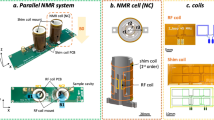
Abstract
Nuclear magnetic resonance (NMR) spectroscopy is a principal analytical technique used for the structure elucidation of molecules. This Primer covers different approaches to accelerate data acquisition and increase sensitivity of NMR measurements through parallelization, enabled by hardware design and/or pulse sequence development. Starting with hardware-based methods, we discuss coupling multiple detectors to multiple samples so each detector/sample combination provides unique information. We then cover spatio-temporal encoding, which uses magnetic field gradients and frequency-selective manipulations to parallelize multidimensional acquisition and compress it into a single shot. We also consider the parallel manipulation of different magnetization reservoirs within a sample to yield new, information-rich pulse schemes using either homonuclear or multinuclear detection. The Experimentation section describes the set-up of parallel NMR techniques. Practical examples revealing improvements in speed and sensitivity offered by the parallel methods are demonstrated in Results. Examples of use of parallelization in small-molecule analysis are discussed in Applications, with experimental constraints addressed under the Limitations and optimizations and Reproducibility and data deposition sections. The most promising future developments are considered in the Outlook, where the largest gains are expected to emerge once the discussed techniques are combined.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
$99.00 per year
only $99.00 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout












Similar content being viewed by others
References
Tucker, T., Marra, M. & Friedman, J. M. Massively parallel sequencing: the next big thing in genetic medicine. Am. J. Hum. Genet. 85, 142–154 (2009).
Roemer, P. B., Edelstein, W. A., Hayes, C. E., Souza, S. P. & Mueller, O. M. The NMR phased array. Magn. Reson. Med. 16, 192–225 (1990).
Pruessmann, K. P., Weiger, M., Scheidegger, M. B. & Boesiger, P. SENSE: sensitivity encoding for fast MRI. Magn. Reson. Med. 42, 952–962 (1999).
Griswold, M. A. et al. Generalized autocalibrating partially parallel acquisitions (GRAPPA). Magn. Reson. Med. 47, 1202–1210 (2002).
Webb, A. G., Sweedler, J. V. & Raftery, D. in On-Line LC-NMR and Related Techniques (ed. Albert, K.) 259–279 (Wiley, 2002).
Webb, A. G. Radiofrequency microcoils for magnetic resonance imaging and spectroscopy. J. Magn. Reson. 229, 55–66 (2013).
Frydman, L., Scherf, T. & Lupulescu, A. The acquisition of multidimensional NMR spectra within a single scan. Proc. Natl Acad. Sci. USA 99, 15858–15862 (2002). This paper introduces the basic idea underlying the execution of 2D NMR acquisitions in a single shot by spatio-temporal encoding.
Gal, M. & Frydman, L. in Multidimensional NMR Methods for the Solution State (eds Morris, G. A. & Emsley, J. W.) 43–60 (Wiley, 2009). This paper includes numerous practical details on how to set up and process 2D homonuclear and heteronuclear NMR acquisitions in commercial spectrometers.
Mishkovsky, M. & Frydman, L. Principles and progress in ultrafast multidimensional nuclear magnetic resonance. Ann. Rev. Phys. Chem. 60, 429–448 (2009).
Tal, A. & Frydman, L. Single-scan multidimensional magnetic resonance. Progr. NMR Spectrosc. 57, 241–292 (2010). This paper contains descriptions of the physical principles underlying the main tools involved in single-scan spatio-temporally encoded NMR and MRI, paying attention to the effects of the frequency-swept manipulations underlying these experiments.
Giraudeau, P. & Frydman, L. Ultrafast 2D NMR: an emerging tool in analytical spectroscopy. Ann. Rev. Anal. Chem. 7, 129–161 (2014). This paper highlights various applications enabled by single-scan 2D NMR for chemical and biophysical scenarios.
Ardenkjaer-Larsen, J. H. et al. Increase in signal-to-noise ratio of >10,000 times in liquid-state NMR. Proc. Natl Acad. Sci. USA 100, 10158–10163 (2003).
Kupče, Ē., Freeman, R. & John, R. B. K. Parallel acquisition of two-dimensional NMR spectra of several nuclear species. J. Am. Chem. Soc 128, 9606–9607 (2006). This paper introduces the direct multinuclear detection and parallel NMR spectroscopy (PANSY) technique, with PANSY–COSY and HETCOR/TOCSY experiments used as a proof of principle.
Kovacs, H. & Kupče, Ē. Parallel NMR spectroscopy with simultaneous detection of 1H and 19F nuclei. Magn. Reson. Chem. 54, 544–560 (2016). This paper modifies various conventional small-molecule experiments to include direct multinuclear detection involving protons and 19F. Basic principles of multinuclear detection techniques are discussed.
Nolis, P., Pérez-Trujillo, M. & Parella, T. Multiple FID acquisition of complementary HMBC data. Angew. Chem. Int. Ed. 46, 7495–7497 (2007).
Kupče, Ē. & Claridge, T. D. W. NOAH: NMR supersequences for small molecule analysis and structure elucidation. Angew. Chem. Int. Ed. 56, 11779–11783 (2017). This paper introduces the NOAH technique, and describes how various heteronuclear and homonuclear 2D NMR experiments can be combined into a supersequence by careful manipulation of magnetization pools.
