm’s law method or impedance spectroscopy, using either microelectrodes integrated into the device during its fabrication127,128,129 or inserted into the device before an experiment129,130. Impedance spectroscopy, when combined with a fitting algorithm, provides a more accurate representation of TEER values than the Ohm’s law method, whereas the Ohm’s law method is easier to apply. Impedance spectroscopy further allows measurement of tissue size and integrity131. For both types of measurement, the electrodes must be placed accurately, and the surface area used in the calculation must be appropriate as a uniform current density must be generated throughout the entire barrier tissue. Applications of TEER to OoC devices have been reviewed previously128,132,133,134.
Microelectrode arrays and cantilevers
Other functional measurements include microelectrode arrays (MEAs) to measure electrical activity in cells and tissues, and cantilevers to measure force generation by cells133,135. The MEA system is much more suitable for application to OoCs than the patch clamp. This method typically relies on a 2D array of cells that allows, using the heart as an example, estimates of conduction velocity, beat frequency and field potential duration as an analogue for the QT interval136. MEAs can be applied to the heart, neuromuscular junctions, skeletal muscle contractions and neuronal systems137,138,139,140. For instance, action potential propagation between presynaptic and postsynaptic chambers connected by microtunnels has been described141. Mechanical forces such as contractile force generation by cells and/or tissues is also critical to many organ functions. They can be measured non-destructively and in near real time by integrating microcantilevers in OoC devices, in which cells are patterned onto silicon cantilevers with optical or electronical detection of their deflection142,143. Meanwhile, a recent study showed that both MEAs and electrodes for TEER measurements can be integrated into chips during their fabrication to achieve multiple functional measurement in a TEER–MEA heart chip144.
The choice of read-outs will depend heavily on the issues to be addressed. Generally speaking, the advantage of most OoC in vitro systems is that they are easier to probe directly than animal models are, irrespective of the analytical approach chosen. OoCs lend themselves to microscopic imaging and small-volume sampling for both offline and online analysis. There has been a remarkable upsurge recently in the availability of small sensors for real-time probing of biological systems. Their application to real-time in situ monitoring of incubation conditions, biomarkers and organ function in OoC has obvious advantages not only for the development of improved OoC but also for the range of experiments that become possible with OoC systems.
Applications
The majority of OoCs are developed with an intended application in mind. In this section, we highlight the various advantageous features of current OoCs for use by commercial organizations interested in testing and predicting compound efficacy and side effects, and biological researchers looking to use OoCs to mimic the complexity of normal or diseased states in humans.
Most OoC prototypes at the pre-commercialization developmental stage generally cannot fulfil the dual purposes of multiplexing and biological complexity simultaneously. As an in vitro system, OoCs also cannot comprehensively capture the entire physiology of an organ or body. Hence, the final commercialized form factor of an OoC system is often informed by the tissue functions and read-outs that are essential for the intended application (Fig. 5). Consequently, one should discern between the necessary and desirable features of an OoC device at different stages of its translational process. This should ideally be done in consultation with the intended end users.
Complexity and form factor of OoCs are dependent on its intended application as illustrated by the liver OoC. Different applications of liver OoC may require varying complexity in culture configuration from hepatocyte monocultures to co-culture with other hepatic cells that better mimic liver physiology. Aa–e | Toxicology testing requires multiplexed arrays for increased throughput. Ba–b | Liver OoCs that mimic liver-bioactivation of drugs or nutraceuticals involved co-culturing liver with the tissue targeted by the drug. Ca–d | Liver disease models often require increased biological complexity with both parenchymal and non-parenchymal cells to recapitulate disease mechanisms. Da–c | Drug pharmacodynamics (PD) and pharmacokinetics (PK) models will include the liver OoC as the metabolizing organ alongside other organs involved in drug absorption, distribution and excretion connected in a recirculating circuit. EBs, embryoid bodies; NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis; PEARL, perfusion array liver system. Part Aa adapted from ref.331, CC BY 3.0 (https://creativecommons.org/licenses/by/3.0/). Part Ab adapted with permission from ref.332, Elsevier. Part Ac adapted from ref.333, CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/). Part Ad adapted with permission from ref.334, RSC. Part Ae ©Emulate. Part Ba adapted with permission from ref.77, RSC. Part Bb adapted from ref.335, CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/). Part Ca courtesy of InSphero AG, Schlieren. Part Cb adapted with permission from ref.336, Wiley. Part Cc “PhysioMimix™ Liver-on-a-Chip (MPS-LC12) consumable plate”. Part Cd adapted from ref.303, CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/). Part Da adapted from ref.337, Springer Nature Limited. Part Db adapted from ref.107, CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/).
Commercial compound testing
Pharmaceutical and chemical compounds
Commercial use of OoC systems has been focused primarily on drug development36,145,146,147. The ability to estimate both efficacy and toxicity for humans in preclinical trials is a huge advantage in allowing a company to choose candidates that have a higher chance of becoming approved drugs. OoC systems can be used throughout preclinical and clinical trials, and coupling OoCs with physiologically based pharmacokinetic (PK) or pharmacodynamic (PD) models offers a rational basis to guide this process37.
The appropriate OoC system changes from relatively simple to increasingly complex due to the different goals of each stage of drug development. Typically, simpler and more highly focused systems that provide relatively high throughput are needed in mid-preclinical trials, whereas multi-organ human-based models are needed towards the end of preclinical trials to predict both efficacy and toxicity. The complexity is not only in the device itself and integrated analytics but also in the complexity of the biological organ module. The majority of OoC companies focus on single-organ modules, although some provide multi-organ systems. Human multi-organ models alone or when coupled with a mathematical model37,148,149,150 have the potential to provide both efficacy and toxicity information on human response in preclinical studies108.
There are numerous issues in terms of scaling data of a multi-organ OoC system to predict human response, including direct scaling, residence time-based scaling, allometric scaling and multifunctional scaling140,151,152,153. Each of these approaches has advantages, although residence-time approaches are the simplest to use. The residence time, defined as the volume of the reactor divided by the total flow rate, is easily applied and controls the degree of conversion of a reactant and formation of metabolites independent of reactor size (which can range from a few microlitres up to tens of thousands of litres). A residence-time approach should be effective if the microphysiological system uses organ chambers sized to be in correct physiological ratios with an appropriate ratio of flow of a blood surrogate to each organ.
Biomaterial testing
Many medical treatment modalities rely on the use of biomaterials, including surgical and medical devices (such as catheters, surgical plates, screws or extracorporeal systems), implants and artificial tissue replacements. OoC platforms can be used to study the biocompatibility and efficacy of biomaterials in an environment that is representative of the (patho)physiology of the organ or tissue to be replaced, repaired or regenerated. An important consideration is the integration of various biomaterials into the microfluidic OoC systems for fast and parallelized biocompatibility or biofunctionality testing154,155. Numerous different solutions for facile and versatile integration of biomaterials of various geometries156,157 or bonding strategies have been reported158. Biomaterial samples that have to be integrated into OoC systems require miniaturization, which may be challenging for some material types. Moreover, it is important to prove that the effects observed with miniaturized samples are representative of the situation in the body.
The ability of OoC systems to achieve precise control of environmental factors are of great use for understanding structural–functional relationships of a biomaterial. By controlling the presentation of various cell microenvironmental cues in the OoC, one can conduct a systematic study of how these environmental factors interact with specific biomaterial properties, such as roughness159,160, stiffness161,162, wettability163 and topography164,165,166,167, which in turn regulate cells’ behaviours, such as adhesion, proliferation and differentiation on the biomaterial.
Biological research
Disease modelling
The use of OoC systems for disease modelling enables the investigation of both inherited and acquired disease in a human setting, overcoming inter-species differences that hamper data extrapolation. Specifically, many of the genetic networks implicated in human disease differ substantially between human and murine models, the most common animal model deployed, which greatly limits their utility for disease modelling168. Similarly, the immune systems of mice and humans show significant differences in both innate and adaptive immunity, limiting their use in evaluating immune-related diseases such as multiple sclerosis169. OoC systems provide a human-specific experimental platform to mechanistically study human diseases with complex aetiologies because they can integrate genetic factors (through use of patient-derived or genetically engineered stem cells harbouring the disease-driving genetic mutations or predispositions), environmental factors (such as exposure to drugs or mechanical stresses170 through precise control over the cell microenvironment) and systemic crosstalk with other organ systems, including immune cells and the microbiome (through engineering multi-OoCs with recirculatory flows). There are already some demonstrated examples where OoC systems have been able to demonstrate the importance of interplay between different aetiological factors in the manifestation of clinically relevant disease phenotypes. For example, engineered heart tissues made from diseased cells harbouring a desmoplakin mutation only show clinical arrhythmogenic cardiomyopathy when exposed to dynamic mechanical loading171. Another example demonstrated that the inclusion of regulatory T cells and T helper 17 immune cells and environmental agents (short-chain fatty acids) into a gut–liver multi-OoC could successfully recapitulate paradoxical modulation of inflammatory bowel disease that was dependent on activated CD4+ T cells172.
The development of disease models is of increasing importance for the clinical development of efficacious therapeutics and provides a model for rare diseases where clinical development and trials may be limited by small patient cohorts. Additionally, OoC platforms can enable studies of systemic disease in the human setting for diseases that currently have minimal other models available, including cancer metastasis, inflammation, fibrosis and ageing. It is expected that the inherent complexity of systemic diseases can be mechanistically evaluated to a greater extent as multi-OoC technology continues to advance. To fully attain this, OoC platforms should be developed in a way that enables spatio-temporal tracking of cell and tissue states, modularity to enable multi-tissue studies for systemic diseases, inclusion of functional stromal cell populations that drive disease progression (such as immune cells, fibroblasts and vasculature) and validated disease cell sources. Benchmarking of diseased cells against healthy cells in the same system should serve as a control to interpret the differences in the diseased phenotype from those related to the in vitro setting. Biomarkers of clinical relevance should similarly be evaluated within the OoC platforms, enabling a more direct comparison with the clinical phenotype for enhanced benchmarking of platform utility.
Probing and mimicking the cell microenvironment
Microenvironmental control of cells is a well-established concept in cell biology. OoC systems have been used extensively to manipulate different environmental factors (such as shear stress, autocrine/paracrine soluble factors, cell–cell and cell–ECM interactions) at physiologically relevant length and timescales so as to elucidate their effects on cell phenotypes and functions. This idea is particularly well demonstrated in the context of the stem cell and tumour microenvironments, as evidenced by the number of reviews on these topics173,174,175,176. We cover some of the common design strategies that are used in OoC devices to control and probe the functional roles of biochemical and mechanical environmental factors.
Soluble biochemical factors, such as morphogens and cytokines, can be controlled in OoC systems by manipulating fluid mass transport properties at microscale resolution, and therefore OoC systems offer a more precise method to study the effects of paracrine and autocrine signalling compared with conventional techniques, such as conditioned medium or changing cell density177. By selectively changing channel dimensions and flow velocity, one can tune between diffusion-dominated (Pe < 1) transport, where secreted factors remain in the cell vicinity for binding to receptors, and convection-dominated transport, where secreted factors are removed from the cell vicinity177. Soluble factors can also be applied exogenously to microfluidic cell cultures with spatio-temporal control over the concentrations and compositions that cells are exposed to. This is often accomplished by coupling a convection-based concentration gradient generator178,179 or combinatorial mixer180,181 to the cell culture chambers. Alternatively, static diffusion-based gradients can be applied to shear-sensitive cell types without exposing them to direct flow. This can be achieved by sandwiching a porous matrix between the source and sink reservoirs182 or balancing the pressures between multiple channels that feed into a static chamber where concentration gradients are being established178,179,183.
Devices that are designed to study cell–cell interactions often attempt to control the spatial localization and relative abundance of different cell types using cell patterning184,185,186 or geometrical microstructures (such as microwells) to physically constrain the cells187,188. This allows for better control over the number of interacting partners as well as the spatio-temporal dynamics of the interaction process compared with random co-cultures. Control over cell–cell and cell–ECM interactions becomes coupled when using cell patterning techniques to modulate the number of neighbouring cells because this is reliant on patterning adhesive ECM proteins on the substrates to which the cells then attach. Proper experimental designs to vary cell–cell interactions while maintaining cell–ECM interactions to be consistent, such as uniform coating with a well-defined ECM protein such as fibronectin, can help circumvent this problem. OoC devices for probing cell–ECM interactions aim to modulate either the biochemical composition of the ECM proteins being patterned onto the substrate189 or the substrate’s mechanical properties, such as stiffness or topography. The latter usually involves replacing glass with other materials — such as PDMS or polyacrylamide hydrogel — as the cell culture substrates in the OoC device, whereby the stiffness190,191 or surface topographies192,193 of the substrates can be precisely controlled.
OoC devices are well suited for applying a range of physical environmental stimuli on cells. As fluid flow is inherent in many OoC systems, these systems are used to mimic physiological shear stresses exerted by blood and interstitial fluids. By designing multiplexed channels with varying geometries, one can simultaneously apply shear stress over a range of magnitudes to probe the effects of shear stress on cell proliferation, differentiation and functions119,194,195. Alternatively, fluid shear is used to enhance the functional maturity of microvasculatures in many organotypic tissues. OoC devices can also be designed to apply mechanical tensile or compressive forces to cells for mimicking breathing-induced stretching of the airway, peristalsis-like motions of the gut or contractility of cardiac tissues196. This is often achieved by growing cells on thin flexible PDMS membranes that are incorporated into the OoC device such that they can be flexed cyclically by vacuum or pressure pneumatic actuators. To better recapitulate the electrophysiological functions of neural and cardiac tissues, electrical stimulation has been built into OoC devices by integrating in situ MEAs that are microfabricated as part of the device197 or electrodes that are inserted into the OoC system196.
Single-organ tissue functions
Liver
The cell source, composition and culture configuration of the liver OoC dictates the extent of liver-specific functions that the device can recapitulate198,199,200. Key liver-specific functions that should be present in in vitro liver models include expression of phase I or II metabolizing enzymes (such as CYP450, UGT and GST), albumin synthesis and biliary excretory functions201,202. Ideally, the synthetic and metabolic functions of liver OoCs — characterized by albumin secretion levels and CYP450 activities, respectively — should be benchmarked to that of freshly thawed cryopreserved primary human hepatocytes over different culture periods. This provides a clear indication of the application utility of the liver OoC system (together with the selected choice of cell source such as iPSC-derived hepatocytes) as cryopreserved primary human hepatocytes are currently the gold standard in drug metabolism studies203. Hepatocyte monocultures in a conducive configuration, such as 3D spheroid and sandwich cultures, can maintain synthetic and metabolic functions for a short period of time (3–7 days) and, to some extent, exhibit hepatocyte repolarization and biliary excretory functions204,205,206,207,208. Co-cultures with non-parenchymal liver cells such as liver sinusoidal endothelial cells, Kupffer cells and hepatic stellate cells are essential for longer-term (~14 days) maintenance of metabolic and synthetic functions and enhanced biliary excretion72,209,210,211,212. These multi-culture liver OoCs can more comprehensively capture the full repertoire of liver-specific functions important for more accurate prediction of human responses in both pharmaceutical preclinical testing and liver disease modelling. Kupffer and stellate cells aid in modelling non-alcoholic fatty liver disease and liver cancer because these diseases often involve inflammation and fibrosis processes mediated by liver-specific macrophages (Kupffer cells) and pericytes (stellate cells)212,213.
