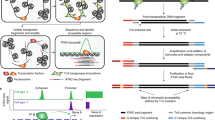
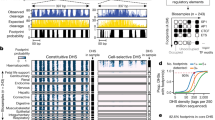
Abstract
Chromatin accessibility, or the physical access to chromatinized DNA, is a widely studied characteristic of the eukaryotic genome. As active regulatory DNA elements are generally ‘accessible’, the genome-wide profiling of chromatin accessibility can be used to identify candidate regulatory genomic regions in a tissue or cell type. Multiple biochemical methods have been developed to profile chromatin accessibility, both in bulk and at the single-cell level. Depending on the method, enzymatic cleavage, transposition or DNA methyltransferases are used, followed by high-throughput sequencing, providing a view of genome-wide chromatin accessibility. In this Primer, we discuss these biochemical methods, as well as bioinformatics tools for analysing and interpreting the generated data, and insights into the key regulators underlying developmental, evolutionary and disease processes. We outline standards for data quality, reproducibility and deposition used by the genomics community. Although chromatin accessibility profiling is invaluable to study gene regulation, alone it provides only a partial view of this complex process. Orthogonal assays facilitate the interpretation of accessible regions with respect to enhancer–promoter proximity, functional transcription factor binding and regulatory function. We envision that technological improvements including single-molecule, multi-omics and spatial methods will bring further insight into the secrets of genome regulation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
$99.00 per year
only $99.00 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Klemm, S. L., Shipony, Z. & Greenleaf, W. J. Chromatin accessibility and the regulatory epigenome. Nat. Rev. Genet. 20, 207–220 (2019).
Kornberg, R. D. Chromatin structure: a repeating unit of histones and DNA. Science 184, 868–871 (1974).
Mazia, D. Enzyme studies on chromosomes. Cold Spring Harb. Symp. Quant. Biol. 9, 40–46 (1941).
Luger, K., Mäder, A. W., Richmond, R. K., Sargent, D. F. & Richmond, T. J. Crystal structure of the nucleosome core particle at 2.8 Å resolution. Nature 389, 251–260 (1997).
Woodcock, C. L., Safer, J. P. & Stanchfield, J. E. Structural repeating units in chromatin. I. Evidence for their general occurrence. Exp. Cell Res. 97, 101–110 (1976).
Lee, C.-K., Shibata, Y., Rao, B., Strahl, B. D. & Lieb, J. D. Evidence for nucleosome depletion at active regulatory regions genome-wide. Nat. Genet. 36, 900–905 (2004).
Ozsolak, F., Song, J. S., Liu, X. S. & Fisher, D. E. High-throughput mapping of the chromatin structure of human promoters. Nat. Biotechnol. 25, 244–248 (2007).
Sheffield, N. C. & Furey, T. S. Identifying and characterizing regulatory sequences in the human genome with chromatin accessibility assays. Genes 3, 651–670 (2012).
The Mouse ENCODE Consortium. et al. A comparative encyclopedia of DNA elements in the mouse genome. Nature 515, 355–364 (2014).
Thurman, R. E. et al. The accessible chromatin landscape of the human genome. Nature 489, 75–82 (2012). This paper represents an extensive map of DHSs, identifying and annotating nearly 3 million DHSs, and thereby demonstrates relationships between chromatin accessibility, transcription and TF binding patterns.
Suzuki, M. M. & Bird, A. DNA methylation landscapes: provocative insights from epigenomics. Nat. Rev. Genet. 9, 465–476 (2008).
Turner, B. M. Defining an epigenetic code. Nat. Cell Biol. 9, 2–6 (2007).
Boija, A. et al. Transcription factors activate genes through the phase-separation capacity of their activation domains. Cell 175, 1842–1855.e16 (2018).
Cook, P. R. The organization of replication and transcription. Science 284, 1790–1795 (1999).
Clapier, C. R., Iwasa, J., Cairns, B. R. & Peterson, C. L. Mechanisms of action and regulation of ATP-dependent chromatin-remodelling complexes. Nat. Rev. Mol. Cell Biol. 18, 407–422 (2017).
Gillette, T. G. & Hill, J. A. Readers, writers, and erasers: chromatin as the whiteboard of heart disease. Circ. Res. 116, 1245–1253 (2015).
Ho, L. & Crabtree, G. R. Chromatin remodelling during development. Nature 463, 474–484 (2010).
Boeger, H., Griesenbeck, J., Strattan, J. S. & Kornberg, R. D. Nucleosomes unfold completely at a transcriptionally active promoter. Mol. Cell 11, 1587–1598 (2003).
Reinke, H. & Hörz, W. Histones are first hyperacetylated and then lose contact with the activated PHO5 promoter. Mol. Cell 11, 1599–1607 (2003).
Chaya, D., Hayamizu, T., Bustin, M. & Zaret, K. S. Transcription factor FoxA (HNF3) on a nucleosome at an enhancer complex in liver chromatin. J. Biol. Chem. 276, 44385–44389 (2001).
Cirillo, L. A. & Zaret, K. S. An early developmental transcription factor complex that is more stable on nucleosome core particles than on free DNA. Mol. Cell 4, 961–969 (1999).
Sherwood, R. I. et al. Discovery of directional and nondirectional pioneer transcription factors by modeling DNase profile magnitude and shape. Nat. Biotechnol. 32, 171–178 (2014).
Zaret, K. S. & Carroll, J. S. Pioneer transcription factors: establishing competence for gene expression. Genes Dev. 25, 2227–2241 (2011). This review article describes the main properties of pioneer factors and their important role in establishing the chromatin landscape and in enabling cellular reprogramming.