Ernst, R. R., Bodenhausen, G. & Wokaun, A. Principles of Nuclear Magnetic Resonance in One and Two Dimensions 148–158 (Oxford Univ. Press, 1987).
deGraaf, R. A. In Vivo NMR Spectroscopy, Principles and Techniques 6–8 (Wiley, 1998).
Kovacs, H., Moskau, D. & Spraul, M. Cryogenically cooled probes — a leap in NMR technology. Prog. NMR Spectrosc. 46, 131–155 (2005).
Martin, G. E. Small-sample cryoprobe NMR applications. Encycl. Magnetic Reson. https://doi.org/10.1002/9780470034590.emrstm1300 (2012).
Cheatham, S., Gierth, P., Bermel, W. & Kupče, Ē. HCNMBC — a pulse sequence for H–(C)–N multiple bond correlations at natural isotopic abundance. J. Magn. Reson. 247, 38–41 (2014).
Aramini, J. M., Rossi, P., Anklin, C., Xiao, R. & Montelione, G. T. Microgram-scale protein structure determination by NMR. Nat. Methods 4, 491–493 (2007).
MacNamara, E., Hou, T., Fisher, G., Williams, S. & Raftery, D. Multiplex sample NMR: an approach to high-throughput NMR using a parallel coil probe. Anal. Chim. Acta. 397, 9–16 (1999). This paper shows for the first time that multiple samples can be studied at once using an arrangement in which radiofrequency coils are connected in parallel.
Hou, T., Smith, J., MacNamara, E., Macnaughtan, M. & Raftery, D. Analysis of multiple samples using multiplex sample NMR: selective excitation and chemical shift imaging approaches. Anal. Chem. 73, 2541–2546 (2001).
Macnaughtan, M. A., Hou, T., Xu, J. & Raftery, D. High-throughput nuclear magnetic resonance analysis using a multiple coil flow probe. Anal. Chem. 75, 5116–5123 (2003).
Macnaughtan, M. A., Hou, T., MacNamara, E., Santini, R. & Raftery, D. NMR difference probe: a dual-coil probe for NMR difference spectroscopy. J. Magn. Reson. 156, 97–103 (2002).
Dumez, J.-N. Spatial encoding and spatial selection methods in high-resolution NMR spectroscopy. Prog. Nucl. Magn. Reson. Spectrosc. 116, 101–134 (2018).
Loening, N. M., Thrippleton, M. J., Keeler, J. & Griffin, R. G. Single-scan longitudinal relaxation measurements in high-resolution NMR spectroscopy. J. Magn. Reson. 164, 321–328 (2003).
Pelta, M. D., Morris, G. A., Stchedroff, M. J. & Hammond, S. J. A one-shot sequence for high-resolution diffusion-ordered spectroscopy. Magn. Reson. Chem. 40, S147–S152 (2002).
Zangger, K. Pure shift NMR. Prog. Nucl. Magn. Reson. Spectrosc. 86–87, 1–20 (2015).
Li, Y., Wolters, A., Malaway, P., Sweedler, J. V. & Webb, A. G. Multiple solenoidal microcoil probes for high-sensitivity, high-throughput nuclear magnetic resonance spectroscopy. Anal. Chem. 71, 4815–4820 (1999). This paper is the first to demonstrate that multiple independent coils and samples can be used to acquire high-resolution NMR spectra with full efficiency and S/N.
Zhang, X., Sweedler, J. V. & Webb, A. G. A probe design for the acquisition of homonuclear, heteronuclear, and inverse detected NMR spectra from multiple samples. J. Magn. Reson. 153, 254–258 (2001). This paper is the first to show that heteronuclear 2D NMR spectra can be obtained from different protein samples simultaneously.
Wang, H., Ciobanu, L., Edison, A. S. & Webb, A. G. An eight-coil high-frequency probehead design for high-throughput nuclear magnetic resonance spectroscopy. J. Magn. Reson. 170, 206–212 (2004).
Wolters, A. M., Jayawickrama, D. A., Webb, A. G. & Sweedler, J. V. NMR detection with multiple solenoidal microcoils for continuous-flow capillary electrophoresis. Anal. Chem. 74, 5550–5555 (2002).
Ciobanu, L., Jayawickrama, D. A., Zhang, X., Webb, A. G. & Sweedler, J. V. Measuring reaction kinetics by using multiple microcoil NMR spectroscopy. Angew. Chem. Int. Ed. 42, 4669–4672 (2003).
Frydman, L., Lupulescu, A. & Scherf, T. Principles and features of single-scan two-dimensional NMR spectroscopy. J. Am. Chem. Soc. 125, 9204–9217 (2003).
Shrot, Y. & Frydman, L. Single-scan NMR spectroscopy at arbitrary dimensions. J. Am. Chem. Soc. 125, 11385–11396 (2003).
Jeener, J. Pulse pair technique in high resolution NMR. Lecture presented at Ampere International Summer School II, Basko Polje, Yugoslavia (1971).
Aue, W. P., Bartholdi, E. & Ernst, R. R. Two-dimensional spectroscopy. Application to nuclear magnetic resonance. J. Chem. Phys. 64, 2229–2246 (1976).
Mansfield, P. Spatial mapping of the chemical shift in NMR. Magn. Reson. Med. 1, 370–386 (1984).