Heart
Cardiac OoCs should ideally facilitate the maturation of cardiomyocytes and their coupling with supporting cells. The two functions to aim for are the robust and synchronous contractile function and electrical activity associated with a positive force–frequency relationship and fast calcium handling. These functions are evaluated using a set of key read-outs that include the beat rate, force of contraction, excitation threshold, maximum capture rate and conduction velocity. To achieve electromechanical maturity, heart tissues need to display adult-like gene expression profiles, ultrastructure (orderly registers of sarcomeres, high density of mitochondria, networks of transverse tubules) and oxidative metabolism.
The choice of cell source, culture configuration and environmental stimulus all have a profound impact on recapitulating a functional cardiac tissue. For a long time, immature phenotypes of human heart muscle derived from iPSCs have limited their utility for biological and medical research. Human heart muscle has been grown from iPSC-derived cardiomyocytes and fibroblasts in hydrogel anchored at the two ends that also allowed the measurement of the contraction force through the deflection of PDMS posts or cantilevers. Electrical stimulation applied with gradually increasing intensity, causing macroscopic contractions of the forming muscle, has markedly advanced tissue maturation214. Other studies have shown that the atrial versus ventricular specification of engineered cardiac tissues could be achieved by gradually increasing the stimulation frequency215. Both tissue models were used to investigate genetic and acquired cardiac diseases.
BBB and nervous tissues
The BBB is an important structure that maintains brain homeostasis and protects the brain from exposure to molecules or pathogens that are circulating in the bloodstream. Drugs for brain diseases need to cross this barrier to arrive at the site of action, whereas other drugs should not cross the BBB58,216. BBB OoCs need to recapitulate the function of this specialized barrier tissue by recreating the compartmentalization of multiple cell types. BBB OoCs often consist of two compartments separated by a thin membrane, with the vasculature compartment lined with brain endothelial cells, whereas the brain compartment is formed with pericytes and astrocytes57,217,218,219. TEER values across BBB OoCs are typically obtained as a measure of barrier integrity. BBB OoCs should be engineered to have TEER values that fall within the appropriate range (~1,500–8,000 Ω.cm2) for it to have physiological value in functional drug studies or disease modelling. Performing studies across longer time frames requires culturing the BBB OoCs in hypoxic conditions to maintain BBB integrity for longer periods of time (up to a week). In comparison, BBB OoC cultures in normoxia lost barrier integrity after 2 days220.
Spinal cord OoCs recreate the blood–spinal cord barrier similar to BBB chips. Although spinal cord OoCs may share a similar compartmentalized design as BBB OoCs due to the similar roles of the blood–spinal cord barrier and BBB, key biological differences prevent interchangeable use221. Rather than using pericytes and astrocytes in the brain compartment, spinal cord OoCs incorporate cells that can differentiate into spinal motor neurons, such as iPSC-derived spinal neural progenitor cells25.
Applications involving neurotoxicity testing or modelling human brain diseases will require OoC systems that not only maintain the survival of neurons and glial cells but also their electrophysiological functions. Electrophysiological functions from neurons can typically be visualized through calcium fluxes, whereas the presence of glial cells can be checked by screening for their respective neural markers. Major central nervous system glial cell types include astrocytes and microglia, which can be identified by screening for GFAP and CD45, respectively. Myelin-producing cell types include oligodendrocytes in the central nervous system and Schwann cells in the peripheral nervous system, both of which can be characterized by screening for myelin basic protein222. Recapitulation of fully functional neural networks in vitro from isolated cells is extremely challenging due to the complex cellular organization of brain tissues, and so OoC systems increasingly look to integrate organotypic models such as brain organoids or organotypic brain slices223,224,225 instead of single cells226,227,228. Peripheral nervous system OoCs or nerve OoCs mostly focus on strategies and culture configurations that promote motor neuron regeneration as indicated by neurite growth and myelination by Schwann cells229, which can then be applied to see how drugs230 or pathogens231 modulate these processes.
Epithelium
Epithelium forms a protective layer on the upper respiratory tract, skin and the corneal layer of the eye, while also serving to facilitate the exchange of substances in the gut and lung. Most epithelium OoC systems for the respiratory tract10,232,233,234,235,236, skin73,237,238,239, gut14,55,240,241 and cornea242,243,244 have a multi-compartment design where two distinct fluidic channels are separated by a porous membrane or hydrogel. Epithelial cells from the respective organs are then used to seed one side of the porous membrane to mimic the epithelial layer. For airway and skin OoCs, the epithelium is cultured under an air–liquid interface to mimic in vivo conditions10,245. Epithelial barrier functions can be characterized in multiple ways, the first of which is to screen for tight junction markers such as claudin and occludin, which are ubiquitous in all epithelia246. Another way is to characterize the TEER across the epithelium barrier, similar to that of BBB OoCs. Compared with BBB OoCs, in vivo TEER reference values are not currently very well characterized for epithelial tissues. In vitro TEER values, on the other hand, have been obtained for lung-on-chip (500–900 Ω.cm2)10,247,248, gut-on-chip (400–4,000 Ω.cm2)14,249,250, skin-on-chip (1,000–6,000 Ω.cm2)239,251,252 and cornea-on-chip (500–1,000 Ω.cm2)242,244,253. The large variation in TEER values for gut-on-chip and skin-on-chip could be due to variations in the cell source and microenvironmental culture conditions used in the different OoC models. Epithelium of the lung, gut and cornea are constantly exposed to mechanical forces within the body, namely cyclic stretching during lung respiration and gut peristalsis, along with blinking-generated shear stresses in the cornea. Exposure to these mechanical forces has been shown to greatly improve epithelium maturity of on-chip skin237,239, gut14,241 and cornea242. Other functional characteristics of the epithelium include mucus secretion by goblet cells232,233,234,235 and the presence of specialized protrusion structures on the epithelium surfaces, namely beating cilia on the upper airway epithelium that sweep mucous-coated foreign particles out of the airway232,235, and villus crypt-like structures on the gut epithelium to increase the surface area for nutrient absorption14,55,241. In the case of the skin, multiple layers of epithelium stack above one another to produce a stratified layer that contributes to the protective properties of skin254.
Multi-organ functions in ADME-Tox
Predicting human ADME-Tox in preclinical trials using an OoC system (most likely with physiologically based PK models) allows measurement of the response of the target organ (the efficacy) as well as any side effects on other organs (the toxicity)35,255. Many organ functions need to be considered for drug ADME-Tox applications. Absorption of drugs can occur not only through the gut but also the skin, lungs and direct injection, and all of these have been modelled with multi-organ OoC systems. Adipose tissue is particularly important for distribution of lipophilic compounds256 and is, unfortunately, neglected in many multi-organ microphysiological models. For a multi-OoC model to mimic distribution of any chemical or drug, a compartment for other tissues is necessary to capture the retention of any compound in tissues not specifically included in the model; no OoC system can explicitly capture every organ or tissue type in the body257. Further adsorption/absorption occurs through internal barriers such as the BBB and these are critical to consider. The liver is essential as it is the primary site for metabolism, although the gut is also important as it houses many of the CYP450 enzymes and a gut–liver model is particularly important for orally ingested drugs and chemicals258. Although elimination occurs through several routes, the kidney is particularly important and also a challenge to fully model; many kidney OoC models focus on the proximal tube, but neglect other aspects of kidney operation259,260. Toxicological side effects can occur in many tissues although toxicity in the liver, heart, kidney, bone marrow and immune systems are the most common. Thus, a multi-organ OoC system that can accurately capture multiple aspects of ADME-Tox is quite demanding. Many multi-organ OoC systems focus on some selected aspects of ADME-Tox using only 3 or 4 organs, although it is possible to construct and operate a multi-organ OoC system with up to 14 organ compartments261.
Reproducibility and data deposition
Development and deployment of OoCs require the coordination of various multidisciplinary elements, such as cells, biomaterials, engineering controls, interconnects and sensors. Reproducibility of OoC systems largely depends on the correct matching and interplay of the different elements as well as their isolated reproducibility. The more variability single elements present, the more the error propagates, and the more difficult consistent outcomes become. In general, modularity and the possibility of individual and timed quality control of the single elements greatly increases system yield and directly dependent reproducibility of experiments using OoC systems. Understanding robustness and producing data of statistical relevance additionally require a minimal number of replicates per conditions. Increasing physiological complexity needs consideration of operational complexity and potential limited scalability.
Sources of variability
A major challenge for reproducibility consists of minimizing system or background variability to maximally resolve the systems’ responses to stimuli and test parameters, such as compounds or mechanical cues. The manufacturing and assembly of microfluidic chips and components need to adhere to operation processes — in which checklists are used to verify dimensions and device characteristics, and their compliance with predefined specifications — rather than relying on manual fabrication processes. PDMS replica moulding used for prototyping provides great design flexibility and short iteration times to explore new approaches and is central in the early stage of the product life cycle. The overall manual process and assembly tends to have a high user dependency and laboratory dependency, making reproducibility more challenging. Industrial processes such as injection moulding are highly standardized, thus enabling reproducible mass fabrication; however, they require a substantial initial investment fixing the device design, and have some feature restrictions (minimal dimensions, limited aspect ratio, de-moulding draft). It is thus important that developers keep these requirements and limitations of industrial manufacturing processes in mind to ensure transferability once design features of prototypes are set and commercialization becomes an option262. Variability increases with the number of components of a system. Process monitoring sensors and their feedback to flow, temperature and gas concentration control ensure stability in long-term experiments263.
The central — and also most variable — components are the cells and tissues and their extracellular natural or synthetic matrix or scaffold. Cell line sourcing, culturing and expansion can be well controlled and has been thoroughly characterized over the years, but the physiological relevance of cell lines is limited in OoC systems and they show genetic instability2. Primary cells closely resemble their tissue of origin. Optimized culturing protocols and the embedding of primary cells in a near in vivo environment as, for example, stroma, ECM and so on have resulted in increased lifespan of the cells and preserved tissue-specific function and phenotype. Major challenges lie in ensuring continuous access and control of donor-to-donor variability and batch-to-batch variability. Adult stem cells or pluripotent stem cells can be expanded indefinitely in culture and can give rise to many cell types. Substantial effort is made to reduce line-to-line variability across different iPSC lines and ensure better maturation of differentiated derivatives. Ensuring reproducibility on the cell level, tissue level and biomaterial level require staged quality and functional control of the source material, before it is introduced into the OoC system prior to an experiment. Quality standards and procedures are currently being discussed and need to be defined in a tissue-specific manner.
Technical issues such as bubbles and contamination are a major challenge to reproducibility in device set-up and operation. System assembly and initialization need to follow defined protocols with minimal user dependency. Transferring the majority of processes to robotic systems is preferred. Predefined kits including all materials and cells or assay-ready products that are shipped ready to use are options for simplifying or even eliminating OoC device assembly and preparation prior to the actual experiment for the end user.
Standardization
Standardization is a central tool to ensure reproducibility in an OoC system’s internal interfaces (cell–surface interactions and matrix composition), as well as its external interfaces. These external interfaces include, but are not limited to, external instruments such as liquid handling, plate automation, operation systems and read-out instruments including microscopy and analytical instruments; interfaces to other OoC devices to create higher-order multi-tissue systems by their connection though tubing or liquid ports; comparability on the functional level and experimental outcome of different organ-on-a-chip systems representing the same organ type (between systems, between laboratories, over time and with respect to historical data); data outcome structure, format and conditions as inputs to in silico models; and data format and content allowing translation to clinical and animal data. Defining tissue-specific functional parameters with unified normalization and commonly agreed reference compounds is a current project for different overarching organizations (for example, ECVAM264,265,266, IQ-MPS, NA3Rs).
Standardization has to be implemented on different levels. Cell-based and technical standards will help improve compatibility between instruments and ensure reliable operation and controlled supply chains. Organ type-based standards, such as defined functional parameters, minimal requirements on viability and lifetime or standardized quality criteria, allow better selection and compatibility between different providers as well as better fitting the purpose. Finally, the system’s quality and performance need to comply with application-based standards, including for example a correct prediction of liver toxicity of a set of reference compounds or true replication of a mechanism of action of a substance.
Data and validation
The wide range of available OoC devices and approaches requires the production and accessibility of reference compound and clinical data for verifying and validating findings. The public availability of this type of data will enable comparison of different OoC devices in order to select the best system for a given study, and for regulatory bodies to classify and value data originating from OoC systems. An example is the University of Pittsburgh Microphysiology Systems Database (MPS Db), which has been designed to aggregate, manage and integrate data obtained from OoC systems with human and animal exposure data. Data included in the MPS Db pertain to tissue-specific functional biomarkers, tissue morphology, transcriptomics and proteomics. Reference data for preclinical assays must be reported in assay-specific formats, such as dose–response curves including commonly used analysis parameters such as the half-maximal inhibitory concentration (IC50).
Reporting standards
There are currently no specific reporting standards that have been agreed. However, to enhance academic progress and further the development of OoCs, it would be preferred to publish experimental details as completely as possible. Deposition of experimental raw data including metadata into a central space should enable other laboratories to reproduce results. An example of information that should be shared is the reporting of cell lines, the exact medium composition for culturing, precise incubation times, local cell culture practices, the batch of serum used and passage numbers used, all of which influence cell phenotype and organ functions. Another example is reporting on the selection of the region of interest — both spatially and temporally. This becomes necessary if it is not possible to extract information from the complete device, and if the region is not representative of the entire OoC, it could yield biased results.
Some of the challenges and opportunities were outlined in a recent international consortium effort to standardize and report minimal information for experiments in 3D cell culture267. Even the most basic of information needed for reproducibility such as media type are often unreported or ambiguous. For example, the properties of a 3D cell spheroid are completely different depending on whether the same culture medium, DMEM, contains high or low glucose formulation; this detail is often missing in publications. When experimental protocols are shared and followed, variability is significantly reduced, although still not completely eliminated, even with cell line culture. Given the system complexity of OoCs, development of reporting standards is even more significant and challenging than it is for 3D cell culture. Advances in standardized commercial OoC hardware, defined media and human-relevant iPSCs enable a broader availability and are helping the community to base their research on a common ground. Based on this, there is currently a high need for developing basic and application-specific assay protocols and minimal experiment information reporting standards for OoCs.