Zhu, F. et al. The interaction landscape between transcription factors and the nucleosome. Nature 562, 76–81 (2018).
Hendrich, B. & Bickmore, W. Human diseases with underlying defects in chromatin structure and modification. Hum. Mol. Genet. 10, 2233–2242 (2001).
Matsumoto, L. et al. CpG demethylation enhances α-synuclein expression and affects the pathogenesis of Parkinson’s disease. PLoS ONE 5, e15522 (2010).
Schwartzentruber, J. et al. Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature 482, 226–231 (2012).
Vinagre, J. et al. Frequency of TERT promoter mutations in human cancers. Nat. Commun. 4, 2185 (2013).
Maurano, M. T. et al. Systematic localization of common disease-associated variation in regulatory DNA. Science 337, 1190–1195 (2012).
Davis, C. A. et al. The Encyclopedia of DNA Elements (ENCODE): data portal update. Nucleic Acids Res. 46, D794–D801 (2018).
Stunnenberg, H. G. et al. The International Human Epigenome Consortium: a blueprint for scientific collaboration and discovery. Cell 167, 1145–1149 (2016).
Bernstein, B. E. et al. The NIH Roadmap Epigenomics Mapping Consortium. Nat. Biotechnol. 28, 1045–1048 (2010).
Adams, D. et al. BLUEPRINT to decode the epigenetic signature written in blood. Nat. Biotechnol. 30, 224–226 (2012).
Barski, A. et al. High-resolution profiling of histone methylations in the human genome. Cell 129, 823–837 (2007).
Boyle, A. P. et al. High-resolution mapping and characterization of open chromatin across the genome. Cell 132, 311–322 (2008). This study is the first to apply genome-wide sequencing to profile chromatin accessibility, by means of DNase-seq.
Buenrostro, J. D., Giresi, P. G., Zaba, L. C., Chang, H. Y. & Greenleaf, W. J. Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA-binding proteins and nucleosome position. Nat. Methods 10, 1213–1218 (2013).
Corces, M. R. et al. Lineage-specific and single-cell chromatin accessibility charts human hematopoiesis and leukemia evolution. Nat. Genet. 48, 1193–1203 (2016).
Corces, M. R. et al. An improved ATAC-seq protocol reduces background and enables interrogation of frozen tissues. Nat. Methods 14, (2017).
Giresi, P. G., Kim, J., McDaniell, R. M., Iyer, V. R. & Lieb, J. D. FAIRE (formaldehyde-assisted isolation of regulatory elements) isolates active regulatory elements from human chromatin. Genome Res. 17, 877–885 (2007).
Hesselberth, J. R. et al. Global mapping of protein–DNA interactions in vivo by digital genomic footprinting. Nat. Methods 6, 283–289 (2009).
Kelly, T. K. et al. Genome-wide mapping of nucleosome positioning and DNA methylation within individual DNA molecules. Genome Res. 22, 2497–2506 (2012). This study develops the first genome-wide assay for single-molecule chromatin accessibility profiling, relying on methyltransferase enzymes that preferentially modify accessible DNA.
Schones, D. E. et al. Dynamic regulation of nucleosome positioning in the human genome. Cell 132, 887–898 (2008).
Taberlay, P. C. et al. Polycomb-repressed genes have permissive enhancers that initiate reprogramming. Cell 147, 1283–1294 (2011).
Wu, C., Bingham, P. M., Livak, K. J., Holmgren, R. & Elgin, S. C. The chromatin structure of specific genes: I. Evidence for higher order domains of defined DNA sequence. Cell 16, 797–806 (1979).
Weintraub, H. & Groudine, M. Chromosomal subunits in active genes have an altered conformation. Science 193, 848–856 (1976). This pioneering work in the field of regulatory genomics shows that genomic regions of active transcription are particularly sensitive to digestion by DNase I, indicating a more permissive form of the chromatin.
Hewish, D. R. & Burgoyne, L. A. Chromatin sub-structure. The digestion of chromatin DNA at regularly spaced sites by a nuclear deoxyribonuclease. Biochem. Biophys. Res. Commun. 52, 504–510 (1973).
Galas, D. J. & Schmitz, A. DNAse footprinting: a simple method for the detection of protein–DNA binding specificity. Nucleic Acids Res. 5, 3157–3170 (1978). This article establishes DNase footprinting as a method to study the sequence-specific binding of proteins to DNA.
Kemper, B., Jackson, P. D. & Felsenfeld, G. Protein-binding sites within the 5′ DNase I-hypersensitive region of the chicken α d-globin gene. Mol. Cell. Biol. 7, 2059–2069 (1987).
Vierstra, J. et al. Global reference mapping of human transcription factor footprints. Nature 583, 729–736 (2020).
Yan, F., Powell, D. R., Curtis, D. J. & Wong, N. C. From reads to insight: a hitchhiker’s guide to ATAC-seq data analysis. Genome Biol. 21, 22 (2020).
Banerji, J., Rusconi, S. & Schaffner, W. Expression of a β-globin gene is enhanced by remote SV40 DNA sequences. Cell 27, 299–308 (1981).
West, J. A. et al. Nucleosomal occupancy changes locally over key regulatory regions during cell differentiation and reprogramming. Nat. Commun. 5, 4719 (2014). This work shows that changes in nucleosome occupancy during cellular differentiation are enriched at regulatory regions and co-localize with binding sites of key developmental regulators.
Reddington, J. et al. Lineage resolved enhancer and promoter usage during a time-course of embryogenesis. Dev. Cell 55, 648–664 (2020).
Al-Ali, R. et al. Single-nucleus chromatin accessibility reveals intratumoral epigenetic heterogeneity in IDH1 mutant gliomas. Acta Neuropathol. Commun. 7, 201 (2019).