Smith, P. E. S. et al. T1 relaxation measurements: probing molecular properties in real time. ChemPhysChem 14, 3138–3145 (2013).
Shrot, Y. & Frydman, L. Single scan 2D DOSY NMR. J. Magn. Reson. 195, 226–231 (2008).
Shrot, Y. & Frydman, L. The effects of molecular diffusion in single-scan 2D NMR. J. Chem. Phys. 128, 164513 (2008).
Yon, M. et al. Diffusion tensor distribution imaging of an in vivo mouse brain at ultra-high magnetic field by spatiotemporal encoding. NMR Biomed, 33, e4355 (2020).
Solomon, E. et al. Diffusion-weighted MR breast imaging with submillimeter resolution and immunity to artifacts by spatio-temporal encoding at 3T. Magn. Reson. Med. 84, 1391–1403 (2020).
Bao, Q., Solomon, E., Liberman, G. & Frydman, L. High-resolution diffusion MRI studies of development in pregnant mice visualized by novel spatiotemporal encoding schemes. NMR Biomed. 33, e4208 (2020).
Cousin, S. F., Liberman, G., Solomon, E., Otikovs, M. & Frydman, L. A regularized reconstruction pipeline for high definition diffusion MRI in challenging regions incorporating a per-shot image correction. Magn. Reson. Med. 81, 3080–3093 (2019).
Liberman, G., Solomon, E., Lustig, M. & Frydman, L. Mulitple coil k-space interpolation enhances resolution in single-shot spatiotemporal MRI. Magn. Reson. Med. 79, 796–805 (2018).
Solomon, E., Liberman, G., Zhang, Z. & Frydman, L. Diffusion MRI measurements in challenging head and brain regions via cross-term spatiotemporally encoding. Sci. Rep. 7, 18010 (2017).
Garbow, J. R., Weitekamp, D. P. & Pines, A. Bilinear rotation decoupling of homonuclear scalar interactions. Chem. Phys. Lett. 93, 504–509 (1982).
Wimperis, S. & Freeman, R. An excitation sequence which discriminates between direct and long-range CH coupling. J. Magn. Reson. 58, 348–353 (1984).
Briand, J. & Sørensen, O. W. Simultaneous and independent rotations with arbitrary flip angles and phases for I, ISα, and ISβ spin systems. J. Magn. Reson. 135, 44–49 (1998).
Kupče, Ē. & Claridge, T. D. W. Molecular structure from a single NMR supersequence. Chem. Commun. 54, 7139–7142 (2018). This paper is the first demonstration of how parallelized NMR experiments can be combined with computer-assisted structural elucidation, allowing unknown molecular frameworks to be determined in a far shorter time than previously possible.
Kupče, Ē. & Claridge, T. D. W. New NOAH modules for structure elucidation at natural isotopic abundance. J. Magn. Reson. 307, 106568 (2019).
Parella, T. & Nolis, P. Time-shared NMR experiments. Concepts Magn. Reson. 36A, 1–23 (2010). This review on time-shared sequences with a focus on small-molecule applications goes into detail about sensitivity considerations as well as numerous experimental variants and improvements on the basic time-shared concept.
Sørensen, O. W. Aspects and prospects of multidimensional time-domain spectroscopy. J. Magn. Reson. 89, 210–216 (1990).
Claridge, T. D. W., Mayzel, M. & Kupče, Ē. Triplet NOAH supersequences optimised for small molecule structure characterisation. Magn. Reson. Chem. 57, 946–952 (2019).
Kakita, V. M. R., Rachineni, K., Bopardikar, M. & Hosur, R. V. NMR supersequences with real-time homonuclear broadband decoupling: sequential acquisition of protein and small molecule spectra in a single experiment. J. Magn. Reson. 297, 108–112 (2018).
Kakita, V. M. R. & Hosur, R. V. All-in-one NMR spectroscopy of small organic molecules: complete chemical shift assignment from a single NMR experiment. RSC Adv. 10, 21174–21179 (2020).
Cavanagh, J. & Rance, M. Sensitivity-enhanced NMR techniques for the study of biomolecules. Annu. Rep. NMR Spectrosc. 27, 1–58 (1993).
Palmer, A. G., Cavanagh, J., Wright, P. E. & Rance, M. Sensitivity improvement in proton-detected two-dimensional heteronuclear correlation NMR spectroscopy. J. Magn. Reson. 93, 151–170 (1991).
Cavanagh, J. & Rance, M. Sensitivity improvement in isotropic mixing (TOCSY) experiments. J. Magn. Reson. 88, 72–85 (1990).
Nolis, P., Motiram-Corral, K., Pérez-Trujillo, M. & Parella, T. Interleaved dual NMR acquisition of equivalent transfer pathways in TOCSY and HSQC experiments. ChemPhysChem 20, 356–360 (2019).
Nolis, P. & Parella, T. Practical aspects of the simultaneous collection of COSY and TOCSY spectra. Magn. Reson. Chem. 57, S85–S94 (2019).
Nolis, P., Motiram-Corral, K., Pérez-Trujillo, M. & Parella, T. Simultaneous acquisition of two 2D HSQC spectra with different 13C spectral widths. J. Magn. Reson. 300, 1–7 (2019).