Limitations and optimizations
OoCs are useful in vitro models bridging the gap between conventional cell cultures and animal models or human subjects. Although many organ systems and their functions can be modelled using OoC devices, the physiological relevance of OoCs is generally less validated than that of organoids and other in vitro models268. To match the existing capabilities of OoC technology with the best applications, it is important to understand some of their current limitations and challenges for the field269: OoC designs, manufacturing and operating procedures have not yet been standardized, and so end users need to invest time and resources to set up the system and customize the testing assays, which inevitably limits the experimental throughput of OoCs; data acquisition from OoC systems remains overly reliant on end point assays, which limits the spatio-temporal resolution of biological information that is important to minimize the effects of sample-to-sample variability and allow dynamic studies; and biological information extracted from OoCs needs to be validated with those of existing in vitro and animal models to determine how accurately OoCs can mimic human responses.
Although OoCs promise to simulate human physiological processes, there are engineering limitations to reaching the full complexity of human physiology. The numbers of vessels, tubes and ducts in human tissues and organs are still too complex to be fully recreated in engineered systems. Even relatively simple channel networks can be challenging to operate robustly and efficiently with throughput required to cover variabilities that arise from biological heterogeneity. To contribute to discovery-driven research and drug discovery, the high cost of OoCs typically necessitates prescreening to create a smaller pool of test conditions or candidate molecules in advance.
Many OoC system designs and operations are focused on trying to reproduce physiological tissue architectures and functions, and therefore less emphasis is placed on testing assays and read-outs. However, for OoCs to be routinely deployed for standardized testing of drugs and chemicals, such as those stipulated in OECD testing guidelines, the compatibility of the designed OoCs with common laboratory instruments for data acquisition and downstream analyses is an important practical consideration. If OoC devices can be designed to have reagent interfacing and cell imaging layouts that are similar to those of standard culture plasticware and glassware, the technology will be more approachable for biological end users as the workflow and associated hardware and software for analyses would be familiar and mostly already present in the laboratory. An example involves fitting 20 microfluidic devices in a 96-well PS plate and using a plate reader to quantify luminescence in each device270.
Manually connecting devices to pumps can be challenging and decrease instrument compatibility. Use of gravity-driven flow — which can be established by tilting a device back and forth to drive fluid between two reservoirs at different ends of the device — can eliminate the need for external tubing and reduce the risk of introducing hard to remove bubbles in the circuit219,270,271. Use of such gravity-driven flow, however, may introduce unwanted variability or bidirectional shear to the cells. An alternative strategy is to implement bespoke periphery benchtop instruments with graphical user interfaces to automate and scale up liquid handling in OoC devices89,107.
In terms of data collection, many commercial analysis tools are still difficult to adapt for OoCs. For example, commercial chopstick electrodes used for measuring TEER will not provide accurate reading of in-channel cell barriers in most cases. Some have fabricated and integrated sensors and assays that are customized for OoC systems. Multiple biosensors have been integrated to monitor multiple biochemical factors, and integration of bubble traps has allowed for long-term operation of multi-organ systems38. For monitoring real-time insulin secretion in an islet-on-chip in response to a change in glucose concentration, fluorescence anisotropy was used to measure the degree of binding between FITC-insulin and insulin antibody272. This no-wash sensing technique allowed on-chip analysis of very small volumes of samples in real time. Chip-integrated sensors with high spatio-temporal resolution are needed for monitoring the distribution of signalling molecules within the cell culture compartment. The reader is referred to other reviews for more in-depth analysis of challenges and opportunities for integrating various on-chip biosensors263,273,274,275.
It is imperative that biological results obtained from OoCs, such as drug testing responses, are systematically validated with those obtained from existing in vitro and animal models or even clinical data. This is to ensure that responses obtained from OoC experiments are due to differences in cell functionality, and not a result of experimental artefacts that can arise even with correct instrumentation and calibration of sensors. For example, a less sensitive drug dose response in an OoC may be later found to be due to adsorption of the drug to the PDMS surface48. Applying a surface coating to PDMS or using a different substrate may prevent this issue276,277. Channel design, including the dimensions, curvature and surface topography, is an important factor affecting cell responses such as cell organization, alignment and the degree of differentiation278,279. Media perfusion in OoCs not only subjects cells to shear stress but also alters effective cell to media volume ratios, which can have unintended effects on cells. For example, fluid shear stress applied to epithelial cells in the intestine-on-chip was thought to be driving the stronger differentiation of the cells and formation of 3D topology compared with static cultures; however, it was found that the continuous dilution of the Wnt antagonist DKK1 was responsible for the more pronounced formation of villi under conditions of flow. The same effect could be achieved in the absence of flow by simply having a larger basal compartment to mitigate the inhibitory effect of DKK1 on villi morphogenesis280. Thus, the opportunity and challenge of OoCs are that the same cells and reagents can give rise to different cellular responses compared with conventional cultures.
Validation studies using paradigm compounds with well-characterized mechanisms of action or responses in humans are needed to discern between real biological differences and experimental artefacts due to changes in materials or operating conditions in OoCs. The number of test compounds required for validation studies depends on the application of interest. For example, the predictive accuracy of a hepatotoxicity screening OoC model may be validated with a large number (>10–20) of positive and negative drugs281 or 2–3 pipeline compounds that are known to fail because of hepatotoxicity during preclinical trials72. A brain OoC model of Alzheimer disease can be validated with two or three known compounds that target specific cell types or signalling processes282. The OoC system should be developed to a stage where there is sufficient throughput and reproducibility to meet the needs of the required validation studies.
Outlook
OoC technology is on track to becoming widely accepted as a human-specific experimental platform for preclinical research and therapeutics testing. This shift is largely based on the results of in vitro studies using these platforms becoming increasingly predictive of clinical data for integrated human physiology. Not surprisingly, numerous OoC start-ups launched from academic laboratories are starting to fill the commercial space for drug testing in the pharmaceutical industry45, including GSK283, Roche and Pfizer284. Nonetheless, there are some important challenges that still need to be overcome269, and so we foresee that future developments for OoC technology will likely focus on making the platform (1) more standardized, (2) compatible with imaging, analytical instruments, robotics and mass production, and (3) user-friendly so that it can be widely adopted by non-specialist end users. We anticipate increased efforts to develop OoCs that allow incorporation of the immune system285, metabolism286, microbiome287 and innervation288,289. These systems impact the health and functionality of organ systems in the body and are needed for achieving the desired functionality in (patho)physiological studies, but are recapitulated in animal models only to a limited extent290.
Platform development
OoCs have mostly been developed in academic laboratories at production throughputs of just a set of devices per day, so that the standardization and quality control remain limited. The major obstacle to general acceptance of OoCs to commercial or academic end users is, ultimately, determined by the quality and quantity of biological data. Many OoCs lack the robustness, ease of use and level of throughput to generate the large amounts of reliable biological data required for OoC validation.
To support broader use in biological research and drug development, standardized OoCs need to become as available as cell culture plates, implying that they need to be reliably manufactured on an industrial scale269. Approaches such as injection moulding, long established in the manufacture of high volumes of plastic products at low cost, are attractive for OoC manufacture where their context of use demands high throughput50,51. As OoCs often consist of several layers, the ability to manufacture many plastic chips may need to be coupled to parallel developments in the automation of chip alignment, assembly and bonding. With increased awareness and emphasis on manufacturability, we are also seeing more OoC systems being fabricated from new materials using additive manufacturing techniques such as 3D printing291. These techniques are constantly being improved with respect to production speed, and provide a largely assembly-free approach to device construction. They are thus a promising alternative for high-volume OoC production. The other major advantage of additive manufacturing is the ability to rapidly prototype new OoC designs. This can have a profound influence on how OoC designers develop their systems, as iterative refinement can be performed far more rapidly and effectively.
Higher availability of inexpensive OoCs would facilitate the adoption of this technology by end users in the life sciences. Future technology efforts should also focus on the development of more user-friendly OoC platforms that require less skill and experience to operate2. OoC prototypes typically are surrounded by a battery of syringe pumps connected to the OoC by tubing and valve arrays, as well as multiple reservoirs of medium, heating elements and other apparatus to maintain tissue viability and function; all of this peripheral equipment requires maintenance. Moreover, it makes the step towards experiments in parallel OoC more difficult, as each additional OoC often requires additional pumps, valves and tubing. Ready-to-use solutions will involve better design of OoC interfaces with peripheral instrumentation to reduce experimental footprints, as well as miniaturization of the actual OoC. This, in turn, will make OoC platforms more robust, easier to use and inherently more automatable.
Finally, OoC technology will be more readily adopted if it fits into the current biological research ecosystem, so that more molecular and functional data can be measured using existing analytical and imaging instruments. Currently, OoC approaches are mostly used to address questions where low to moderate throughput is sufficient. High-throughput applications, such as the screening of new chemical entities in the drug discovery process, are based on simpler cell cultures that are tested in well plate formats using robotic liquid handling. However, the use of OoC platforms at this early stage to produce more predictive tissue responses has been proposed. This will improve the selection of new chemical entities based on their safety, pharmacological and efficacy properties, minimizing the costly attrition of compounds at later stages283. We expect that some current OoC technologies based on relatively simple designs will be scaled up according to the standardized multiwell plate format292. These systems will find good use in many present-day screening applications besides drug development. Robotics and machine learning will also be implemented to automate operation and data analysis for these OoC systems11. Clearly, further instrumental development of more complex OoCs for improved throughput will also benefit the wider implementation of these platforms. We also anticipate that a new generation of OoC systems will integrate physical and biological sensors for in situ real-time dynamic stimulation and measurement of cell and tissue responses at a molecular or functional level.
Multi-OoCs are noteworthy in that they need to be configurable so that the types and the order of tissues can be selected based on the biological question studied. Therefore, we will likely see more innovation in modular OoC designs with reversible interconnections between different organ systems77,293,294 so that users can easily configure the number and order of interacting organs in a single-pass or recirculatory perfusion system. These approaches are modular, allowing organ tissue cultures to be established before linking different organs to one another. In one approach, researchers have developed a translational organ-on-a-chip platform, which uses a fluidic circuit board containing integrated channels as a baseplate into which microfluidic building blocks (the OoC) can be plugged in any desired configuration. This platform has as one of its objectives the standardization of OoC technology295. A different concept for modularity involves the design of a device with interconnected chambers, into which glass coverslips bearing separately prepared cell cultures or gel mixtures with cells are introduced108. Recently, a novel multi-organ chip was reported in which matured human heart, liver, bone and skin tissue niches are linked by recirculating vascular flow, to allow for the recapitulation of interdependent organ functions. Each tissue is cultured in its own optimized environment and is separated from the common vascular flow by a selectively permeable endothelial barrier. The interlinked tissues were shown to maintain their molecular, structural and functional phenotypes over 4 weeks of culture, recapitulate the PK and PD profiles of doxorubicin in humans, and allow for the identification of early microRNA biomarkers of cardiotoxicity27.
Biological applications
A broad area where we expect to see continued innovation is the development of multi-OoC systems to mimic systemic physiology of the human body. This utility was previously developed mostly in the context of evaluating the safety and efficacy of pharmaceutical compounds, which we expect to be extended to other therapeutic agents, including nanomedicine, cell therapies and engineered tissue replacements. With new knowledge being generated on the systemic nature of many chronic human diseases, there are also burgeoning opportunities for OoC systems to replace animals to model these diseases in a human-specific context. An example in this area is modelling metabolic crosstalk. Multi-OoCs offer a unique way to model the interactions between metabolically active organs, such as skeletal muscle, the liver, adipose tissue, the pancreas and the gut, to regulate the use and storage of energy through metabolic homeostasis296,297. Dysfunction in this metabolic crosstalk gives rise to metabolic diseases such as type 2 diabetes and non-alcoholic fatty liver disease, which is a growing global health concern298. Liver–gut multi-OoC systems have been created to model the gut–liver axis in hepatic steatosis299,300,301, and metabolic crosstalk in muscle–adipose16 and pancreas–liver302,303 systems has already been modelled in multi-OoC systems. The challenge for the next generation of metabolic multi-OoCs will be to incorporate more organs into the crosstalk, hence recapitulating more physiological metabolic phenotypes. The incorporation of patient microbiota through the gut OoCs can potentially offer us the opportunity to experimentally investigate various gut microbiome dysbiosis, which have been correlated to many chronic diseases through metagenome-wide association studies304,305.
Every tissue within the human body contains immune cells and their homeostasis determines tissue health and pathology. With the advent of immunotherapies and improvement in protocols to expand and maintain immune cell populations in vitro, there is increasing impetus to incorporate both adaptive and innate immune components into OoC systems306,307. In the context of cancer immunotherapy, researchers have started utilizing OoCs to investigate cancer cell–immune cell interactions in 3D environments308. There are also efforts to form lymphatic vessels within OoCs to study how cancer cells remodel the tumour microenvironment309. Recent studies also highlight the roles of endothelial–immune cell or pathogen crosstalk in modulating immune response310,311. Given significant species differences in the immune system and the high sensitivity of immune cells to a multitude of molecular, cellular and mechanical factors, human cell-based OoCs with highly controlled microenvironments are positioned to fill many important unmet needs. The complexity of the immune system presents many challenges as well as opportunities. Contributions towards these needs can span from innate immune response-mimicking biomaterials312 that can be easily incorporated into any cell culture to sophisticated long-term OoCs that recreate diseases involving multiple types of immune cell285. Overall, OoC models that adequately include human immune systems stand to provide a much needed human-specific context, as the majority of studies are carried out in murine models which often fall short of mimicking human-specific immune physiology. Innervation may also be required to model diseases resulting from neurological disorders, and to advance the biological fidelity of tissues that are normally innervated288,289.
Finally, OoC systems that are based on the use of iPSCs and organoids offer an unprecedented opportunity to study patient diversity (racial and ethnic background, sex, age, state of health or disease) as a biological variable, and to conduct patient-specific studies of the progression of disease and effects of treatment. By looking for commonalities between the clinical and in vitro data, we can identify early-stage biomarkers, monitor disease progression and determine optimal therapeutic treatment regimens in a personalized manner. However, the technicalities for routine integration of iPSC-derived organoids with microfluidic devices remain to be resolved. Existing methods often generate large organoids (approximately millimetres in diameter) and require extended (approximately weeks) differentiation times. Therefore, OoC systems need to either significantly alter their design and dimensions to accommodate large preformed organoids313 or invest substantial efforts to adapt and optimize differentiation protocols for in situ organoid differentiation on-chip314. iPSCs may also start playing a larger role in filling the need for reliable access to functional immune cells. Given the universal importance of patient-specific immune cells for many diseases and their treatment, it will be important to incorporate immune organs (such as bone marrow and lymph nodes) into the OoC platforms285 to provide renewable sources of both the innate and the adaptive immune cells, rather than adding the patient’s immune cells into the tissues or perfusate. Another approach would be to use a common HLA-null iPSC line to generate agnostic tissues that can be combined with the patient’s immune cells269.