Cusanovich, D. A. et al. The cis-regulatory dynamics of embryonic development at single-cell resolution. Nature 555, 538–542 (2018).
Cusanovich, D. A. et al. A single-cell atlas of in vivo mammalian chromatin accessibility. Cell 174, 1309–1324.e18 (2018).
Fullard, J. F. et al. An atlas of chromatin accessibility in the adult human brain. Genome Res. 28, 1243–1252 (2018).
Jin, W. et al. Genome-wide detection of DNase I hypersensitive sites in single cells and FFPE tissue samples. Nature 528, 142–146 (2015).
Lake, B. B. et al. Integrative single-cell analysis of transcriptional and epigenetic states in the human adult brain. Nat. Biotechnol. 36, 70–80 (2017).
Pijuan-Sala, B. et al. Single-cell chromatin accessibility maps reveal regulatory programs driving early mouse organogenesis. Nat. Cell Biol. 22, 487–497 (2020).
Preissl, S. et al. Single-nucleus analysis of accessible chromatin in developing mouse forebrain reveals cell-type-specific transcriptional regulation. Nat. Neurosci. 21, 432–439 (2018).
Kwasnieski, J. C., Fiore, C., Chaudhari, H. G. & Cohen, B. A. High-throughput functional testing of ENCODE segmentation predictions. Genome Res. 24, 1595–1602 (2014).
Wang, X. et al. High-resolution genome-wide functional dissection of transcriptional regulatory regions and nucleotides in human. Nat. Commun. 9, 5380 (2018).
Bravo González-Blas, C. et al. Identification of genomic enhancers through spatial integration of single-cell transcriptomics and epigenomics. Mol. Syst. Biol. 16, e9438 (2020).
Graybuck, L. T. et al. Enhancer viruses and a transgenic platform for combinatorial cell subclass-specific labeling. Preprint at bioRxiv https://doi.org/10.1101/525014 (2019).
Hafez, D. et al. McEnhancer: predicting gene expression via semi-supervised assignment of enhancers to target genes. Genome Biol. 18, 199 (2017).
Kempfer, R. & Pombo, A. Methods for mapping 3D chromosome architecture. Nat. Rev. Genet. 21, 207–226 (2020).
Moore, J. E., Pratt, H. E., Purcaro, M. J. & Weng, Z. A curated benchmark of enhancer–gene interactions for evaluating enhancer–target gene prediction methods. Genome Biol. 21, 17 (2020).
Ricci, W. A. et al. Widespread long-range cis-regulatory elements in the maize genome. Nat. Plants 5, 1237–1249 (2019).
Ron, G., Globerson, Y., Moran, D. & Kaplan, T. Promoter–enhancer interactions identified from Hi-C data using probabilistic models and hierarchical topological domains. Nat. Commun. 8, 2237 (2017).
Sanyal, A., Lajoie, B. R., Jain, G. & Dekker, J. The long-range interaction landscape of gene promoters. Nature 489, 109–113 (2012).
Song, L. & Crawford, G. E. DNase-seq: a high-resolution technique for mapping active gene regulatory elements across the genome from mammalian cells. Cold Spring Harb. Protoc. 5, 1–12 (2010).
Lu, F. et al. Establishing chromatin regulatory landscape during mouse preimplantation development. Cell 165, 1375–1388 (2016).
Cooper, J., Ding, Y., Song, J. & Zhao, K. Genome-wide mapping of DNase I hypersensitive sites in rare cell populations using single-cell DNase sequencing. Nat. Protoc. 12, 2342–2354 (2017).
Lazarovici, A. et al. Probing DNA shape and methylation state on a genomic scale with DNase I. Proc. Natl Acad. Sci. USA 110, 6376–6381 (2013).
Suck, D., Lahm, A. & Oefner, C. Structure refined to 2A of a nicked DNA octanucleotide complex with DNase I. Nature 332, 464–468 (1988).
He, H. H. et al. Refined DNase-seq protocol and data analysis reveals intrinsic bias in transcription factor footprint identification. Nat. Methods 11, 73–78 (2014).
Sung, M.-H., Baek, S. & Hager, G. L. Genome-wide footprinting: ready for prime time? Nat. Methods 13, 222–228 (2016).
Adey, A. et al. Rapid, low-input, low-bias construction of shotgun fragment libraries by high-density in vitro transposition. Genome Biol. 11, R119 (2010).
Goryshin, I. Y. & Reznikoff, W. S. Tn5 in vitro transposition. J. Biol. Chem. 273, 7367–7374 (1998).
Qu, K. et al. Chromatin accessibility landscape of cutaneous T cell lymphoma and dynamic response to HDAC inhibitors. Cancer Cell 32, 27–41.e4 (2017).
Wu, J. et al. The landscape of accessible chromatin in mammalian preimplantation embryos. Nature 534, 652–657 (2016).
Wu, J. et al. Chromatin analysis in human early development reveals epigenetic transition during ZGA. Nature 557, 256–260 (2018).
Chen, X. et al. ATAC-see reveals the accessible genome by transposase-mediated imaging and sequencing. Nat. Methods 13, 1013–1020 (2016).
Lu, Z., Hofmeister, B. T., Vollmers, C., DuBois, R. M. & Schmitz, R. J. Combining ATAC-seq with nuclei sorting for discovery of cis-regulatory regions in plant genomes. Nucleic Acids Res. 45, e41 (2017).
Meyer, C. A. & Liu, X. S. Identifying and mitigating bias in next-generation sequencing methods for chromatin biology. Nat. Rev. Genet. 15, 709–721 (2014).