Nagy, T. M., Kövér, K. E. & Sørensen, O. W. Double and adiabatic BANGO for concatenating two NMR experiments relying on the same pool of magnetization. J. Magn. Reson. 316, 106767 (2020).
Motiram-Corral, K., Pérez-Trujillo, M., Nolis, P. & Parella, T. Implementing one-shot multiple-FID acquisition into homonuclear and heteronuclear NMR experiments. Chem. Commun. 54, 13507–13510 (2018).
Bermel, W., Bertini, I., Felli, I. C., Piccioli, M. & Pierattelli, R. 13C-detected protonless NMR spectroscopy of proteins in solution. Prog. NMR Spectrosc. 48, 25–45 (2006).
Pervushin, K., Vögeli, B. & Eletsky, A. Longitudinal 1H relaxation optimization in TROSY NMR spectroscopy. J. Am. Chem. Soc. 124, 12898–12902 (2002).
Edison, A. S., Le Guennec, A., Delaglio F. & Kupče, Ē. in NMR-based Metabolomics: Methods and Protocols (eds Gowda, G. A. N. & Raftery, D.) 69–96 (Humana Press, 2019).
Kupče, Ē. & Freeman, R. Molecular structure from a single NMR sequence (fast-PANACEA). J. Magn. Reson. 206, 147–153 (2010).
van de Ven, F. J. M. Multidimensional NMR in Liquids—Basic Principles and Experimental Methods 165–171 (VCH, 1995).
Kitevski-LeBlanc, J. L. & Prosser, R. S. Current applications of 19F NMR to studies of protein structure and dynamics. Prog. NMR Spectrosc. 62, 1–33 (2012).
Wan, Y.-B. & Li, X.-H. Two-dimensional nuclear magnetic resonance spectroscopy with parallel acquisition of 1H–1H and 19F–19F correlations. Chin. J. Anal. Chem. 43, 1203–1209 (2015).
Gonen, O. et al. Simultaneous and interleaved multinuclear chemical-shift imaging, a method for concurrent, localized spectroscopy. J. Magn. Reson. 104B, 26–33 (1994).
Bellstedt, P. et al. Sequential acquisition of multi-dimensional heteronuclear chemical shift correlation spectra with 1H detection. Sci. Rep. 4, 4490 (2014).
Wiedemann, C. et al. Sequential protein NMR assignments in the liquid state via sequential data acquisition. J. Magn. Reson. 239, 23–28 (2014).
Gierth, P., Codina, A., Schumann, F., Kovacs, H. & Kupče, Ē. Fast experiments for structure elucidation of small molecules: Hadamard NMR with multiple receivers. Magn. Reson. Chem. 53, 940–944 (2015).
Pudakalakatti, S. M. et al. A fast NMR method for resonance assignments: application to metabolomics. J. Biomol. NMR 58, 165–173 (2014).
Pudakalakatti, S. M., Dubey, A. & Atreya, H. S. Simultaneous acquisition of three NMR spectra in a single experiment for rapid resonance assignments in metabolomics. J. Chem. Sci. 127, 1091–1097 (2015).
Kupče, Ē. & Freeman, R. Resolving ambiguities in two-dimensional NMR spectra: the ‘TILT’ experiment. J. Magn. Reson. 172, 329–332 (2005).
Freeman, R. & Kupče, Ē. Distant echoes of the accordion: reduced dimensionality, GFT-NMR, and projection-reconstruction of multidimensional spectra. Concepts Magn. Reson. 23A, 63–75 (2008).
Kupče, Ē. & Freeman, R. Molecular structure from a single NMR experiment. J. Am. Chem. Soc 130, 10788–10792 (2008). This paper introduces the idea of constructing the PANACEA pulse scheme that allows the structure of small organic molecules to be determined unambiguously from a single NMR experiment.
Bax, A., Freeman, R. & Kempsell, S. P. Natural abundance carbon-13–carbon-13 coupling observed via double-quantum coherence. J. Am. Chem. Soc. 102, 4849–4851 (1980).
Meissner, A. & Sørensen, O. W. Exercise in modern NMR pulse sequence design: INADEQUATE CR. Concepts Magn. Reson. 14, 141–154 (2002).
Kupče, Ē., Nishida, T. & Freeman, R. Hadamard NMR spectroscopy. Prog. NMR Spectrosc. 42, 95–122 (2003).
Jeannerat, D. Rapid multidimensional NMR: high resolution by spectral aliasing. Ency. Magn. Reson. https://doi.org/10.1002/9780470034590.emrstm1187 (2011).
Kupče, Ē. & Wrackmeyer, B. Multiple receiver experiments for NMR spectroscopy of organosilicon compounds. Appl. Organometal. Chem. 24, 837–841 (2010).
Claridge, T. D. W. High-resolution NMR Techniques in Organic Chemistry 3rd edn 171–202 (Elsevier, 2016).
Elyashberg, M. E., Williams, A. J. & Martin, G. E. Computer-assisted structure verification and elucidation tools in NMR-based structure elucidation. Prog. NMR Spectrosc. 53, 1–104 (2008).