In summary, the goal of this Primer was to explore the use of OoC in biological research and highlight the emerging opportunities and the unmet challenges. We described the design and applications of OoCs representing a single tissue unit and those representing multi-tissue units linked by microfluidic flow to recapitulate complex physiological functions such as cancer metastasis, inflammation and infection. Although OoCs consist of relatively simple tissues, compared with their native counterparts, they are able to approximate one or few organ-level functions: barrier function of the lung, contractile function of the heart or filtration in the kidney. We conclude that OoCs are poised to become broadly accepted in biological research, as they offer biologic fidelity along with experimental control in human tissue settings.
References
Bhatia, S. N. & Ingber, D. E. Microfluidic organs-on-chips. Nat. Biotechnol. 32, 760–772 (2014).
Low, L. A., Mummery, C., Berridge, B. R., Austin, C. P. & Tagle, D. A. Organs-on-chips: into the next decade. Nat. Rev. Drug. Discov. 20, 345–361 (2021).
Manz, A., Graber, N. & Widmer, H. M. Miniaturized total chemical analysis systems: a novel concept for chemical sensing. Sens. Actuator B Chem. 1, 244–248 (1990). This pioneering paper introduces the concept of micro total analysis systems integrated into microfluidic chips, which led, in turn, to the introduction of OoCs and integrated read-out systems.
Harrison, D. J., Manz, A., Fan, Z. H., Ludi, H. & Widmer, H. M. Capillary electrophoresis and sample injection systems integrated on a planar glass chip. Anal. Chem. 64, 1926–1932 (1992).
Whitesides, G. M. The origins and the future of microfluidics. Nature 442, 368–373 (2006).
Sahlgren, C. et al. Tailored approaches in drug development and diagnostics: from molecular design to biological model systems. Adv. Healthc. Mater. 6, 1700258 (2017).
Jackson, E. L. & Lu, H. Three-dimensional models for studying development and disease: moving on from organisms to organs-on-a-chip and organoids. Integr. Biol. 8, 672–683 (2016).
Sin, A., Baxter, G. & Shuler, M. Animal on a Chip: A Microscale Cell Culture Analog Device for Evaluating Toxicological and Pharmacological Profiles Vol. 4560 PWM (SPIE, 2001).
Sin, A. et al. The design and fabrication of three-chamber microscale cell culture analog devices with integrated dissolved oxygen sensors. Biotechnol. Prog. 20, 338–345 (2004). This paper is the first to provide a detailed description of the construction of a multi-OoC system based on a physiologically based PK model.
Huh, D. et al. Reconstituting organ-level lung functions on a chip. Science 328, 1662–1668 (2010). This paper is one of the pioneering works on lung OoC, which demonstrates a functional alveolar–capillary interface that is responsive to pathogen infection.
Ronaldson-Bouchard, K. & Vunjak-Novakovic, G. Organs-on-a-chip: a fast track for engineered human tissues in drug development. Cell Stem Cell 22, 310–324 (2018).
World Economic Forum. Top 10 emerging technologies of 2016. World Economic Forum https://www.weforum.org/reports/top-10-emerging-technologies-of-2016 (2016).
Jang, K.-J. & Suh, K.-Y. A multi-layer microfluidic device for efficient culture and analysis of renal tubular cells. Lab Chip 10, 36–42 (2010).
Kim, H. J., Huh, D., Hamilton, G. & Ingber, D. E. Human gut-on-a-chip inhabited by microbial flora that experiences intestinal peristalsis-like motions and flow. Lab Chip 12, 2165–2174 (2012). This work is one of the first gut OoC papers showing that physical stretching of the cell culture substrate and fluid perfusion can enhance the differentiation of Caco-2 cells into an intestinal epithelium with physiological architectures and functions.
Bokhari, M., Carnachan, R. J., Cameron, N. R. & Przyborski, S. A. Culture of HepG2 liver cells on three dimensional polystyrene scaffolds enhances cell structure and function during toxicological challenge. J. Anat. 211, 567–576 (2007).
Shahin-Shamsabadi, A. & Selvaganapathy, P. R. A 3D self-assembled in vitro model to simulate direct and indirect interactions between adipocytes and skeletal muscle cells. Adv. Biosyst. 4, 2000034 (2020).
Wang, Y., Wang, L., Guo, Y., Zhu, Y. & Qin, J. Engineering stem cell-derived 3D brain organoids in a perfusable organ-on-a-chip system. RSC Adv. 8, 1677–1685 (2018).
Lee, S.-R. et al. Modeling neural circuit, blood–brain barrier, and myelination on a microfluidic 96 well plate. Biofabrication 11, 035013 (2019).
Jang, J. M., Lee, J., Kim, H., Jeon, N. L. & Jung, W. One-photon and two-photon stimulation of neurons in a microfluidic culture system. Lab Chip 16, 1684–1690 (2016).
Park, S. M. et al. Reconstruction of in vivo-like in vitro model: enabling technologies of microfluidic systems for dynamic biochemical/mechanical stimuli. Microelectron. Eng. 203–204, 6–24 (2019).
Herzog, N., Katzenberger, N., Martin, F., Schmidtke, K. U. & Küpper, J.-H. Generation of cytochrome P450 3A4-overexpressing HepG2 cell clones for standardization of hepatocellular testosterone 6β-hydroxylation activity. J. Cell. Biotechnol. 1, 15–26 (2015).
Moysidou, C.-M., Barberio, C. & Owens, R. M. Advances in engineering human tissue models. Front. Bioeng. Biotechnol. 8, 620962 (2021).
Gallagher, L. B. et al. Pre-culture of mesenchymal stem cells within RGD-modified hyaluronic acid hydrogel improves their resilience to ischaemic conditions. Acta Biomater. 107, 78–90 (2020).
Ramme, A. P. et al. Autologous induced pluripotent stem cell-derived four-organ-chip. Future Sci. OA 5, FSO413 (2019).
Sances, S. et al. Human iPSC-derived endothelial cells and microengineered organ-chip enhance neuronal development. Stem Cell Rep. 10, 1222–1236 (2018).
Rowe, R. G. & Daley, G. Q. Induced pluripotent stem cells in disease modelling and drug discovery. Nat. Rev. Genet. 20, 377–388 (2019).
Ronaldson-Bouchard K., et al. Inter-organ chips with matured tissue niches linked by vascular perfusion. Nat. Biomed. Eng. (in the press). This paper reports a multi-tissue chip system in which matured human tissues are linked by recirculating vascular flow, allowing for the maintenance of their phenotypes, communication across endothelial barriers and recapitulation of interdependent organ functions.
Duffy, D. C., McDonald, J. C., Schueller, O. J. A. & Whitesides, G. M. Rapid prototyping of microfluidic systems in poly(dimethylsiloxane). Anal. Chem. 70, 4974–4984 (1998).
Xia, Y. & Whitesides, G. M. Soft lithography. Annu. Rev. Mater. Sci. 28, 153–184 (1998).
Reyes, D. R., Iossifidis, D., Auroux, P.-A. & Manz, A. Micro total analysis systems. 1. Introduction, theory, and technology. Anal. Chem. 74, 2623–2636 (2002).
Verpoorte, E. M. J. et al. Three-dimensional micro flow manifolds for miniaturized chemical analysis systems. J. Micromech. Microeng. 4, 246–256 (1994).
Dittrich, P. S. & Manz, A. Lab-on-a-chip: microfluidics in drug discovery. Nat. Rev. Drug. Discov. 5, 210–218 (2006).
Meyvantsson, I. & Beebe, D. J. Cell culture models in microfluidic systems. Annu. Rev. Anal. Chem. 1, 423–449 (2008).
Kim, L., Toh, Y.-C., Voldman, J. & Yu, H. A practical guide to microfluidic perfusion culture of adherent mammalian cells. Lab Chip 7, 681–694 (2007). This paper offers practical help for setting up a microfluidic perfusion cell culture system, such as dealing with bubbles, proper control of temperature and gas equilibration in media.
Sung, J. H. et al. Recent advances in body-on-a-chip systems. Anal. Chem. 91, 330–351 (2019).
Allwardt, V. et al. Translational roadmap for the organs-on-a-chip industry toward broad adoption. Bioengineering 7, 112 (2020).
Abaci, H. E. & Shuler, M. L. Human-on-a-chip design strategies and principles for physiologically based pharmacokinetics/pharmacodynamics modeling. Integr. Biol. 7, 383–391 (2015).
Zhang, Y. S. et al. Multisensor-integrated organs-on-chips platform for automated and continual in situ monitoring of organoid behaviors. Proc. Natl Acad. Sci. USA 114, E2293–E2302 (2017).
Frey, O., Misun, P. M., Fluri, D. A., Hengstler, J. G. & Hierlemann, A. Reconfigurable microfluidic hanging drop network for multi-tissue interaction and analysis. Nat. Commun. 5, 4250 (2014). This paper introduces interconnected hanging drops and unifies spheroid aggregation of different organ types and their cross-communication in a parallel format.
Santbergen, M. J. C., van der Zande, M., Bouwmeester, H. & Nielen, M. W. F. Online and in situ analysis of organs-on-a-chip. TrAC Trends Anal. Chem. 115, 138–146 (2019).
Misun, P. M., Rothe, J., Schmid, Y. R. F., Hierlemann, A. & Frey, O. Multi-analyte biosensor interface for real-time monitoring of 3D microtissue spheroids in hanging-drop networks. Microsyst. Nanoeng. 2, 16022 (2016).
Hargrove-Grimes, P., Low, L. A. & Tagle, D. A. Microphysiological systems: stakeholder challenges to adoption in drug development. Cell Tissues Organs 211, 69–81 (2022).
FDA-NIH Biomarker Working Group. BEST (Biomarkers, EndpointS, and other Tools) Resource (US Food and Drug Administration & National Institutes of Health, 2016).
US Food and Drug Administration. Advancing alternative methods at FDA. US Food and Drug Administration https://www.fda.gov/science-research/about-science-research-fda/advancing-alternative-methods-fda#:~:text=FDA%20Definitions&text=MPS%20platforms%20may%20comprise%20mono,inclusion%20of%20organoid%20cell%20formations (2022).
Zhang, B. & Radisic, M. Organ-on-a-chip devices advance to market. Lab Chip 17, 2395–2420 (2017). This review discusses the commercialization trajectory of OoC technologies into the drug discovery market.
Verpoorte, E. et al. in 2015 Transducers — 2015 18th International Conference on Solid-State Sensors, Actuators and Microsystems (TRANSDUCERS) 224–227 (IEEE, 2015).
Shin, J. et al. Monolithic digital patterning of polydimethylsiloxane with successive laser pyrolysis. Nat. Mater. 20, 100–107 (2021). This study describes a novel laser-based fabrication technique for PDMS devices; a high-resolution technique suitable for rapid prototyping and production of a small series of devices (10–100 devices), while also being a good alternative to replication moulding.
Toepke, M. W. & Beebe, D. J. PDMS absorption of small molecules and consequences in microfluidic applications. Lab Chip 6, 1484–1486 (2006).
Wang, J. D., Douville, N. J., Takayama, S. & ElSayed, M. Quantitative analysis of molecular absorption into PDMS microfluidic channels. Ann. Biomed. Eng. 40, 1862–1873 (2012).
Ko, J. et al. Tumor spheroid-on-a-chip: a standardized microfluidic culture platform for investigating tumor angiogenesis. Lab Chip 19, 2822–2833 (2019).
Lee, Y. et al. Microfluidics within a well: an injection-molded plastic array 3D culture platform. Lab Chip 18, 2433–2440 (2018). This paper presents the basis for high-throughput microfluidic organ-on-a-chip design that is amenable for 3D co-cultures. Furthermore, the device designs described here are compatible with 3D printing and injection moulding such that they offer an alternative to traditional PDMS-based chips.
Homan, K. A. et al. Flow-enhanced vascularization and maturation of kidney organoids in vitro. Nat. Methods 16, 255–262 (2019).
Berthier, E., Young, E. W. K. & Beebe, D. Engineers are from PDMS-land, biologists are from Polystyrenia. Lab Chip 12, 1224–1237 (2012). This paper highlights how differences in the inherent material properties of PS and PDMS can lead to disparity between conventional and microfluidic cell cultures.
Jensen, C. & Teng, Y. Is it time to start transitioning from 2D to 3D cell culture? Front. Mol. Biosci. 7, 33 (2020).
Kim, H. J., Li, H., Collins, J. J. & Ingber, D. E. Contributions of microbiome and mechanical deformation to intestinal bacterial overgrowth and inflammation in a human gut-on-a-chip. Proc. Natl Acad. Sci. USA 113, E7–E15 (2016).
Maoz, B. M. et al. A linked organ-on-chip model of the human neurovascular unit reveals the metabolic coupling of endothelial and neuronal cells. Nat. Biotechnol. 36, 865–874 (2018).
Brown, J. A. et al. Recreating blood–brain barrier physiology and structure on chip: a novel neurovascular microfluidic bioreactor. Biomicrofluidics 9, 054124 (2015).
Ahn, S. I. et al. Microengineered human blood–brain barrier platform for understanding nanoparticle transport mechanisms. Nat. Commun. 11, 175 (2020).
Esch, M. B. et al. On chip porous polymer membranes for integration of gastrointestinal tract epithelium with microfluidic ‘body-on-a-chip’ devices. Biomed. Microdevices 14, 895–906 (2012).
Wang, Y. et al. Capture and 3D culture of colonic crypts and colonoids in a microarray platform. Lab Chip 13, 4625–4634 (2013).
Uemura, M. et al. Matrigel supports survival and neuronal differentiation of grafted embryonic stem cell-derived neural precursor cells. J. Neurosci. Res. 88, 542–551 (2010).
Afshar Bakooshli, M. et al. A 3D culture model of innervated human skeletal muscle enables studies of the adult neuromuscular junction. eLife 8, e44530 (2019).
Horowitz, L. F., Rodriguez, A. D., Ray, T. & Folch, A. Microfluidics for interrogating live intact tissues. Microsyst. Nanoeng. 6, 69 (2020). This article is a reference for readers interested in the integration of live intact tissue slices into a microfluidic system.
Shi, Y., Inoue, H., Wu, J. C. & Yamanaka, S. Induced pluripotent stem cell technology: a decade of progress. Nat. Rev. Drug Discov. 16, 115–130 (2017).
van den Berg, A., Mummery, C. L., Passier, R. & van der Meer, A. D. Personalised organs-on-chips: functional testing for precision medicine. Lab Chip 19, 198–205 (2019).