Sato, S. et al. Biochemical analysis of nucleosome targeting by Tn5 transposase. Open Biol. 9, 190116 (2019).
Karabacak Calviello, A., Hirsekorn, A., Wurmus, R., Yusuf, D. & Ohler, U. Reproducible inference of transcription factor footprints in ATAC-seq and DNase-seq datasets using protocol-specific bias modeling. Genome Biol. 20, 42 (2019).
Davie, K. et al. Discovery of transcription factors and regulatory regions driving in vivo tumor development by ATAC-seq and FAIRE-seq open chromatin profiling. PLoS Genet. 11, 1–24 (2015).
Montefiori, L. et al. Reducing mitochondrial reads in ATAC-seq using CRISPR/Cas9. Sci. Rep. 7, 2451 (2017).
Sos, B. C. et al. Characterization of chromatin accessibility with a transposome hypersensitive sites sequencing (THS-seq) assay. Genome Biol. 17, 20 (2016).
Chereji, R. V., Bryson, T. D. & Henikoff, S. Quantitative MNase-seq accurately maps nucleosome occupancy levels. Genome Biol. 20, 198 (2019).
Chang, P., Gohain, M., Yen, M.-R. & Chen, P.-Y. Computational methods for assessing chromatin hierarchy. Comput. Struct. Biotechnol. J. 16, 43–53 (2018).
Kensche, P. R. et al. The nucleosome landscape of Plasmodium falciparum reveals chromatin architecture and dynamics of regulatory sequences. Nucleic Acids Res. 44, 2110–2124 (2016).
Lai, W. K. M. & Pugh, B. F. Understanding nucleosome dynamics and their links to gene expression and DNA replication. Nat. Rev. Mol. Cell Biol. 18, 548–562 (2017).
Lai, B. et al. Principles of nucleosome organization revealed by single-cell micrococcal nuclease sequencing. Nature 562, 281–285 (2018).
Carvin, C. D., Dhasarathy, A., Friesenhahn, L. B., Jessen, W. J. & Kladde, M. P. Targeted cytosine methylation for in vivo detection of protein–DNA interactions. Proc. Natl Acad. Sci. USA 100, 7743–7748 (2003).
Jessen, W. J. et al. Mapping chromatin structure in vivo using DNA methyltransferases. Methods 33, 68–80 (2004).
Kladde, M. P., Xu, M. & Simpson, R. T. Direct study of DNA–protein interactions in repressed and active chromatin in living cells. EMBO J. 15, 6290–6300 (1996).
Xu, M., Kladde, M. P., Van Etten, J. L. & Simpson, R. T. Cloning, characterization and expression of the gene coding for a cytosine-5-DNA methyltransferase recognizing GpC. Nucleic Acids Res. 26, 3961–3966 (1998).
Pardo, C. E., Nabilsi, N. H., Darst, R. P. & Kladde, M. P. Integrated DNA methylation and chromatin structural analysis at single-molecule resolution. Methods Mol. Biol. 1288, 123–141 (2015).
Darst, R. P., Nabilsi, N. H., Pardo, C. E., Riva, A. & Kladde, M. P. DNA methyltransferase accessibility protocol for individual templates by deep sequencing. Methods Enzymol. 513, 185–204 (2012).
Shipony, Z. et al. Long-range single-molecule mapping of chromatin accessibility in eukaryotes. Nat. Methods 17, 319–327 (2020).
Krebs, A. R. et al. Genome-wide single-molecule footprinting reveals high RNA polymerase II turnover at paused promoters. Mol. Cell 67, 411–422.e4 (2017).
Sönmezer, C. et al. Molecular co-occupancy identifies transcription factor binding cooperativity in iivo. Mol. Cell 20, S1097–2765 (2020).
Yang, Y. et al. Quantitative and multiplexed DNA methylation analysis using long-read single-molecule real-time bisulfite sequencing (SMRT-BS). BMC Genomics 16, 350 (2015).
Payne, A., Holmes, N., Rakyan, V. & Loose, M. BulkVis: a graphical viewer for Oxford Nanopore bulk FAST5 files. Bioinformatics 35, 2193–2198 (2019).
Tyler, A. D. et al. Evaluation of Oxford Nanopore’s minion sequencing device for microbial whole genome sequencing applications. Sci. Rep. 8, 10931 (2018).
Mahmoud, M., Zywicki, M., Twardowski, T. & Karlowski, W. M. Efficiency of PacBio long read correction by 2nd generation Illumina sequencing. Genomics 111, 43–49 (2019).
Pfeiffer, F. et al. Systematic evaluation of error rates and causes in short samples in next-generation sequencing. Sci. Rep. 8, 10950 (2018).
Amarasinghe, S. L. et al. Opportunities and challenges in long-read sequencing data analysis. Genome Biol. 21, 30 (2020).
Rhoads, A. & Au, K. F. PacBio sequencing and its applications. Genomics Proteom. Bioinforma. 13, 278–289 (2015).
Lee, I. et al. Simultaneous profiling of chromatin accessibility and methylation on human cell lines with nanopore sequencing. Nat. Methods 17, 1191–1199 (2020).
Wang, Y. et al. Single-molecule long-read sequencing reveals the chromatin basis of gene expression. Genome Res. 29, 1329–1342 (2019).
Liu, Y. et al. Accurate targeted long-read DNA methylation and hydroxymethylation sequencing with TAPS. Genome Biol. 21, 54 (2020).
Stergachis, A. B., Debo, B. M., Haugen, E., Churchman, L. S. & Stamatoyannopoulos, J. A. Single-molecule regulatory architectures captured by chromatin fiber sequencing. Science 368, 1449–1454 (2020).