Sattler, M., Maurer, M., Schleucher, J. & Griesinger, C. A simultaneous 15N,1H- and 13C,1H-HSQC with sensitivity enhancement and a heteronuclear gradient echo. J. Biomol. NMR 5, 97–102 (1995). This paper is an early demonstration of how simultaneous acquisition can be used to accelerate acquisition of heteronuclear correlation spectra, and also provides an illuminating discussion of the theory underpinning the construction of pulse sequence elements for time-shared experiments.
Nolis, P., Pérez-Trujillo, M. & Parella, T. Time-sharing evolution and sensitivity enhancements in 2D HSQC–TOCSY and HSQMBC experiments. Magn. Reson. Chem. 44, 1031–1036 (2006).
Haasnoot, C. A. G., van de Ven, F. J. M. & Hilbers, C. W. COCONOSY. Combination of 2D correlated and 2D nuclear Overhauser enhancement spectroscopy in a single experiment. J. Magn. Reson. 56, 343–349 (1984).
Gurevich, A. Z., Barsukov, I. L., Arseniev, A. S. & Bystrov, V. F. Combined COSY–NOESY experiment. J. Magn. Reson. 56, 471–478 (1984).
Viegas, A. et al. UTOPIA NMR: activating unexploited magnetization using interleaved low-gamma detection. J. Biomol. NMR 64, 9–15 (2016).
Schiavina, M. et al. Taking simultaneous snapshots of intrinsically disordered proteins in action. Biophys. J. 117, 46–55 (2019).
Kupče, Ē. & Freeman, R. High-resolution NMR correlation experiments in a single measurement (HR-PANACEA). Magn. Reson. Chem. 48, 333–336 (2010).
Giraudeau, P., Shrot, Y. & Frydman, L. Multiple ultrafast, broadband 2D NMR spectra of hyperpolarized natural products. J. Am. Chem. Soc. 131, 13902–13903 (2009).
Donovan, K. J., Kupče, Ē. & Frydman, L. Multiple parallel 2D NMR acquisitions in a single scan. Angew. Chem. Int. Ed. 52, 4152–4155 (2013).
Purea, A., Neuberger, T. & Webb, A. G. Simultaneous NMR microimaging of multiple single-cell samples. Concepts Magn. Reson. 22B, 7–14 (2004).
Lee, H., Sun, E., Ham, D. & Weissleder, R. Chip-NMR biosensor for detection and molecular analysis of cells. Nat. Med. 14, 869–874 (2008).
Shapira, B., Shetty, K., Brey, W. W., Gan, Z. & Frydman, L. Single scan 2D NMR spectroscopy on a 25 T bitter magnet. Chem. Phys. Lett. 442, 478–482 (2007).
Gal, M., Mishkovsky, M. & Frydman, L. Real-time monitoring of chemical transformations by ultrafast 2D NMR spectroscopy. J. Am. Chem. Soc. 128, 951–956 (2006).
Herrera, A. et al. Real-time monitoring of organic reactions with two-dimensional ultrafast TOCSY NMR spectroscopy. Angew. Chem. Int. Ed. 48, 6274–6277 (2009).
Pardo, Z. D. et al. Monitoring mechanistic details in the synthesis of pyrimidines via real-time, ultrafast multidimensional NMR spectroscopy. J. Am. Chem. Soc. 134, 2706–2715 (2012).
Queiroz, L. H. K. Jr., Giraudeau, P., dos Santos, F. A. B., Oliveira, K. T. & Ferreira, A. G. Real-time mechanistic monitoring of an acetal hydrolysis using ultrafast 2D NMR. Magn. Reson. Chem. 50, 496–501 (2012).
Gal, M., Schanda, P., Brutscher, B. & Frydman, L. UltraSOFAST HMQC NMR and the repetitive acquisition of 2D protein spectra at Hz rates. J. Am. Chem. Soc. 129, 1372–1377 (2007).
Gal, M., Kern, T., Schanda, P., Frydman, L. & Brutscher, B. An improved ultrafast 2D NMR experiment: towards atom-resolved real-time studies of protein kinetics at multi-Hz rates. J. Biomol. NMR. 43, 1–10 (2009).
Shapira, B., Karton, A., Aronzon, D. & Frydman, L. Real-time 2D NMR identification of analytes undergoing continuous chromatographic separation. J. Am. Chem. Soc. 126, 1262–1265 (2004).
Queiroz, L. H. K. Jr., Queiroz, D. P. K., Dhooghe, L., Ferreira, A. G. & Giraudeau, P. Real-time separation of natural products by ultrafast 2D NMR coupled to on-line HPLC. Analyst 137, 2357–2361 (2012).
Shapira, B., Morris, E., Muszkat, A. K. & Frydman, L. Sub-second 2D NMR spectroscopy at sub-mM concentrations. J. Am. Chem. Soc. 126, 11756–11757 (2004).
Frydman, L. & Blazina, D. Ultrafast two-dimensional nuclear magnetic resonance spectroscopy of hyperpolarized solutions. Nat. Phys. 3, 415–419 (2007).
Mishkovsky, M. & Frydman, L. Progress in hyperpolarized ultrafast 2D NMR spectroscopy. ChemPhysChem. 9, 2340–2348 (2008).
Lloyd, L. S. et al. Utilization of SABRE-derived hyperpolarization to detect low-concentration analytes via 1D and 2D NMR methods. J. Am. Chem. Soc. 134, 12904–12907 (2012).