Diederichs, S. & Tuan, R. S. Functional comparison of human-induced pluripotent stem cell-derived mesenchymal cells and bone marrow-derived mesenchymal stromal cells from the same donor. Stem Cell Dev. 23, 1594–1610 (2014).
Verma, A., Verma, M. & Singh, A. in Animal Biotechnology 2nd edn (eds Verma, A. S. & Singh, A.) 269–293 (Academic, 2020).
Yeste, J., Illa, X., Alvarez, M. & Villa, R. Engineering and monitoring cellular barrier models. J. Biol. Eng. 12, 18 (2018).
Yang, F., Cohen, R. N. & Brey, E. M. Optimization of co-culture conditions for a human vascularized adipose tissue model. Bioengineering 7, 114 (2020).
Chang, S.-Y. et al. Human liver–kidney model elucidates the mechanisms of aristolochic acid nephrotoxicity. JCI Insight 2, e95978 (2017).
Zhang, C., Zhao, Z., Abdul Rahim, N. A., van Noort, D. & Yu, H. Towards a human-on-chip: culturing multiple cell types on a chip with compartmentalized microenvironments. Lab Chip 9, 3185–3192 (2009).
Jang, K. J. et al. Reproducing human and cross-species drug toxicities using a liver-chip. Sci. Transl. Med. 11, eaax5516 (2019).
Wufuer, M. et al. Skin-on-a-chip model simulating inflammation, edema and drug-based treatment. Sci. Rep. 6, 37471 (2016).
Even, M. S., Sandusky, C. B. & Barnard, N. D. Serum-free hybridoma culture: ethical, scientific and safety considerations. Trends Biotechnol. 24, 105–108 (2006).
Kongsuphol, P. et al. In vitro micro-physiological model of the inflamed human adipose tissue for immune-metabolic analysis in type II diabetes. Sci. Rep. 9, 4887 (2019).
Massa, S. et al. Bioprinted 3D vascularized tissue model for drug toxicity analysis. Biomicrofluidics 11, 044109 (2017).
Ong, L. J. Y. et al. Self-aligning Tetris-Like (TILE) modular microfluidic platform for mimicking multi-organ interactions. Lab Chip 19, 2178–2191 (2019). This paper demonstrates a modular design of assembling pre-established tissue chips into a recirculating multi-OoC system.
Satoh, T. et al. A pneumatic pressure-driven multi-throughput microfluidic circulation culture system. Lab Chip 16, 2339–2348 (2016).
Lee, K. K. et al. Human stomach-on-a-chip with luminal flow and peristaltic-like motility. Lab Chip 18, 3079–3085 (2018).
Ong, L. J. Y. et al. A pump-free microfluidic 3D perfusion platform for the efficient differentiation of human hepatocyte-like cells. Biotechnol. Bioeng. 114, 2360–2370 (2017).
Yu, F. et al. A pump-free tricellular blood–brain barrier on-a-chip model to understand barrier property and evaluate drug response. Biotechnol. Bioeng. 117, 1127–1136 (2020).
Lohasz, C., Rousset, N., Renggli, K., Hierlemann, A. & Frey, O. Scalable microfluidic platform for flexible configuration of and experiments with microtissue multiorgan models. SLAS Technol. 24, 79–95 (2019). This paper describes a scalable and automation-compatible multi-spheroid culture system in a microtitre plate format based on gravity-driven flow that is actuated by tilting.
Wang, Y. I. & Shuler, M. L. UniChip enables long-term recirculating unidirectional perfusion with gravity-driven flow for microphysiological systems. Lab Chip 18, 2563–2574 (2018).
Oleaga, C. et al. Multi-organ toxicity demonstration in a functional human in vitro system composed of four organs. Sci. Rep. 6, 20030 (2016).
Chen, L., Yang, Y., Ueno, H. & Esch, M. B. Body-in-a-cube: a microphysiological system for multi-tissue co-culture with near-physiological amounts of blood surrogate. Microphys. Syst. 4, 1 (2020).
Yang, Y. et al. Pumpless microfluidic devices for generating healthy and diseased endothelia. Lab Chip 19, 3212–3219 (2019).
Maschmeyer, I. et al. A four-organ-chip for interconnected long-term co-culture of human intestine, liver, skin and kidney equivalents. Lab Chip 15, 2688–2699 (2015).
Vernetti, L. et al. Functional coupling of human microphysiology systems: intestine, liver, kidney proximal tubule, blood–brain barrier and skeletal muscle. Sci. Rep. 7, 42296 (2017). This paper combines multiple single-OoCs by manual transfer of culture medium from one organ to the next to simulate multi-organ functions; termed ‘functional’ coupling rather than direct coupling of organs in a flow-through system.
Novak, R. et al. Robotic fluidic coupling and interrogation of multiple vascularized organ chips. Nat. Biomed. Eng. 4, 407–420 (2020).
van Midwoud, P. M. et al. On-line HPLC analysis system for metabolism and inhibition studies in precision-cut liver slices. Anal. Chem. 83, 84–91 (2011).
Young, E. W. & Beebe, D. J. Fundamentals of microfluidic cell culture in controlled microenvironments. Chem. Soc. Rev. 39, 1036–1048 (2010). This review covers fundamental concepts on how microfluidics alter physical and biochemical microenvironmental factors, which are important for successful microscale cell culture.
Blagovic, K., Kim, L. Y. & Voldman, J. Microfluidic perfusion for regulating diffusible signaling in stem cells. PLoS ONE 6, e22892 (2011).
Przybyla, L. M. & Voldman, J. Attenuation of extrinsic signaling reveals the importance of matrix remodeling on maintenance of embryonic stem cell self-renewal. Proc. Natl Acad. Sci. USA 109, 835–840 (2012).
Bhattacharjee, N. & Folch, A. Large-scale microfluidic gradient arrays reveal axon guidance behaviors in hippocampal neurons. Microsyst. Nanoeng. 3, 17003 (2017).
McCarty, W. J., Usta, O. B. & Yarmush, M. L. A microfabricated platform for generating physiologically-relevant hepatocyte zonation. Sci. Rep. 6, 26868 (2016).
Tilles, A. W., Baskaran, H., Roy, P., Yarmush, M. L. & Toner, M. Effects of oxygenation and flow on the viability and function of rat hepatocytes cocultured in a microchannel flat-plate bioreactor. Biotechnol. Bioeng. 73, 379–389 (2001).
Lindner, M., Laporte, A., Block, S., Elomaa, L. & Weinhart, M. Physiological shear stress enhances differentiation, mucus-formation and structural 3D organization of intestinal epithelial. Cell Vitro. Cell 10, 2062 (2021).
Marrero, D. et al. Gut-on-a-chip: mimicking and monitoring the human intestine. Biosens. Bioelectron. 181, 113156 (2021).
Trieu, D., Waddell, T. K. & McGuigan, A. P. A microfluidic device to apply shear stresses to polarizing ciliated airway epithelium using air flow. Biomicrofluidics 8, 064104 (2014).
Arora, S., Srinivasan, A., Leung, M. C. & Toh, Y.-C. Bio-mimicking shear stress environments for enhancing mesenchymal stem cell differentiation. Curr. Stem Cell Res. Ther. 15, 414–427 (2020).
Ergir, E., Bachmann, B., Redl, H., Forte, G. & Ertl, P. Small force, big impact: next generation organ-on-a-chip systems incorporating biomechanical cues. Front. Physiol. 9, 1417 (2018).
Takano, A., Tanaka, M. & Futai, N. On-chip multi-gas incubation for microfluidic cell cultures under hypoxia. Biomicrofluidics 8, 061101 (2014).
Toh, Y.-C. et al. A novel 3D mammalian cell perfusion-culture system in microfluidic channels. Lab Chip 7, 302–309 (2007).
Imura, Y., Asano, Y., Sato, K. & Yoshimura, E. A microfluidic system to evaluate intestinal absorption. Anal. Sci. 25, 1403–1407 (2009).
Bein, A. et al. Enteric coronavirus infection and treatment modeled with an immunocompetent human intestine-on-a-chip. Front. Pharmacol. 12, 718484 (2021).
Viravaidya, K., Sin, A. & Shuler, M. L. Development of a microscale cell culture analog to probe naphthalene toxicity. Biotechnol. Prog. 20, 316–323 (2004).
Edington, C. D. et al. Interconnected microphysiological systems for quantitative biology and pharmacology studies. Sci. Rep. 8, 4530 (2018).
McAleer, C. W. et al. Multi-organ system for the evaluation of efficacy and off-target toxicity of anticancer therapeutics. Sci. Transl. Med. https://doi.org/10.1126/scitranslmed.aav1386 (2019). Together with Viravaidya et al. (2004) and Edington et al. (2018), this paper and the above-mentioned papers have been selected as case studies to highlight evolution in the design of multi-OoC systems for ADME-Tox studies.
Grist, S. M., Chrostowski, L. & Cheung, K. C. Optical oxygen sensors for applications in microfluidic cell culture. Sensors 10, 9286–9316 (2010).
Rivera, K. R., Yokus, M. A., Erb, P. D., Pozdin, V. A. & Daniele, M. Measuring and regulating oxygen levels in microphysiological systems: design, material, and sensor considerations. Analyst 144, 3190–3215 (2019).
Oomen, P. E., Skolimowski, M. D. & Verpoorte, E. Implementing oxygen control in chip-based cell and tissue culture systems. Lab Chip 16, 3394–3414 (2016).
Brennan, M. D., Rexius-Hall, M. L., Elgass, L. J. & Eddington, D. T. Oxygen control with microfluidics. Lab Chip 14, 4305–4318 (2014).
Scheidecker, B. et al. Induction of in vitro metabolic zonation in primary hepatocytes requires both near-physiological oxygen concentration and flux. Front. Bioeng. Biotechnol. 8, 524 (2020).
Farzaneh, Z. et al. Dissolved oxygen concentration regulates human hepatic organoid formation from pluripotent stem cells in a fully controlled bioreactor. Biotechnol. Bioeng. 117, 3739–3756 (2020).
Kietzmann, T. Metabolic zonation of the liver: the oxygen gradient revisited. Redox Biol. 11, 622–630 (2017).
Sung, J. H., Choi, J. R., Kim, D. & Shuler, M. L. Fluorescence optical detection in situ for real-time monitoring of cytochrome P450 enzymatic activity of liver cells in multiple microfluidic devices. Biotechnol. Bioeng. 104, 516–525 (2009).
Choi, J. R., Sung, J. H., Shuler, M. L. & Kim, D. Investigation of portable in situ fluorescence optical detection for microfluidic 3D cell culture assays. Opt. Lett. 35, 1374–1376 (2010).
Weltin, A. et al. Cell culture monitoring for drug screening and cancer research: a transparent, microfluidic, multi-sensor microsystem. Lab Chip 14, 138–146 (2014).
Toh, Y. C. & Voldman, J. Fluid shear stress primes mouse embryonic stem cells for differentiation in a self-renewing environment via heparan sulfate proteoglycans transduction. FASEB J. 25, 1208–1217 (2011).
Vit, F. F. et al. A modular, reversible sealing, and reusable microfluidic device for drug screening. Analytica Chim. Acta 1185, 339068 (2021).
Morsink, M. A. J., Willemen, N. G. A., Leijten, J., Bansal, R. & Shin, S. R. Immune organs and immune cells on a chip: an overview of biomedical applications. Micromachines 11, 849 (2020).
Chen, Y. Y. et al. Clarifying intact 3D tissues on a microfluidic chip for high-throughput structural analysis. Proc. Natl Acad. Sci. USA 113, 14915–14920 (2016).
Wardwell-Swanson, J. et al. A framework for optimizing high-content imaging of 3D models for drug discovery. SLAS Discov. 25, 709–722 (2020).
Chen, Z. et al. Automated evaluation of tumor spheroid behavior in 3D culture using deep learning-based recognition. Biomaterials 272, 120770 (2021).
Wolff, A., Antfolk, M., Brodin, B. & Tenje, M. In vitro blood-brain barrier models — an overview of established models and new microfluidic approaches. J. Pharm. Sci. 104, 2727–2746 (2015).
Brown, C. D. A. et al. Characterisation of human tubular cell monolayers as a model of proximal tubular xenobiotic handling. Toxicol. Appl. Pharmacol. 233, 428–438 (2008).
Henry, O. Y. F. et al. Organs-on-chips with integrated electrodes for trans-epithelial electrical resistance (TEER) measurements of human epithelial barrier function. Lab Chip 17, 2264–2271 (2017).
van der Helm, M. W. et al. Direct quantification of transendothelial electrical resistance in organs-on-chips. Biosens. Bioelectron. 85, 924–929 (2016).
Demircan Yalcin, Y. & Luttge, R. Electrical monitoring approaches in 3-dimensional cell culture systems: toward label-free, high spatiotemporal resolution, and high-content data collection in vitro. Organs-on-a-Chip 3, 100006 (2021).
Elbakary, B. & Badhan, R. K. S. A dynamic perfusion based blood–brain barrier model for cytotoxicity testing and drug permeation. Sci. Rep. 10, 3788 (2020).
Bürgel, S. C., Diener, L., Frey, O., Kim, J. Y. & Hierlemann, A. Automated, multiplexed electrical impedance spectroscopy platform for continuous monitoring of microtissue spheroids. Anal. Chem. 88, 10876–10883 (2016).
Srinivasan, B. et al. TEER measurement techniques for in vitro barrier model systems. J. Lab. Autom. 20, 107–126 (2015). This paper reviews different methods of making TEER measurements in barrier tissues, and their strengths and weaknesses, and addresses issues where the technique is applied incorrectly.
Soucy, J. R., Bindas, A. J., Koppes, A. N. & Koppes, R. A. Instrumented microphysiological systems for real-time measurement and manipulation of cellular electrochemical processes. iScience 21, 521–548 (2019).
Ferrari, E., Palma, C., Vesentini, S., Occhetta, P. & Rasponi, M. Integrating biosensors in organs-on-chip devices: a perspective on current strategies to monitor microphysiological systems. Biosensors 10, 110 (2020). This review covers the integration of sensors into OoC devices for real-time measurements of oxygen, soluble metabolites (for example, glucose or lactate cytokines), TEER and electrical activity.
Oleaga, C. et al. A human in vitro platform for the evaluation of pharmacology strategies in cardiac ischemia. APL. Bioeng. 3, 036103 (2019).
Kussauer, S., David, R. & Lemcke, H. hiPSCs derived cardiac cells for drug and toxicity screening and disease modeling: what micro-electrode-array analyses can tell us. Cells 8, 1331 (2019).
van de Wijdeven, R. et al. A novel lab-on-chip platform enabling axotomy and neuromodulation in a multi-nodal network. Biosens. Bioelectron. 140, 111329 (2019).