Abdulhay, N. J. et al. Massively multiplex single-molecule oligonucleosome footprinting. eLife 9, e59404 (2020).
Johnson, D. S., Mortazavi, A., Myers, R. M. & Wold, B. Genome-wide mapping of in vivo protein-DNA interactions. Science 316, 1497–1502 (2007).
Mikkelsen, T. S. et al. Genome-wide maps of chromatin state in pluripotent and lineage-committed cells. Nature 448, 553–560 (2007).
Robertson, G. et al. Genome-wide profiles of STAT1 DNA association using chromatin immunoprecipitation and massively parallel sequencing. Nat. Methods 4, 651–657 (2007).
Bannister, A. J. & Kouzarides, T. Regulation of chromatin by histone modifications. Cell Res. 21, 381–395 (2011).
Strahl, B. D. & Allis, C. D. The language of covalent histone modifications. Nature 403, 41–45 (2000).
Bysani, M. et al. ATAC-seq reveals alterations in open chromatin in pancreatic islets from subjects with type 2 diabetes. Sci. Rep. 9, 7785 (2019).
Shu, W., Chen, H., Bo, X. & Wang, S. Genome-wide analysis of the relationships between DNaseI HS, histone modifications and gene expression reveals distinct modes of chromatin domains. Nucleic Acids Res. 39, 7428–7443 (2011).
Lara-Astiaso, D. et al. Chromatin state dynamics during blood formation. Science 345, 943–949 (2014).
Bonn, S. et al. Tissue-specific analysis of chromatin state identifies temporal signatures of enhancer activity during embryonic development. Nat. Genet. 44, 148–156 (2012).
Calo, E. & Wysocka, J. Modification of enhancer chromatin: what, how, and why? Mol. Cell 49, 825–837 (2013).
Roadmap Epigenomics Consortium. et al. Integrative analysis of 111 reference human epigenomes. Nature 518, 317–330 (2015).
Kuo, M. H. & Allis, C. D. In vivo cross-linking and immunoprecipitation for studying dynamic Protein:DNA associations in a chromatin environment. Methods 19, 425–433 (1999).
O’Neill, L. P. & Turner, B. M. Immunoprecipitation of native chromatin: NChIP. Methods 31, 76–82 (2003).
Orlando, V. Mapping chromosomal proteins in vivo by formaldehyde-crosslinked-chromatin immunoprecipitation. Trends Biochem. Sci. 25, 99–104 (2000).
Brind’Amour, J. et al. An ultra-low-input native ChIP–seq protocol for genome-wide profiling of rare cell populations. Nat. Commun. 6, 6033 (2015).
Dahl, J. A. et al. Broad histone H3K4me3 domains in mouse oocytes modulate maternal-to-zygotic transition. Nature 537, 548–552 (2016).
Ng, J.-H. et al. In vivo epigenomic profiling of germ cells reveals germ cell molecular signatures. Dev. Cell 24, 324–333 (2013).
Zhang, B. et al. Allelic reprogramming of the histone modification H3K4me3 in early mammalian development. Nature 537, 553–557 (2016).
Schmidl, C., Rendeiro, A. F., Sheffield, N. C. & Bock, C. ChIPmentation: fast, robust, low-input ChIP–seq for histones and transcription factors. Nat. Methods 12, 963–965 (2015).
Carter, B. et al. Mapping histone modifications in low cell number and single cells using antibody-guided chromatin tagmentation (ACT-seq). Nat. Commun. 10, 3747 (2019).
Harada, A. et al. A chromatin integration labelling method enables epigenomic profiling with lower input. Nat. Cell Biol. 21, 287–296 (2019).
Skene, P. J. & Henikoff, S. An efficient targeted nuclease strategy for high-resolution mapping of DNA binding sites. eLife 6, e21856 (2017).
Kaya-Okur, H. S. et al. CUT&Tag for efficient epigenomic profiling of small samples and single cells. Nat. Commun. 10, 1930 (2019).
Ku, W. L. et al. Single-cell chromatin immunocleavage sequencing (scChIC-seq) to profile histone modification. Nat. Methods 16, 323–325 (2019).
Wang, Q. et al. CoBATCH for high-throughput single-cell epigenomic profiling. Mol. Cell 76, 206–216.e7 (2019).
Hainer, S. J., Boskovic, A., Rando, O. J. & Fazzio, T. G. Profiling of pluripotency factors in individual stem cells and early embryos. Cell https://doi.org/10.1101/286351 (2018).
Rotem, A. et al. Single-cell ChIP–seq reveals cell subpopulations defined by chromatin state. Nat. Biotechnol. 33, 1165–1172 (2015).
Grosselin, K. et al. High-throughput single-cell ChIP–seq identifies heterogeneity of chromatin states in breast cancer. Nat. Genet. 51, 1060–1066 (2019).
Buenrostro, J. D. et al. Single-cell chromatin accessibility reveals principles of regulatory variation. Nature 523, 486–490 (2015). This study is one of the first to perform genome-wide chromatin accessibility profiling at a single-cell level, thus spearheading the now rising use of scATAC-seq.
Chen, X., Miragaia, R. J., Natarajan, K. N. & Teichmann, S. A. A rapid and robust method for single cell chromatin accessibility profiling. Nat. Commun. 9, 5345 (2018).
Cusanovich, D. A. et al. Multiplex single-cell profiling of chromatin accessibility by combinatorial cellular indexing. Science 348, 910–914 (2015). This study is one of the first to perform genome-wide chromatin accessibility profiling at a single-cell level, thus spearheading the now rising use of scATAC-seq.
Lareau, C. A. et al. Droplet-based combinatorial indexing for massive-scale single-cell chromatin accessibility. Nat. Biotechnol. 37, 916–924 (2019).