Shrot, Y. & Frydman, L. Spatially-resolved multidimensional NMR spectroscopy within a single scan. J. Magn. Reson. 167, 42–48 (2004).
Tal, A. & Frydman, L. Spectroscopic imaging from spatially-encoded single-scan multidimensional MRI data. J. Magn. Reson. 189, 46–58 (2007).
Schmidt, R. & Frydman, L. In vivo 3D spatial/1D spectral imaging by spatiotemporal encoding: a new single-shot experimental and processing approach. Magn. Reson. Med. 70, 382–391 (2013).
Schmidt, R. et al. In vivo single-shot 13C spectroscopic imaging of hyperpolarized metabolites by spatiotemporal encoding. J. Magn. Reson. 240, 8–15 (2014).
Solomon, E. et al. Removing silicone artifacts in diffusion-weighted breast MRI via shift-resolved spatiotemporally encoding. Magn. Reson. Med. 75, 2064–2071 (2016).
Jacquemmoz, C., Giraud, F. & Dumez, J.-N. Online reaction monitoring by single-scan 2D NMR under flow conditions. Analyst 145, 478–485 (2020).
Rouger, L. et al. Ultrafast acquisition of 1H–1H dipolar correlation experiments in spinning elastomers. J. Magn. Reson. 277, 30–35 (2017).
Kiryutin, A. S. et al. Ultrafast single-scan 2DNMR spectroscopic detection of a PHIP–hyperpolarized protease inhibitor. Chem. Eur. J. 25, 4025–4230 (2019).
Gouilleux, B. et al. High-throughput authentication of edible oils with benchtop ultrafast 2D NMR. Food Chem. 244, 153–158 (2018).
Kupče, Ē. & Sørensen, O. W. 2BOB — extracting an H2BC and an HSQC-type spectrum from the same data set, and H2OBC — a fast experiment delineating the protonated 13C backbone. Magn. Reson. Chem. 55, 515–518 (2017).
Nagy, T. M., Gyöngyösi, T., Kövér, K. E., Sørensen, O. W. & BANGO, S. E. A. XLOC/HMBC–H2OBC: complete heteronuclear correlation within minutes from one NMR pulse sequence. Chem. Commun. 55, 12208–12211 (2019).
Bingol, K., Li, D. W., Zhang, B. & Bruschweiler, R. Comprehensive metabolite identification strategy using multiple two-dimensional NMR spectra of a complex mixture implemented in the COLMARm web server. Anal. Chem. 88, 12411–12418 (2016).
Xella, S. et al. Embryo quality and implantation rate in two different culture media: ISM1 versus universal IVF medium. Fertil. Steril. 93, 1859–1863 (2010).
Kupče, Ē., Kay, L. E. & Freeman, R. Detecting the afterglow of 13C NMR in proteins using multiple receivers. J. Am. Chem. Soc. 132, 18008–18011 (2010).
Kupče, Ē. & Kay, L. E. Parallel acquisition of multi-dimensional spectra in protein NMR. J. Biomol. NMR 54, 1–7 (2012).
Marchand, J. et al. A multidimensional 1H NMR lipidomics workflow to address chemical food safety issues. Metabolomics 14, 60 (2018).
Jézéquel, T. et al. Absolute quantification of metabolites in tomato fruit extracts by fast 2D NMR. Metabolomics 11, 1231–1242 (2015).
Pupier, M. et al. NMReDATA, a standard to report the NMR assignment and parameters of organic compounds. Magn. Reson. Chem. 56, 703–715 (2018).
Hall, S. R. The STAR file: a new format for electronic data transfer and archiving. J. Chem. Inf. Model. 31, 326–333 (1991).
Hall, S. R. & Spadaccini, N. The STAR file: detailed specifications. J. Chem. Inf. Model. 34, 505–508 (1994).
Spadaccini, N. & Hall, S. R. Extensions to the STAR file syntax. J. Chem. Inf. Model. 52, 1901–1906 (2012).
Ulrich, E. L. et al. BioMagResBank. Nucleic Acids Res. 36, D402–D408 (2007).
Ulrich, E. L. et al. NMR-STAR: comprehensive ontology for representing, archiving and exchanging data from nuclear magnetic resonance spectroscopic experiments. J. Biomol. NMR 73, 5–9 (2019).
Schober, D. et al. nmrML: a community supported open data standard for the description, storage, and exchange of NMR data. Anal. Chem. 90, 649–656 (2018).
Kautz, R. A., Goetzinger, W. K. & Karger, B. L. High-throughput microcoil NMR of compound libraries using zero-dispersion segmented flow analysis. J. Comb. Chem. 7, 14–20 (2005).
Shapira, B., Lupulescu, A., Shrot, Y. & Frydman, L. Line shape considerations in ultrafast 2D NMR. J. Magn. Reson. 166, 152–163 (2004).
Mishkovsky, M. & Frydman, L. Interlaced Fourier transformation of ultrafast 2D NMR data. J. Magn. Reson. 173, 344–350 (2005).
Mishkovsky, M., Kupče, Ē. & Frydman, L. Ultrafast-based projection-reconstruction 3D nuclear magnetic resonance spectroscopy. J. Chem. Phys. 127, 034507 (2007).
Pérez-Trujillo, M., Nolis, P., Bermel, W. & Parella, T. Optimizing sensitivity and resolution in time-shared NMR experiments. Magn. Reson. Chem. 45, 325–329 (2007).