Oiwa, K. et al. A device for co-culturing autonomic neurons and cardiomyocytes using micro-fabrication techniques. Integr. Biol. 8, 341–348 (2016).
Yuan, X. et al. Versatile live-cell activity analysis platform for characterization of neuronal dynamics at single-cell and network level. Nat. Commun. 11, 4854 (2020).
Sung, J. H., Wang, Y. & Shuler, M. L. Strategies for using mathematical modeling approaches to design and interpret multi-organ microphysiological systems (MPS). APL Bioeng. 3, 021501 (2019). This paper provides a useful guide to design principles and scaling rules for multi-OoC systems to be used for PK and PD studies.
Moutaux, E., Charlot, B., Genoux, A., Saudou, F. & Cazorla, M. An integrated microfluidic/microelectrode array for the study of activity-dependent intracellular dynamics in neuronal networks. Lab Chip 18, 3425–3435 (2018).
Stancescu, M. et al. A phenotypic in vitro model for the main determinants of human whole heart function. Biomaterials 60, 20–30 (2015).
Coln, E. A. et al. Piezoelectric BioMEMS cantilever for measurement of muscle contraction and for actuation of mechanosensitive cells. MRS Commun. 9, 1186–1192 (2019).
Maoz, B. M. et al. Organs-on-chips with combined multi-electrode array and transepithelial electrical resistance measurement capabilities. Lab Chip 17, 2294–2302 (2017).
Balijepalli, A. & Sivaramakrishan, V. Organs-on-chips: research and commercial perspectives. Drug. Discov. Today 22, 397–403 (2017).
Livingston, C. A., Fabre, K. M. & Tagle, D. A. Facilitating the commercialization and use of organ platforms generated by the microphysiological systems (Tissue Chip) program through public–private partnerships. Comput. Struct. Biotechnol. J. 14, 207–210 (2016).
Reyes, D. R. et al. Accelerating innovation and commercialization through standardization of microfluidic-based medical devices. Lab Chip 21, 9–21 (2021).
Fabre, K. et al. Introduction to a manuscript series on the characterization and use of microphysiological systems (MPS) in pharmaceutical safety and ADME applications. Lab Chip 20, 1049–1057 (2020).
Sung, J. H., Kam, C. & Shuler, M. L. A microfluidic device for a pharmacokinetic–pharmacodynamic (PK–PD) model on a chip. Lab Chip 10, 446–455 (2010).
Tatosian, D. A. & Shuler, M. L. A novel system for evaluation of drug mixtures for potential efficacy in treating multidrug resistant cancers. Biotechnol. Bioeng. 103, 187–198 (2009).
Stokes, C. L., Cirit, M. & Lauffenburger, D. A. Physiome-on-a-chip: the challenge of “scaling” in design, operation, and translation of microphysiological systems. CPT Pharmacomet. Syst. Pharmacol. 4, 559–562 (2015).
Wikswo, J. P. et al. Scaling and systems biology for integrating multiple organs-on-a-chip. Lab Chip 13, 3496–3511 (2013).
Yu, J. et al. Quantitative systems pharmacology approaches applied to microphysiological systems (MPS): data interpretation and multi-MPS integration. CPT Pharmacomet. Syst. Pharmacol. 4, 585–594 (2015).
Bai, J. et al. A novel 3D vascular assay for evaluating angiogenesis across porous membranes. Biomaterials 268, 120592 (2021).
Carter, S. D., Barbe, L., Tenje, M. & Mestres, G. Exploring microfluidics as a tool to evaluate the biological properties of a titanium alloy under dynamic conditions. Biomater. Sci. 8, 6309–6321 (2020).
Chueh, B. H. et al. Leakage-free bonding of porous membranes into layered microfluidic array systems. Anal. Chem. 79, 3504–3508 (2007).
Winkler, T. E., Feil, M., Stronkman, E., Matthiesen, I. & Herland, A. Low-cost microphysiological systems: feasibility study of a tape-based barrier-on-chip for small intestine modeling. Lab Chip 20, 1212–1226 (2020).
Schneider, S., Gruner, D., Richter, A. & Loskill, P. Membrane integration into PDMS-free microfluidic platforms for organ-on-chip and analytical chemistry applications. Lab Chip 21, 1866–1885 (2021).
Kuo, C. H., Xian, J., Brenton, J. D., Franze, K. & Sivaniah, E. Complex stiffness gradient substrates for studying mechanotactic cell migration. Adv. Mater. 24, 6059–6064 (2012).
Zink, C., Hall, H., Brunette, D. M. & Spencer, N. D. Orthogonal nanometer–micrometer roughness gradients probe morphological influences on cell behavior. Biomaterials 33, 8055–8061 (2012).
Kim, T. H. et al. Creating stiffness gradient polyvinyl alcohol hydrogel using a simple gradual freezing–thawing method to investigate stem cell differentiation behaviors. Biomaterials 40, 51–60 (2015).
Oh, S. H., An, D. B., Kim, T. H. & Lee, J. H. Wide-range stiffness gradient PVA/HA hydrogel to investigate stem cell differentiation behavior. Acta Biomater. 35, 23–31 (2016).
Mei, Y. et al. Combinatorial development of biomaterials for clonal growth of human pluripotent stem cells. Nat. Mater. 9, 768–778 (2010).
Jana, S., Levengood, S. K. & Zhang, M. Anisotropic materials for skeletal-muscle-tissue engineering. Adv. Mater. 28, 10588–10612 (2016).
Kim, D.-H. et al. Mechanosensitivity of fibroblast cell shape and movement to anisotropic substratum topography gradients. Biomaterials 30, 5433–5444 (2009).
Park, J. et al. Directed migration of cancer cells guided by the graded texture of the underlying matrix. Nat. Mater. 15, 792–801 (2016).
Zhou, Q. et al. Screening platform for cell contact guidance based on inorganic biomaterial micro/nanotopographical gradients. ACS Appl. Mater. Interfaces 9, 31433–31445 (2017).
Perlman, R. L. Mouse models of human disease: an evolutionary perspective. Evol. Med. Public Health 2016, 170–176 (2016).
Mestas, J. & Hughes, C. C. W. Of mice and not men: differences between mouse and human immunology. J. Immunol. 172, 2731–2738 (2004).
Dellaquila, A., Thomée, E. K., McMillan, A. H. & Lesher-Pérez, S. C. in Organ-on-a-Chip (eds Hoeng, J., Bovard, D. & Peitsch, M. C.) 133–180 (Academic, 2020).
Bliley, J. M. et al. Dynamic loading of human engineered heart tissue enhances contractile function and drives a desmosome-linked disease phenotype. Sci. Transl. Med. 13, eabd1817 (2021).
Trapecar, M. et al. Gut–liver physiomimetics reveal paradoxical modulation of IBD-related inflammation by short-chain fatty acids. Cell Syst. 10, 223–239.e9 (2020).
Ahn, J., Sei, Y. J., Jeon, N. L. & Kim, Y. Tumor microenvironment on a chip: the progress and future perspective. Bioengineering 4, 64 (2017).
Park, D., Lim, J., Park, J. Y. & Lee, S. H. Concise review: stem cell microenvironment on a chip: current technologies for tissue engineering and stem cell biology. Stem Cell Transl. Med. 4, 1352–1368 (2015).
Selimović, Š., Kaji, H., Bae, H. & Khademhosseini, A. in Microfluidic Cell Culture Systems 2nd edn (eds Borenstein, J. T., Tandon, V., Tao, S. L & Charest, J. L) 31–63 (Elsevier, 2019).
Shang, M., Soon, R. H., Lim, C. T., Khoo, B. L. & Han, J. Microfluidic modelling of the tumor microenvironment for anti-cancer drug development. Lab Chip 19, 369–386 (2019).
Przybyla, L. & Voldman, J. Probing embryonic stem cell autocrine and paracrine signaling using microfluidics. Annu. Rev. Anal. Chem. 5, 293–315 (2012). This review paper explains the physics governing the use of microfluidic cell culture to control the presentation of soluble factors to cells in order to investigate paracrine and autocrine signalling.
Toh, A. G. G., Wang, Z. P., Yang, C. & Nguyen, N.-T. Engineering microfluidic concentration gradient generators for biological applications. Microfluidics Nanofluidics 16, 1–18 (2014).
Wang, X., Liu, Z. & Pang, Y. Concentration gradient generation methods based on microfluidic systems. RSC Adv. 7, 29966–29984 (2017).
Gómez-Sjöberg, R., Leyrat, A. A., Pirone, D. M., Chen, C. S. & Quake, S. R. Versatile, fully automated, microfluidic cell culture system. Anal. Chem. 79, 8557–8563 (2007).
Titmarsh, D. M. et al. Induction of human iPSC-derived cardiomyocyte proliferation revealed by combinatorial screening in high density microbioreactor arrays. Sci. Rep. 6, 24637 (2016).
Cosson, S. & Lutolf, M. P. Hydrogel microfluidics for the patterning of pluripotent stem cells. Sci. Rep. 4, 4462 (2014).
Manfrin, A. et al. Engineered signaling centers for the spatially controlled patterning of human pluripotent stem cells. Nat. Methods 16, 640–648 (2019).
Rhee, S. W. et al. Patterned cell culture inside microfluidic devices. Lab Chip 5, 102–107 (2005).
Chiu, D. T. et al. Patterned deposition of cells and proteins onto surfaces by using three-dimensional microfluidic systems. Proc. Natl Acad. Sci. USA 97, 2408–2413 (2000).
Jeon, K. J. et al. Combined effects of flow-induced shear stress and micropatterned surface morphology on neuronal differentiation of human mesenchymal stem cells. J. Biosci. Bioeng. 117, 242–247 (2014).
Tu, C. et al. A microfluidic chip for cell patterning utilizing paired microwells and protein patterns. Micromachines 8, 1 (2017).
Skelley, A. M., Kirak, O., Suh, H., Jaenisch, R. & Voldman, J. Microfluidic control of cell pairing and fusion. Nat. Methods 6, 147–152 (2009).
Flaim, C. J., Chien, S. & Bhatia, S. N. An extracellular matrix microarray for probing cellular differentiation. Nat. Methods 2, 119–125 (2005).
McCain, M. L., Yuan, H., Pasqualini, F. S., Campbell, P. H. & Parker, K. K. Matrix elasticity regulates the optimal cardiac myocyte shape for contractility. Am. J. Physiol. Heart Circul. Physiol. 306, H1525–H1539 (2014).
Vázquez-Victorio, G. et al. Building a microfluidic cell culture platform with stiffness control using Loctite 3525 glue. Lab Chip 19, 3512–3525 (2019).
Pasman, T., Grijpma, D., Stamatialis, D. & Poot, A. Flat and microstructured polymeric membranes in organs-on-chips. J. R. Soc. Interface 15, 20180351 (2018).
Arora, S., Lin, S., Cheung, C., Yim, E. K. F. & Toh, Y.-C. Topography elicits distinct phenotypes and functions in human primary and stem cell derived endothelial cells. Biomaterials 234, 119747 (2020).
Chau, L., Doran, M. & Cooper-White, J. A novel multishear microdevice for studying cell mechanics. Lab Chip 9, 1897–1902 (2009).
Arora, S., Lam, A. J. Y., Cheung, C., Yim, E. K. F. & Toh, Y.-C. Determination of critical shear stress for maturation of human pluripotent stem cell-derived endothelial cells towards an arterial subtype. Biotechnol. Bioeng. 116, 1164–1175 (2019).
Visone, R. et al. A microscale biomimetic platform for generation and electro-mechanical stimulation of 3D cardiac microtissues. APL. Bioeng. 2, 046102 (2018).
Duc, P. et al. Human neuromuscular junction on micro-structured microfluidic devices implemented with a custom micro electrode array (MEA). Lab Chip 21, 4223–4236 (2021).
Beckwitt, C. H. et al. Liver ‘organ on a chip’. Exp. Cell Res. 363, 15–25 (2018).
Deng, J. et al. Engineered liver-on-a-chip platform to mimic liver functions and its biomedical applications: a review. Micromachines 10, 676 (2019).
Starokozhko, V. & Groothuis, G. M. Judging the value of ‘liver-on-a-chip’ devices for prediction of toxicity. Expert Opin. Drug Metab. Toxicol. 13, 125–128 (2017).
Khetani, S. R. & Bhatia, S. N. Microscale culture of human liver cells for drug development. Nat. Biotechnol. 26, 120–126 (2008).
Sivaraman, A. et al. A microscale in vitro physiological model of the liver: predictive screens for drug metabolism and enzyme induction. Curr. Drug Metab. 6, 569–591 (2005).
Boon, R. et al. Amino acid levels determine metabolism and CYP450 function of hepatocytes and hepatoma cell lines. Nat. Commun. 11, 1393 (2020).
Chao, P., Maguire, T., Novik, E., Cheng, K. C. & Yarmush, M. L. Evaluation of a microfluidic based cell culture platform with primary human hepatocytes for the prediction of hepatic clearance in human. Biochem. Pharmacol. 78, 625–632 (2009).
Goral, V. N. et al. Perfusion-based microfluidic device for three-dimensional dynamic primary human hepatocyte cell culture in the absence of biological or synthetic matrices or coagulants. Lab Chip 10, 3380–3386 (2010).
Hegde, M. et al. Dynamic interplay of flow and collagen stabilizes primary hepatocytes culture in a microfluidic platform. Lab Chip 14, 2033–2039 (2014).
Lee, P. J., Hung, P. J. & Lee, L. P. An artificial liver sinusoid with a microfluidic endothelial-like barrier for primary hepatocyte culture. Biotechnol. Bioeng. 97, 1340–1346 (2007).
Toh, Y. C. et al. A microfluidic 3D hepatocyte chip for drug toxicity testing. Lab Chip 9, 2026–2035 (2009).
Clark, A. M. et al. A liver microphysiological system of tumor cell dormancy and inflammatory responsiveness is affected by scaffold properties. Lab Chip 17, 156–168 (2016).
Deng, J. et al. A liver-on-a-chip for hepatoprotective activity assessment. Biomicrofluidics 14, 064107 (2020).
Vernetti, L. A. et al. A human liver microphysiology platform for investigating physiology, drug safety, and disease models. Exp. Biol. Med. 241, 101–114 (2016).
Ströbel, S. et al. A 3D primary human cell-based in vitro model of non-alcoholic steatohepatitis for efficacy testing of clinical drug candidates. Sci. Rep. 11, 22765 (2021).
Müller, F. A. & Sturla, S. J. Human in vitro models of nonalcoholic fatty liver disease. Curr. Opin. Toxicol. 16, 9–16 (2019).
Ronaldson-Bouchard, K. et al. Advanced maturation of human cardiac tissue grown from pluripotent stem cells. Nature 556, 239–243 (2018).