Satpathy, A. T. et al. Massively parallel single-cell chromatin landscapes of human immune cell development and intratumoral T cell exhaustion. Nat. Biotechnol. 37, 925–936 (2019).
Mezger, A. et al. High-throughput chromatin accessibility profiling at single-cell resolution. Nat. Commun. 9, 3647 (2018).
Domcke, S. et al. A human cell atlas of fetal chromatin accessibility. Science 370, eaba7612 (2020).
Ma, S. et al. Chromatin potential identified by shared single cell profiling of RNA and chromatin. Cell 183, 1103–1116 (2020).
Yin, Y. et al. High-throughput single-cell sequencing with linear amplification. Mol. Cell 76, 676–690.e10 (2019).
Zhu, C. et al. An ultra high-throughput method for single-cell joint analysis of open chromatin and transcriptome. Nat. Struct. Mol. Biol. 26, 1063–1070 (2019).
Lee, J. et al. Kundajelab/atac_dnase_pipelines: 0.3.0. Zenodo https://doi.org/10.5281/ZENODO.156534 (2016).
Ewels, P., Magnusson, M., Lundin, S. & Käller, M. MultiQC: summarize analysis results for multiple tools and samples in a single report. Bioinformatics 32, 3047–3048 (2016).
Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet.Journal 17, 10 (2011).
Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120 (2014).
Aronesty, E. ea-utils, FASTQ processing utilities https://expressionanalysis.github.io/ea-utils/ (2011).
Pass, D. A. et al. Genome-wide chromatin mapping with size resolution reveals a dynamic sub-nucleosomal landscape in Arabidopsis. PLOS Genet. 13, e1006988 (2017).
Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012).
Li, H. & Durbin, R. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 25, 1754–1760 (2009).
Amemiya, H. M., Kundaje, A. & Boyle, A. P. The ENCODE blacklist: identification of problematic regions of the genome. Sci. Rep. 9, 9354 (2019).
Ou, J. et al. ATACseqQC: a Bioconductor package for post-alignment quality assessment of ATAC-seq data. BMC Genomics 19, 169 (2018).
Robinson, J. T. et al. Integrative genomics viewer. Nat. Biotechnol. 29, 24–26 (2011).
Kent, W. J. et al. The human genome browser at UCSC. Genome Res. 12, 996–1006 (2002).
Yates, A. D. et al. Ensembl 2020. Nucleic Acids Res. 48, 682–688 (2019).
Buels, R. et al. JBrowse: a dynamic web platform for genome visualization and analysis. Genome Biol. 17, 66 (2016).
Hofmeister, B. T. & Schmitz, R. J. Enhanced JBrowse plugins for epigenomics data visualization. BMC Bioinforma. 19, 159 (2018).
Zhang, Y. et al. Model-based analysis of ChIP–seq (MACS). Genome Biol. 9, R137 (2008).
Rashid, N. U., Giresi, P. G., Ibrahim, J. G., Sun, W. & Lieb, J. D. ZINBA integrates local covariates with DNA-seq data to identify broad and narrow regions of enrichment, even within amplified genomic regions. Genome Biol. 12, R67 (2011).
Tarbell, E. D. & Liu, T. HMMRATAC: a Hidden Markov ModeleR for ATAC-seq. Nucleic Acids Res. 47, e91–e91 (2019).
Gaspar, J. M. Genrich: detecting sites of genomic enrichment. Github https://github.com/jsh58/Genrich (2018).
Boyle, A. P., Guinney, J., Crawford, G. E. & Furey, T. S. F-Seq: a feature density estimator for high-throughput sequence tags. Bioinformatic. 24, 2537–2538 (2008).
John, S. et al. Chromatin accessibility pre-determines glucocorticoid receptor binding patterns. Nat. Genet. 43, 264–268 (2011).
Koohy, H., Down, T. A., Spivakov, M. & Hubbard, T. A comparison of peak callers used for DNase-seq data. PLoS ONE 9, e96303 (2014).
Boleu, N., Kundaje, A. & Bickel, P. J. Irreproducible Discovery Rate (IDR). https://github.com/nboley/idr (2016).
Li, Q., Brown, J. B., Huang, H. & Bickel, P. J. Measuring reproducibility of high-throughput experiments. Ann. Appl. Stat. 5, 1752–1779 (2011).
Samb, R. et al. Using informative Multinomial-Dirichlet prior in a t-mixture with reversible jump estimation of nucleosome positions for genome-wide profiling. Stat. Appl. Genet. Mol. Biol. 14, 517–532 (2015).
The ENCODE Project Consortium. et al. Expanded encyclopaedias of DNA elements in the human and mouse genomes. Nature 583, 699–710 (2020). This paper summarizes years of effort from the ENCODE project, which have led to a recourse of almost 1 million human and more than 300,000 mouse candidate regulatory elements.
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2010).
Stark, R. & Brown, G. Differential binding analysis of ChIP- Seq peak data. https://bioconductor.org/packages/release/bioc/html/DiffBind.html (2020).
Heinz, S. et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol. Cell 38, 576–589 (2010).
Liang, K. & Keles, S. Detecting differential binding of transcription factors with ChIP–seq. Bioinformatics 28, 121–122 (2012).
Robinson, M. D. & Oshlack, A. A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol. 11, R25 (2010).
Reske, J. J., Wilson, M. R. & Chandler, R. L. ATAC-seq normalization method can significantly affect differential accessibility analysis and interpretation. Epigenetics Chromatin 13, 22 (2020).
Lun, A. T. L. csaw: a Bioconductor package for differential binding analysis of ChIP–seq data using sliding windows. Nucleic Acids Res. 44, e45 (2016).