Mobli, M. & Hoch, J. C. Nonuniform sampling and non-Fourier signal processing methods in multidimensional NMR. Prog. Nucl. Magn. Reson. Spectrosc. 83, 21–41 (2014).
Shaka, A. J., Lee, C. J. & Pines, A. Iterative schemes for bilinear operators; application to spin decoupling. J. Magn. Reson. 77, 274–293 (1988).
Kupče, Ē., Schmidt, P., Rance, M. & Wagner, G. Adiabatic mixing in the liquid state. J. Magn. Reson. 135, 361–367 (1998).
Kupče, Ē. & Freeman, R. Fast multidimensional NMR by polarization sharing. Magn. Reson. Chem. 45, 2–4 (2007).
Furrer, J. A robust, sensitive, and versatile HMBC experiment for rapid structure elucidation by NMR: IMPACT-HMBC. Chem. Commun. 46, 3396 (2010).
Schulze-Sünninghausen, D., Becker, J. & Luy, B. Rapid heteronuclear single quantum correlation NMR spectra at natural abundance. J. Am. Chem. Soc. 136, 1242–1245 (2014).
Spengler, N. et al. Micro-fabricated Helmholtz coil featuring disposable microfluidic sample inserts for applications in nuclear magnetic resonance. J. Micromech. Microeng. 24, 034004 (2014).
Badilita, V. et al. On-chip three dimensional microcoils for MRI at the microscale. Lab Chip 10, 1387–1390 (2010).
Levitt, M. H. Composite pulses. Prog. Nucl. Magn. Reson. Spectrosc. 18, 61–122 (1986).
Khaneja, N., Reiss, T., Kehlet, C., Schulte-Herbrüggen, T. & Glaser, S. J. Optimal control of coupled spin dynamics: design of NMR pulse sequences by gradient ascent algorithms. J. Magn. Reson. 172, 296–305 (2005).
Glaser, S. J. Unitary control in quantum ensembles: maximizing signal intensity in coherent spectroscopy. Science 280, 421–424 (1998).
Glaser, S. J. et al. Training Schrödinger’s cat: quantum optimal control. Eur. Phys. J. D 69, 279 (2015).
Pascal, S. M., Muhandiram, D. R., Yamazaki, T., Formankay, J. D. & Kay, L. E. Simultaneous acquisition of 15N- and 13C-edited NOE spectra of proteins dissolved in H2O. J. Magn. Reson. Ser. B 103, 197–201 (1994).
Xia, Y., Yee, A., Arrowsmith, C. H. & Gao, X. 1HC and 1HN total NOE correlations in a single 3D NMR experiment. 15N and 13C time-sharing in t1 and t2 dimensions for simultaneous data acquisition. J. Biomol. NMR 27, 193–203 (2003).
Xu, Y., Long, D. & Yang, D. Rapid data collection for protein structure determination by NMR spectroscopy. J. Am. Chem. Soc. 129, 7722–7723 (2007).
Otikovs, M., Olsen, G. L., Kupče, Ē. & Frydman, L. Natural abundance, single-scan 13C–13C-based structural elucidations by dissolution DNP NMR. J. Am. Chem. Soc. 141, 1857–1861 (2019).
Gołowicz, D., Kasprzak, P., Orekhov, V. & Kazimierczuk, K. Fast time-resolved NMR with non-uniform sampling. Prog. Nucl. Magn. Reson. Spectrosc. 116, 40–55 (2020).
Webb, A. G. Microcoil nuclear magnetic resonance spectroscopy. J. Pharm. Biomed. Anal. 10, 892–903 (2005).
Acknowledgements
L.F. acknowledges support from the Israel Science Foundation (grants 965/18) and the generosity of the Perlman Family Foundation. L.F. holds the Bertha and Isadore Gudelsky Professorial Chair and heads the Clore Institute for High-Field Magnetic Resonance Imaging and Spectroscopy, whose support is also acknowledged.
Author information
Authors and Affiliations
Contributions
Introduction (A.G.W., L.F., E.K., T.D.W.C. and J.R.J.Y.); Experimentation (A.G.W., L.F., E.K., T.D.W.C. and J.R.J.Y.); Results (A.G.W., L.F., E.K., T.D.W.C. and J.R.J.Y.); Applications (A.G.W., L.F., E.K., T.D.W.C. and J.R.J.Y.); Reproducibility and data deposition (T.D.W.C. and J.R.J.Y.); Limitations and optimizations (A.G.W., L.F., E.K., T.D.W.C. and J.R.J.Y.); Outlook (A.G.W., L.F., E.K., T.D.W.C. and J.R.J.Y.); Overview of the Primer (A.G.W., L.F., E.K., T.D.W.C. and J.R.J.Y.).
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Methods Primers thanks M. Perez Trujillo and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Bruker User Library: https://www.bruker.com/en/services/bruker-user-library.html
CMC-se: https://www.bruker.com/fr/products-and-solutions/mr/nmr-software/cmc-se.html
Supplementary information
Glossary
- Precessional
-
The process by which nuclei spins rotate (precess) about an applied magnetic field.
- Gradient-based spatial encoding
-
Selective excitation in the presence of magnetic field gradients.