Zhao, Y. et al. A platform for generation of chamber-specific cardiac tissues and disease modeling. Cell 176, 913–927.e918 (2019).
Kaisar, M. A. et al. New experimental models of the blood–brain barrier for CNS drug discovery. Expert Opin. Drug Discov. 12, 89–103 (2017).
Herland, A. et al. Distinct contributions of astrocytes and pericytes to neuroinflammation identified in a 3D human blood–brain barrier on a chip. PLoS ONE 11, e0150360 (2016).
Wang, J. D., Khafagy el, S., Khanafer, K., Takayama, S. & ElSayed, M. E. Organization of endothelial cells, pericytes, and astrocytes into a 3D microfluidic in vitro model of the blood–brain barrier. Mol. Pharm. 13, 895–906 (2016).
Wevers, N. R. et al. A perfused human blood–brain barrier on-a-chip for high-throughput assessment of barrier function and antibody transport. Fluids Barriers CNS 15, 23 (2018).
Park, T.-E. et al. Hypoxia-enhanced blood–brain barrier chip recapitulates human barrier function and shuttling of drugs and antibodies. Nat. Commun. 10, 2621 (2019).
Jin, L. Y. et al. Blood–spinal cord barrier in spinal cord injury: a review. J. Neurotrauma 38, 1203–1224 (2021).
Zheng, W. et al. Differentiation of glial cells from hiPSCs: potential applications in neurological diseases and cell replacement therapy. Front. Cell. Neurosci. 12, 239 (2018).
Chiaradia, I. & Lancaster, M. A. Brain organoids for the study of human neurobiology at the interface of in vitro and in vivo. Nat. Neurosci. 23, 1496–1508 (2020).
Eugène, E. et al. An organotypic brain slice preparation from adult patients with temporal lobe epilepsy. J. Neurosci. Methods 235, 234–244 (2014).
Schwarz, N. et al. Long-term adult human brain slice cultures as a model system to study human CNS circuitry and disease. eLife 8, e48417 (2019).
Cakir, B. et al. Engineering of human brain organoids with a functional vascular-like system. Nat. Methods 16, 1169–1175 (2019).
Liu, J., Pan, L., Cheng, X. & Berdichevsky, Y. Perfused drop microfluidic device for brain slice culture-based drug discovery. Biomed. Microdevices 18, 46 (2016).
Wang, Y., Wang, L., Zhu, Y. & Qin, J. Human brain organoid-on-a-chip to model prenatal nicotine exposure. Lab Chip 18, 851–860 (2018).
Sharma, A. D. et al. Engineering a 3D functional human peripheral nerve in vitro using the nerve-on-a-chip platform. Sci. Rep. 9, 8921 (2019).
Hyung, S. et al. A 3D disease and regeneration model of peripheral nervous system-on-a-chip. Sci. Adv. 7, eabd9749 (2021).
Johnson, B. N. et al. 3D printed nervous system on a chip. Lab Chip 16, 1393–1400 (2016).
Benam, K. H. et al. Small airway-on-a-chip enables analysis of human lung inflammation and drug responses in vitro. Nat. Methods 13, 151–157 (2016).
Humayun, M., Chow, C. W. & Young, E. W. K. Microfluidic lung airway-on-a-chip with arrayable suspended gels for studying epithelial and smooth muscle cell interactions. Lab Chip 18, 1298–1309 (2018).
Shrestha, J. et al. A rapidly prototyped lung-on-a-chip model using 3D-printed molds. Organs-on-a-Chip 1, 100001 (2019).
Si, L. et al. A human-airway-on-a-chip for the rapid identification of candidate antiviral therapeutics and prophylactics. Nat. Biomed. Eng. 5, 815–829 (2021).
Zamprogno, P. et al. Second-generation lung-on-a-chip with an array of stretchable alveoli made with a biological membrane. Commun. Biol. 4, 168 (2021).
Ataç, B. et al. Skin and hair on-a-chip: in vitro skin models versus ex vivo tissue maintenance with dynamic perfusion. Lab Chip 13, 3555–3561 (2013).
Lee, S. et al. Construction of 3D multicellular microfluidic chip for an in vitro skin model. Biomed. Microdevices 19, 22 (2017).
Sriram, G. et al. Full-thickness human skin-on-chip with enhanced epidermal morphogenesis and barrier function. Mater. Today 21, 326–340 (2018).
de Haan, P. et al. A versatile, compartmentalised gut-on-a-chip system for pharmacological and toxicological analyses. Sci. Rep. 11, 4920 (2021).
Kasendra, M. et al. Duodenum intestine-chip for preclinical drug assessment in a human relevant model. eLife 9, e50135 (2020).
Abdalkader, R. & Kamei, K. I. Multi-corneal barrier-on-a-chip to recapitulate eye blinking shear stress forces. Lab Chip 20, 1410–1417 (2020).
Bai, J., Fu, H., Bazinet, L., Birsner, A. E. & D’Amato, R. J. A method for developing novel 3D cornea-on-a-chip using primary murine corneal epithelial and endothelial cells. Front. Pharmacol. 11, 453 (2020).
Bennet, D., Estlack, Z., Reid, T. & Kim, J. A microengineered human corneal epithelium-on-a-chip for eye drops mass transport evaluation. Lab Chip 18, 1539–1551 (2018).
Cao, X. et al. Invited review: human air–liquid-interface organotypic airway tissue models derived from primary tracheobronchial epithelial cells — overview and perspectives. In Vitro Cell Dev. Biol. Anim. 57, 104–132 (2021).
Chiba, H., Osanai, M., Murata, M., Kojima, T. & Sawada, N. Transmembrane proteins of tight junctions. Biochim. Biophys. Acta 1778, 588–600 (2008).
Park, J. Y. et al. Development of a functional airway-on-a-chip by 3D cell printing. Biofabrication 11, 015002 (2018).
Stucki, J. D. et al. Medium throughput breathing human primary cell alveolus-on-chip model. Sci. Rep. 8, 14359 (2018).
Gijzen, L. et al. An intestine-on-a-chip model of plug-and-play modularity to study inflammatory processes. SLAS Technol. 25, 585–597 (2020).
van der Helm, M. W. et al. Non-invasive sensing of transepithelial barrier function and tissue differentiation in organs-on-chips using impedance spectroscopy. Lab Chip 19, 452–463 (2019).
Zoio, P., Lopes-Ventura, S. & Oliva, A. Barrier-on-a-chip with a modular architecture and integrated sensors for real-time measurement of biological barrier function. Micromachines 12, 816 (2021).
Ramadan, Q. & Ting, F. C. W. In vitro micro-physiological immune-competent model of the human skin. Lab Chip 16, 1899–1908 (2016).
Yu, Z., Hao, R., Zhang, Y. & Yang, H. in 2021 IEEE 34th Int. Conf. Micro Electro Mechanical Systems (MEMS) 982–985 (IEEE, 2021).
Roberts, N. & Horsley, V. Developing stratified epithelia: lessons from the epidermis and thymus. WIREs Dev. Biol. 3, 389–402 (2014).
Ma, C., Peng, Y., Li, H. & Chen, W. Organ-on-a-chip: a new paradigm for drug development. Trends Pharmacol. Sci. 42, 119–133 (2021).
Viravaidya, K. & Shuler, M. L. Incorporation of 3T3-L1 cells to mimic bioaccumulation in a microscale cell culture analog device for toxicity studies. Biotechnol. Prog. 20, 590–597 (2004).
Renggli, K. & Frey, O. in Organ-on-a-chip (eds Hoeng, J., Bovard, D. & Peitsch, M. C.) 393–427 (Academic, 2020).
Picollet-D’hahan, N., Zuchowska, A., Lemeunier, I. & Le Gac, S. Multiorgan-on-a-chip: a systemic approach to model and decipher inter-organ communication. Trends Biotechnol. 39, 788–810 (2021). This paper provides a comprehensive review of the various applications of multi-OoCs and the different design approaches of coupling individual organ systems.
Lee, J. & Kim, S. Kidney-on-a-chip: a new technology for predicting drug efficacy, interactions, and drug-induced nephrotoxicity. Curr. Drug Metab. 19, 577–583 (2018).
Lee, J., Kim, K. & Kim, S. in Methods in Cell Biology Vol. 146 (eds Doh, J., Fletcher, D. & Piel, J.) 85–104 (Academic, 2018).
Miller, P. G. & Shuler, M. L. Design and demonstration of a pumpless 14 compartment microphysiological system. Biotechnol. Bioeng. 113, 2213–2227 (2016).
Piergiovanni, M., Leite, S. B., Corvi, R. & Whelan, M. Standardisation needs for organ on chip devices. Lab Chip 21, 2857–2868 (2021).
Kilic, T., Navaee, F., Stradolini, F., Renaud, P. & Carrara, S. Organs-on-chip monitoring: sensors and other strategies. Microphysiol. Syst. 2, 1–32 (2018).
EU Science Hub. European Union Reference Laboratory for alternatives to animal testing. EURL ECVAM. Europrean Commission https://ec.europa.eu/jrc/en/eurl/ecvam (2021).
Piergiovanni, M. et al. Organ on Chip: Building a Roadmap Towards Standardisation (European Commission, 2021).
Piergiovanni, M. et al. Putting Science into Standards workshop on standards for organ-on-chip. Stem Cell Rep. 16, 2076–2077 (2021).
Peirsman, A. et al. MISpheroID: a knowledgebase and transparency tool for minimum information in spheroid identity. Nat. Methods 18, 1294–1303 (2021).
Greaney, A. M. et al. Platform effects on regeneration by pulmonary basal cells as evaluated by single-cell RNA sequencing. Cell Rep. 30, 4250–4265.e6 (2020).
Vunjak-Novakovic, G., Ronaldson-Bouchard, K. & Radisic, M. Organs-on-a-chip models for biological research. Cell 184, 4597–4611 (2021). This article is complementary to the present Primer and provides a perspective of how the tissue engineering paradigm, which involves the integration of cells, scaffolds and bioreactors, has been applied to drive the development of OoCs intended for biological research.
Johnson, B. P. et al. A microphysiological approach to evaluate effectors of intercellular hedgehog signaling in development. Front. Cell Dev. Biol. 9, 621442 (2021).
Komeya, M. et al. Pumpless microfluidic system driven by hydrostatic pressure induces and maintains mouse spermatogenesis in vitro. Sci. Rep. 7, 15459 (2017).
Glieberman, A. L. et al. Synchronized stimulation and continuous insulin sensing in a microfluidic human islet on a chip designed for scalable manufacturing. Lab Chip 19, 2993–3010 (2019).
Modena, M. M., Chawla, K., Misun, P. M. & Hierlemann, A. Smart cell culture systems: integration of sensors and actuators into microphysiological systems. ACS Chem. Biol. 13, 1767–1784 (2018).
Liao, Z. et al. Microfluidic chip coupled with optical biosensors for simultaneous detection of multiple analytes: a review. Biosens. Bioelectron. 126, 697–706 (2019).
Hiramoto, K., Ino, K., Nashimoto, Y., Ito, K. & Shiku, H. Electric and electrochemical microfluidic devices for cell analysis. Front. Chem. 7, 396 (2019).
van Meer, B. J. et al. Small molecule absorption by PDMS in the context of drug response bioassays. Biochem. Biophys. Res. Commun. 482, 323–328 (2017).
Kim, S. et al. Anchor-IMPACT: a standardized microfluidic platform for high-throughput antiangiogenic drug screening. Biotechnol. Bioeng. 118, 2524–2535 (2021).
Werner, M. et al. Surface curvature differentially regulates stem cell migration and differentiation via altered attachment morphology and nuclear deformation. Adv. Sci. 4, 1600347 (2017).
Wang, J. D. Development of a 3D in Vitro Model of the Blood–Brain Barrier in Layered Microfluidic Devices. PhD thesis, Univ. of Michigan (2015).
Shin, W., Hinojosa, C. D., Ingber, D. E. & Kim, H. J. Human intestinal morphogenesis controlled by transepithelial morphogen gradient and flow-dependent physical cues in a microengineered gut-on-a-chip. iScience 15, 391–406 (2019).
Albrecht, W. et al. Prediction of human drug-induced liver injury (DILI) in relation to oral doses and blood concentrations. Arch. Toxicol. 93, 1609–1637 (2019).
Park, J. et al. A 3D human triculture system modeling neurodegeneration and neuroinflammation in Alzheimer’s disease. Nat. Neurosci. 21, 941–951 (2018).
Ekert, J. E. et al. Recommended guidelines for developing, qualifying, and implementing complex in vitro models (CIVMs) for drug discovery. SLAS Discov. 25, 1174–1190 (2020).
Kopec, A. K. et al. Microphysiological systems in early stage drug development: perspectives on current applications and future impact. J. Toxicol. Sci. 46, 99–114 (2021).
Chou, D. B. et al. On-chip recapitulation of clinical bone marrow toxicities and patient-specific pathophysiology. Nat. Biomed. Eng. 4, 394–406 (2020).
LaValley, D. J., Miller, P. G. & Shuler, M. L. Pumpless, unidirectional microphysiological system for testing metabolism-dependent chemotherapeutic toxicity. Biotechnol. Prog. 37, e3105 (2021).
McCracken, K. W. et al. Modelling human development and disease in pluripotent stem-cell-derived gastric organoids. Nature 516, 400–404 (2014).
Park, D., Lee, J., Chung, J. J., Jung, Y. & Kim, S. H. Integrating organs-on-chips: multiplexing, scaling, vascularization, and innervation. Trends Biotechnol. 38, 99–112 (2020).
Vila, O. F. et al. Bioengineered optogenetic model of human neuromuscular junction. Biomaterials 276, 121033 (2021).
Habert, R. et al. Concerns about the widespread use of rodent models for human risk assessments of endocrine disruptors. Reproduction 147, R119–R129 (2014).
Sun, H., Jia, Y., Dong, H., Dong, D. & Zheng, J. Combining additive manufacturing with microfluidics: an emerging method for developing novel organs-on-chips. Curr. Opin. Chem. Eng. 28, 1–9 (2020). This review provides an update on the integration of additive manufacturing technologies such as 3D printing to fabricate OoC devices.
Trietsch, S. J., Israëls, G. D., Joore, J., Hankemeier, T. & Vulto, P. Microfluidic titer plate for stratified 3D cell culture. Lab Chip 13, 3548–3554 (2013). This paper presents a stratified 3D cell culture platform with side-by-side gel and liquid lanes in a microtitre plate format. Cells in neighbouring lanes can interact, and analysis can be done in high throughput.
Esch, M. B., Ueno, H., Applegate, D. R. & Shuler, M. L. Modular, pumpless body-on-a-chip platform for the co-culture of GI tract epithelium and 3D primary liver tissue. Lab Chip 16, 2719–2729 (2016).