Ramírez, F. et al. deepTools2: a next generation web server for deep-sequencing data analysis. Nucleic Acids Res. 44, W160–W165 (2016).
Bravo González-Blas, C. et al. cisTopic: cis-regulatory topic modeling on single-cell ATAC-seq data. Nat. Methods 16, 397–400 (2019).
Gandolfi, F. & Tramontano, A. A computational approach for the functional classification of the epigenome. Epigenetics Chromatin 10, 26 (2017).
McLean, C. Y. et al. GREAT improves functional interpretation of cis-regulatory regions. Nat. Biotechnol. 28, 495–501 (2010).
Yu, G., Wang, L.-G. & He, Q.-Y. ChIPseeker: an R/Bioconductor package for ChIP peak annotation, comparison and visualization. Bioinformatics 31, 2382–2383 (2015).
Zhu, L. J. et al. ChIPpeakAnno: a Bioconductor package to annotate ChIP–seq and ChIP-chip data. BMC Bioinforma. 11, 237 (2010).
Chen, E. Y. et al. Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinforma. 14, 128 (2013).
Herrmann, C., Van De Sande, B., Potier, D. & Aerts, S. i-cisTarget: an integrative genomics method for the prediction of regulatory features and cis-regulatory modules. Nucleic Acids Res. 40, e114 (2012).
Imrichová, H., Hulselmans, G., Kalender Atak, Z., Potier, D. & Aerts, S. i-cisTarget 2015 update: generalized cis-regulatory enrichment analysis in human, mouse and fly. Nucleic Acids Res. 43, W57–W64 (2015).
Layer, R. M. et al. GIGGLE: a search engine for large-scale integrated genome analysis. Nat. Methods 15, 123–126 (2018).
Sheffield, N. C. & Bock, C. LOLA: enrichment analysis for genomic region sets and regulatory elements in R and Bioconductor. Bioinformatics 32, 587–589 (2016).
Ernst, J. & Kellis, M. Chromatin-state discovery and genome annotation with ChromHMM. Nat. Protoc. 12, 2478–2492 (2017).
Mammana, A. & Chung, H.-R. Chromatin segmentation based on a probabilistic model for read counts explains a large portion of the epigenome. Genome Biol. 16, 151 (2015).
Hoffman, M. M. et al. Unsupervised pattern discovery in human chromatin structure through genomic segmentation. Nat. Methods 9, 473–476 (2012).
Bailey, T. L. et al. MEME SUITE: tools for motif discovery and searching. Nucleic Acids Res. 37, W202–W208 (2009).
Stormo, G. D., Schneider, T. D., Gold, L. & Ehrenfeucht, A. Use of the ‘Perceptron’ algorithm to distinguish translational initiation sites in E. coli. Nucleic Acids Res. 10, 2997–3011 (1982). This article presents the first use of a position weight matrix, the currently most widely used model for representing binding sites of a TF.
Fornes, O. et al. JASPAR 2020: update of the open-access database of transcription factor binding profiles. Nucleic Acids Res. 48, 87–92 (2019).
Weirauch, M. T. et al. Determination and inference of eukaryotic transcription factor sequence specificity. Cell 158, 1431–1443 (2014).
Wingender, E., Dietze, P., Karas, H. & Knüppel, R. TRANSFAC: a database on transcription factors and their DNA binding sites. Nucleic Acids Res. 24, 238–241 (1996).
Kulakovskiy, I. V. et al. HOCOMOCO: towards a complete collection of transcription factor binding models for human and mouse via large-scale ChIP–seq analysis. Nucleic Acids Res. 46, D252–D259 (2018).
Thomas-Chollier, M. et al. RSAT peak-motifs: motif analysis in full-size ChIP–seq datasets. Nucleic Acids Res. 40, e31–e31 (2012).
Pavesi, G., Mereghetti, P., Mauri, G. & Pesole, G. Weeder Web: discovery of transcription factor binding sites in a set of sequences from co-regulated genes. Nucleic Acids Res. 32, W199–W203 (2004).
Kelley, D. R., Snoek, J. & Rinn, J. L. Basset: learning the regulatory code of the accessible genome with deep convolutional neural networks. Genome Res. 26, 990–999 (2016).
Zhou, J. & Troyanskaya, O. G. Predicting effects of noncoding variants with deep learning-based sequence model. Nat. Methods 12, 931–934 (2015).
Shrikumar, A., Greenside, P. & Kundaje, A. Learning important features through propagating activation differences. Preprint at ArXiv https://arxiv.org/abs/1704.02685 (2017).
Minnoye, L. et al. Cross-species analysis of enhancer logic using deep learning. Genome Res. 30, 1815–1834 (2020).
Baek, S. & Sung, M.-H. in Statistical Genomics Vol. 1418 (eds Mathé, E. & Davis, S.) 225–240 (Springer, 2016).
Neph, S. et al. An expansive human regulatory lexicon encoded in transcription factor footprints. Nature 489, 83–90 (2012).
Piper, J. et al. Wellington: a novel method for the accurate identification of digital genomic footprints from DNase-seq data. Nucleic Acids Res. 41, e201 (2013).
Gusmao, E. G., Dieterich, C., Zenke, M. & Costa, I. G. Detection of active transcription factor binding sites with the combination of DNase hypersensitivity and histone modifications. Bioinformatics 30, 3143–3151 (2014).
Chen, X., Hoffman, M. M., Bilmes, J. A., Hesselberth, J. R. & Noble, W. S. A dynamic Bayesian network for identifying protein-binding footprints from single molecule-based sequencing data. Bioinformatics 26, i334–i342 (2010).