- Chemical shifts
-
The resonant frequencies of a nucleus relative to those of a defined chemical group within a reference compound.
- Magnetogyric ratio
-
(γ). The ratio of the magnetic moment of a nucleus to its angular momentum.
- Spectral dimensions
-
Frequency dimensions in nuclear magnetic resonance spectra that will typically reflect chemical shifts and/or coupling constants.
- Mass-limited samples
-
Samples of limited amount; the term is used to distinguish from the situation of low concentration due to poor solubility. The sensitivity of nuclear magnetic resonance measurements of mass-limited samples can be improved by using small-diameter probes and higher sample concentrations, prompting use of the term ‘mass sensitivity’ for small-diameter probes.
- Phase shift
-
A change in the phase of a signal or waveform.
- Transient
-
(Also referred to as a scan). The acquisition of a solitary free induction decay.
- COSY
-
(Correlation spectroscopy). A technique for identifying directly scalar coupled (J-coupled) nuclei, most often protons.
- TOCSY
-
(Total correlation spectroscopy). A technique related to COSY that distributes magnetization within a network of mutually scalar coupled protons so as to group them within a structure.
- HSQC
-
(Heteronuclear single-quantum correlation). An experiment used to correlate an insensitive nucleus (such as 13C or 15N) with its directly attached proton(s) via one-bond scalar coupling.
- HMQC
-
(Heteronuclear multiple-quantum correlation). An experiment closely related to HSQC and HMBC used to correlate an insensitive nucleus (such as 13C or 15N) with its directly attached proton(s) via one-bond scalar coupling.
- Dynamic nuclear polarization
-
A technique that uses unpaired electron spins to boost the nuclear magnetic resonance signal by as much as 100,000.
- Free induction decays
-
(FIDs). The observable nuclear magnetic resonance signals generated by non-equilibrium nuclear spin magnetization precessing about the magnetic field.
- Supersequences
-
Sequences of nuclear magnetic resonance experiments (pulse schemes) with a common relaxation delay.
- Pools of magnetization
-
Subsets of nuclear spins, typically defined by their coupling interactions with other nuclear magnetic resonance-active spins.
- NOAH
-
(Nuclear magnetic resonance by ordered acquisition using 1H detection). An experimental scheme for acquiring multiple experiments in one but requiring only a single relaxation delay.
- Polarization transfer
-
The transfer of nuclear polarization between subsets of nuclear spins.
- Magnetization helices
-
Spatially dependent magnetization patterns, where each chemical site’s magnetization subtends a helix whose pitch is linearly proportional to the site’s chemical shift.
- Echoes
-
Signals that peak as a function of the k-domain variable, according to their indirect-domain chemical shift.
- Recovery delay
-
(Also known as relaxation delay). A time period in which spins recover their equilibrium populations between scans.
- Nucleus editing
-
Recording multiple data sets in which signals from separate pools are phase-labelled relative to one another.
- Isotropic mixing
-
Transfer of x, y and z magnetization components (hence, isotropic) between J-coupled spin systems.
- HMBC
-
(Heteronuclear multiple-bond correlation). An experiment that correlates an insensitive nucleus (such as 13C or 15N) with protons that are remote in a molecular structure (typically within two or three bonds) via their long-range scalar coupling.
- PANSY
-
Parallel acquisition nuclear magnetic resonance spectroscopy.
- Polarization
-
The degree of alignment of nuclear spins with the applied magnetic field that gives rise to an observable nuclear magnetic resonance signal.
- HETCOR
-
(Heteronuclear correlation). A technique for correlating an insensitive nucleus (such as 13C) with neighbouring proton(s) via scalar coupling while using direct detection of the insensitive nucleus.
- PANACEA
-
(Parallel acquisition nuclear magnetic resonance). An all-in-one combination of experimental applications: a method that combines three standard pulse sequences (INADEQUATE, HSQC and HMBC) into a single supersequence.
- INADEQUATE
-
(Incredible natural abundance double-quantum transfer experiment). A method for correlating adjacent insensitive nuclei (typically 13C) via one-bond scalar coupling.
- Linewidths
-
Widths of nuclear magnetic resonance peaks at half height, usually defined in hertz.
- NOESY
-
(Nuclear Overhauser effect spectroscopy). A technique for identifying nuclei, most often protons, that are close in space (typically <5 Å) and, hence, share dipolar coupling.
- ROESY
-
(Rotating-frame Overhauser effect spectroscopy). A technique related to NOESY that is also used to identify spatial proximity between protons.
- Time-domain multiplex
-
A method of interfacing a certain number of coils with a smaller number of receive channels for parallelizing multiple NMR experiments.
Rights and permissions
About this article
Cite this article
Kupče, Ē., Frydman, L., Webb, A.G. et al. Parallel nuclear magnetic resonance spectroscopy. Nat Rev Methods Primers 1, 27 (2021). https://doi.org/10.1038/s43586-021-00024-3
Accepted:
Published:
DOI: https://doi.org/10.1038/s43586-021-00024-3
This article is cited by
-
Artificial intelligence-driven shimming for parallel high field nuclear magnetic resonance
Scientific Reports (2023)
-
In vivo NMR spectroscopy
Nature Reviews Methods Primers (2023)
-
In-cell NMR: recent progresses and future challenges
Rendiconti Lincei. Scienze Fisiche e Naturali (2023)