Loskill, P., Marcus, S. G., Mathur, A., Reese, W. M. & Healy, K. E. μOrgano: a lego®-like plug & play system for modular multi-organ-chips. PLoS ONE 10, e0139587 (2015).
Vollertsen, A. R. et al. Facilitating implementation of organs-on-chips by open platform technology. Biomicrofluidics 15, 051301 (2021).
Castillo-Armengol, J., Fajas, L. & Lopez-Mejia, I. C. Inter-organ communication: a gatekeeper for metabolic health. EMBO Rep. 20, e47903 (2019).
Gancheva, S., Jelenik, T., Álvarez-Hernández, E. & Roden, M. Interorgan metabolic crosstalk in human insulin resistance. Physiol. Rev. 98, 1371–1415 (2018).
Ogurtsova, K. et al. IDF Diabetes Atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res. Clin. Pract. 128, 40–50 (2017).
Yang, J. et al. Integrated gut–liver-on-a-chip platform as an in vitro human model of non-alcoholic fatty liver disease. Preprint at bioRxiv https://doi.org/10.1101/2020.06.10.141606 (2020).
Lee, S. Y. & Sung, J. H. Gut–liver on a chip toward an in vitro model of hepatic steatosis. Biotechnol. Bioeng. 115, 2817–2827 (2018).
Jeon, J.-W., Lee, S. H., Kim, D. & Sung, J. H. In vitro hepatic steatosis model based on gut–liver-on-a-chip. Biotechnol. Prog. 37, e3121 (2021).
Essaouiba, A. et al. Development of a pancreas–liver organ-on-chip coculture model for organ-to-organ interaction studies. Biochem. Eng. J. 164, 107783 (2020).
Bauer, S. et al. Functional coupling of human pancreatic islets and liver spheroids on-a-chip: towards a novel human ex vivo type 2 diabetes model. Sci. Rep. 7, 14620 (2017).
Cho, I. & Blaser, M. J. The human microbiome: at the interface of health and disease. Nat. Rev. Genet. 13, 260–270 (2012).
Tan, H.-Y. & Toh, Y.-C. What can microfluidics do for human microbiome research? Biomicrofluidics 14, 051303 (2020). This review highlights OoC systems that specifically model microbial–mammalian host tissue interactions.
Polini, A. et al. Towards the development of human immune-system-on-a-chip platforms. Drug Discov. Today 24, 517–525 (2019). This review provides an overview of key characteristics of the human immune system and how different aspects of human immunity can be modelled by OoC systems.
Miller, C. P., Shin, W., Ahn, E. H., Kim, H. J. & Kim, D.-H. Engineering microphysiological immune system responses on chips. Trends Biotechnol. 38, 857–872 (2020).
Park, D. et al. High-throughput microfluidic 3D cytotoxicity assay for cancer immunotherapy (CACI-IMPACT Platform). Front. Immunol. 10, 1133 (2019).
Lee, S. et al. Modeling 3D human tumor lymphatic vessel network using high-throughput platform. Adv. Biol. 5, 2000195 (2021).
Hind, L. E., Ingram, P. N., Beebe, D. J. & Huttenlocher, A. Interaction with an endothelial lumen increases neutrophil lifetime and motility in response to P. aeruginosa. Blood 132, 1818–1828 (2018).
Hind, L. E. et al. Immune cell paracrine signaling drives the neutrophil response to A. fumigatus in an infection-on-a-chip model. Cell. Mol. Bioeng. 14, 133–145 (2021).
Weerappuli, P. D. et al. Extracellular trap-mimicking DNA–histone mesostructures synergistically activate dendritic cells. Adv. Healthc. Mater. 8, 1900926 (2019).
Tao, T. et al. Microengineered multi-organoid system from hipscs to recapitulate human liver–islet axis in normal and type 2 diabetes. Adv. Sci. 9, e2103495 (2022).
Yin, F. et al. HiPSC-derived multi-organoids-on-chip system for safety assessment of antidepressant drugs. Lab Chip 21, 571–581 (2021).
Osaki, T., Uzel, S. G. M. & Kamm, R. D. On-chip 3D neuromuscular model for drug screening and precision medicine in neuromuscular disease. Nat. Protoc. 15, 421–449 (2020).
Douville, N. J. et al. Fabrication of two-layered channel system with embedded electrodes to measure resistance across epithelial and endothelial barriers. Anal. Chem. 82, 2505–2511 (2010).
Poussin, C. et al. 3D human microvessel-on-a-chip model for studying monocyte-to-endothelium adhesion under flow — application in systems toxicology. Altex 37, 47–63 (2020).
Mori, N., Morimoto, Y. & Takeuchi, S. Skin integrated with perfusable vascular channels on a chip. Biomaterials 116, 48–56 (2017).
Ong, L. J. Y. et al. A 3D printed microfluidic perfusion device for multicellular spheroid cultures. Biofabrication 9, 045005 (2017).
Asif, A., Kim, K. H., Jabbar, F., Kim, S. & Choi, K. Real-time sensors for live monitoring of disease and drug analysis in microfluidic model of proximal tubule. Microfluid. Nanofluidics 24, 1–10 (2020).
Khoshfetrat Pakazad, S., Savov, A., van de Stolpe, A. & Dekker, R. A novel stretchable micro-electrode array (SMEA) design for directional stretching of cells. J. Micromech. Microeng. 24, 034003 (2014).
Liu, Y. et al. Adipose-on-a-chip: a dynamic microphysiological in vitro model of the human adipose for immune-metabolic analysis in type II diabetes. Lab Chip 19, 241–253 (2019).
Huh, D. et al. Microfabrication of human organs-on-chips. Nat. Protoc. 8, 2135–2157 (2013).
Zhang, W., Zhang, H., Williams, S. E. & Zhou, A. Microfabricated three-electrode on-chip PDMS device with a vibration motor for stripping voltammetric detection of heavy metal ions. Talanta 132, 321–326 (2015).
Lee, B. et al. 3D micromesh-based hybrid bioprinting: multidimensional liquid patterning for 3D microtissue engineering. NPG Asia Mater. 14, 6 (2022).
Kang, H.-W. et al. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat. Biotechnol. 34, 312–319 (2016).
Lee, S.-Y., Kim, D.-S., Kim, E.-S. & Lee, D.-W. Nano-textured polyimide cantilever for enhancing the contractile behavior of cardiomyocytes and its application to cardiac toxicity screening. Sens. Actuators B Chem. 301, 126995 (2019).
Riahi, R. et al. Automated microfluidic platform of bead-based electrochemical immunosensor integrated with bioreactor for continual monitoring of cell secreted biomarkers. Sci. Rep. 6, 24598 (2016).
Schmittlein, C., Meier, R. J., Sauer, L., Liebsch, G. & Wegener, J. Monitoring oxygenation in microfluidic cell culture using 2D sensor foils as growth substrate. PreSens https://www.presens.de/knowledge/publications/application-note/monitoring-oxygenation-in-microfluidic-cell-culture-using-2d-sensor-foils-as-growth-substrate-full-text-1681 (2019).
Zervantonakis, I. K. et al. Three-dimensional microfluidic model for tumor cell intravasation and endothelial barrier function. Proc. Natl Acad. Sci. USA 109, 13515–13520 (2012).
LeCluyse, E. L., Witek, R. P., Andersen, M. E. & Powers, M. J. Organotypic liver culture models: meeting current challenges in toxicity testing. Crit. Rev. Toxicol. 42, 501–548 (2012).
Zhang, S. et al. A robust high-throughput sandwich cell-based drug screening platform. Biomaterials 32, 1229–1241 (2011).
Bircsak, K. M. et al. A 3D microfluidic liver model for high throughput compound toxicity screening in the OrganoPlate®. Toxicology 450, 152667 (2021).
Domansky, K. et al. Perfused multiwell plate for 3D liver tissue engineering. Lab Chip 10, 51–58 (2010).
Boos, J. A., Misun, P. M., Michlmayr, A., Hierlemann, A. & Frey, O. Microfluidic multitissue platform for advanced embryotoxicity testing in vitro. Adv. Sci. 6, 1900294 (2019).
Freag, M. S. et al. Human nonalcoholic steatohepatitis on a chip. Hepatol. Commun. 5, 217–233 (2021).
Herland, A. et al. Quantitative prediction of human pharmacokinetic responses to drugs via fluidically coupled vascularized organ chips. Nat. Biomed. Eng. 4, 421–436 (2020).
Acknowledgements
This work was supported by National Institute of Health (NIH) (U10A214300) awarded to M.L.S.; NIH (UG3EB025765, CA249799, P41 EB027062 and R01HL136414), National Science Foundation (NSF1647837), National Aeronautics and Space Administration (NASA NNX16A069A) and BARDA (75A50121C00017) awarded to G.V.-N.; National Research Foundation of Korea (No. 2021R1A3B1077481) awarded to N.L.J.; Australian Research Council (FT180100157, DP200101658) awarded to Y.-C.T.; and National University of Singapore Graduate School (NUSGS) Integrative Sciences and Engineering Programme (ISEP) scholarship awarded to C.M.L.
Author information
Authors and Affiliations
Contributions
Introduction (E.V., Y.-C.T., O.F. and G.V.-N.); Experimentation (E.V., C.M.L., Y.-C.T., P.d.H., K.R.-B., G.V.-N., J.K. and N.L.J.); Results (Y.-C.T., O.F. and M.L.S.); Applications (C.M.L., Y.-C.T., K.R.-B., G.V.-N., M.L.S., Z.C., H.S.R. and P.H.); Reproducibility and data deposition (O.F., S.T. and P.d.H.); Limitations and optimizations (G.-A.K. and S.T.); Outlook (C.M.L., Y.-C.T., G.V.-N., E.V., G.-A.K. and S.T.); Overview of the Primer (E.V., Y.-C.T., C.M.L. and P.d.H.).
Corresponding authors
Ethics declarations
Competing interests
O.F. is member of the management team of InSphero commercializing 3D organ systems. K.R.-B. and G.V.-N. are co-founders of Tara Biosystems, a Columbia University start-up company commercializing organs-on-a-chip with human heart muscle. M.L.S. is CEO and President of Hesperos, Inc. All other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Methods Primers thanks Mandy Esch, Zhongze Gu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Glossary
- Microsystems technology
-
A set of technologies for fabrication of planar devices having microscale and nanoscale features. Organ-on-a-chip (OoC)-containing microfluidic channels and integrated electrical and non-electrical components are often fabricated using microsystems technology.
- Lithographic pattern transfer
-
A microfabrication technique in which micrometre-sized features are transferred from a mould into a silicone polymer, usually poly(dimethylsiloxane) (PDMS).
- Single-organ systems
-
Organ-on-a-chip systems with only one organ or tissue modelled.
- Multi-organ systems
-
Organ-on-a-chip systems with multiple tissues that are representative of organ systems are being modelled. A fluidic circuit is embedded in the device to connect the different organ compartments and allow for inter-organ communication via soluble paracrine signalling factors.
- Solid organ chips
-
Organ-on-a-chip systems that are representative of parenchymal or mesenchymal organ tissues, such as the liver, tumour, pancreas, bone and cartilage. Cells are often cultured as a 3D tissue mass or embedded in an extracellular matrix (ECM) analogue where they may directly interact with one another and with the culture substrate and medium in a defined manner.
- Barrier tissue chips
-
Organ-on-a-chip systems that are representative of endothelial and epithelial tissues, such as the vascular endothelium, gut, and corneal and skin epithelium, that function as a living barrier to regulate active and/or passive transport of molecules. Cells are often attached to a porous surface separating two different compartments.
- Micro-milling
-
A microfabrication technique in which structures are carved into a solid block of material by a computer-controlled milling tool.
- PDMS-based soft lithography
-
A collection of techniques for replicating structures in the elastic silicone rubber, poly(dimethylsiloxane) (PDMS), or using PDMS stamps to print molecules in patterns onto device surfaces.
- Media perfusion
-
The delivery of cell culture medium to the living elements inside a microchannel using convective fluid flow driven by external forces generated by pumps or gravity, in order to supply nutrients and remove its metabolites.
- Shear stress
-
Friction on a cell surface caused by the moving of fluids over that surface.
- Reynolds number
-
(Re). A dimensionless parameter that describes the ratio of a fluid’s inertial forces to viscous forces. Commonly used as a measure to determine whether fluid flow is laminar or turbulent.
- Péclet number
-
(Pe). A dimensionless parameter that describes the ratio of convective to diffusive mass transport. Commonly used as a measure to determine the primary mode of mass transport.
- Damköhler number
-
(Da). A dimensionless parameter that describes the ratio of diffusion to reactive timescales. Commonly used as a measure to determine the overall efficacy of a reaction process.
- Effective culture time
-
The period of time whereby a cell culture is able to receive sufficient biochemical factors essential for survival and maintenance of its phenotype.
- Transepithelial electrical resistance
-
(TEER). The electrical resistance across a cell layer or tissue layer. Generally used as a measure to determine the overall integrity and permeability of the cell/tissue layer; a higher TEER indicates better integrity or cell confluency.
- Excitation threshold
-
The minimal electrical potential required for the tissue to contract in response to electrical signal.
- Maximum capture rate
-
The highest beat rate that is the maximum rate of synchronous contraction under an electric field voltage corresponding to twice the excitation threshold.
- Donor-to-donor variability
-
Variation in phenotypes and functions of primary cells that are due to inherent genetic and physiological differences (such as age, sex, ethnicity) between the donors.
- Batch-to-batch variability
-
Variation that arises from in vitro cell culture processes, such as cell expansion and differentiation. As cell culture is a batch process (as opposed to a continuous process), variation can arise due to different operators, equipment and reagent sources used across the different batches.
- Line-to-line variability
-
Variation that exists between different induced pluripotent stem cell (iPSC) lines due to the inter-patient heterogeneity from which the iPSC lines are derived.
Rights and permissions
About this article
Cite this article
Leung, C.M., de Haan, P., Ronaldson-Bouchard, K. et al. A guide to the organ-on-a-chip. Nat Rev Methods Primers 2, 33 (2022). https://doi.org/10.1038/s43586-022-00118-6
Accepted:
Published:
DOI: https://doi.org/10.1038/s43586-022-00118-6
This article is cited by
-
Modeling the neuroimmune system in Alzheimer’s and Parkinson’s diseases
Journal of Neuroinflammation (2024)
-
Neuropathogenesis-on-chips for neurodegenerative diseases
Nature Communications (2024)
-
Rapid prototyping of PMMA-based microfluidic spheroid-on-a-chip models using micromilling and vapour-assisted thermal bonding
Scientific Reports (2024)
-
Integration of multiple flexible electrodes for real-time detection of barrier formation with spatial resolution in a gut-on-chip system
Microsystems & Nanoengineering (2024)
-
A high-throughput gut-on-chip platform to study the epithelial responses to enterotoxins
Scientific Reports (2024)