Sung, M.-H., Guertin, M. J., Baek, S. & Hager, G. L. DNase footprint signatures are dictated by factor dynamics and DNA sequence. Mol. Cell 56, 275–285 (2014).
Pique-Regi, R. et al. Accurate inference of transcription factor binding from DNA sequence and chromatin accessibility data. Genome Res. 21, 447–455 (2011).
Yardımcı, G. G., Frank, C. L., Crawford, G. E. & Ohler, U. Explicit DNase sequence bias modeling enables high-resolution transcription factor footprint detection. Nucleic Acids Res. 42, 11865–11878 (2014).
Vierstra, J. & Stamatoyannopoulos, J. A. Genomic footprinting. Nat. Methods 13, 213–221 (2016).
Schwessinger, R. et al. Sasquatch: predicting the impact of regulatory SNPs on transcription factor binding from cell- and tissue-specific DNase footprints. Genome Res. 27, 1730–1742 (2017).
Li, Z. et al. Identification of transcription factor binding sites using ATAC-seq. Genome Biol. 20, 45 (2019).
Quach, B. & Furey, T. S. DeFCoM: analysis and modeling of transcription factor binding sites using a motif-centric genomic footprinter. Bioinformatics 33, 956–963 (2017).
Bentsen, M. et al. ATAC-seq footprinting unravels kinetics of transcription factor binding during zygotic genome activation. Nat. Commun. 11, 4267 (2020).
Chen, K. et al. DANPOS: dynamic analysis of nucleosome position and occupancy by sequencing. Genome Res. 23, 341–351 (2013).
Zhou, X., Blocker, A. W., Airoldi, E. M. & O’Shea, E. K. A computational approach to map nucleosome positions and alternative chromatin states with base pair resolution. eLife 5, e16970 (2016).
Schep, A. N. et al. Structured nucleosome fingerprints enable high-resolution mapping of chromatin architecture within regulatory regions. Genome Res. 25, 1757–1770 (2015).
Zhong, J. et al. Mapping nucleosome positions using DNase-seq. Genome Res. 26, 351–364 (2016).
Baker, S. M., Rogerson, C., Hayes, A. & Sharrocks, A. D. Classifying cells with Scasat — a tool to analyse single-cell ATAC-seq. Nucleic Acids Res. 47, e10 (2017).
de Boer, C. G. & Regev, A. BROCKMAN: deciphering variance in epigenomic regulators by k-mer factorization. BMC Bioinforma. 19, (2018).
Fang, R. et al. Fast and accurate clustering of single cell epigenomes reveals cis-regulatory elements in rare cell types. Preprint at bioRxiv https://doi.org/10.1101/615179 (2019).
Granja, J. M. et al. ArchR: an integrative and scalable software package for single-cell chromatin accessibility analysis. Preprint at bioRxiv https://doi.org/10.1101/2020.04.28.066498 (2020).
Ji, Z., Zhou, W. & Ji, H. Single-cell regulome data analysis by SCRAT. Bioinformatics 33, 2930–2932 (2017).
Pliner, H. A. et al. Cicero predicts cis-regulatory DNA interactions from single-cell chromatin accessibility data. Mol. Cell 71, 858–871.e8 (2018).
Schep, A. N., Wu, B., Buenrostro, J. D. & Greenleaf, W. J. chromVAR: inferring transcription-factor-associated accessibility from single-cell epigenomic data. Nat. Methods 14, 975–978 (2017).
Stuart, T. et al. Comprehensive integration of single-cell data. Cell 177, 1888–1902.e21 (2019).
Zamanighomi, M. et al. Unsupervised clustering and epigenetic classification of single cells. Nat. Commun. 9, 2410 (2018).
Wang, C. et al. Integrative analyses of single-cell transcriptome and regulome using MAESTRO. Genome Biol. 21, 198 (2020).
Fang, R. et al. SnapATAC: a comprehensive analysis package for single cell ATAC-seq. Preprint at bioRxiv https://doi.org/10.1101/615179 (2019).
Chen, H. et al. Assessment of computational methods for the analysis of single-cell ATAC-seq data. Genome Biol. 20, 241 (2019).
Butler, A., Hoffman, P., Smibert, P., Papalexi, E. & Satija, R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat. Biotechnol. 36, 411–420 (2018).
Danese, A., Richter, M. L., Fischer, D. S., Theis, F. J. & Colomé-Tatché, M. EpiScanpy: integrated single-cell epigenomic analysis. Preprint at bioRxiv https://doi.org/10.1101/648097 (2019).
Wolf, F. A., Angerer, P. & Theis, F. J. SCANPY: large-scale single-cell gene expression data analysis. Genome Biol. 19, 15 (2018).
Baek, S. & Lee, I. Single-cell ATAC sequencing analysis: from data preprocessing to hypothesis generation. Comput. Struct. Biotechnol. J. 18, 1429–1439 (2020).
Polański, K. et al. BBKNN: fast batch alignment of single cell transcriptomes. Bioinformatics 36, 964–965 (2019).
Hie, B., Bryson, B. & Berger, B. Efficient integration of heterogeneous single-cell transcriptomes using Scanorama. Nat. Biotechnol. 37, 685–691 (2019).
Lopez, R., Regier, J., Cole, M. B., Jordan, M. I. & Yosef, N. Deep generative modeling for single-cell transcriptomics. Nat. Methods 15, 1053–1058 (2018).
Korsunsky, I. et al. Fast, sensitive and accurate integration of single-cell data with Harmony. Nat. Methods 16, 1289–1296 (2019).
Luecken, M. et al. Benchmarking atlas-level data integration in single-cell genomics. Preprint at bioRxiv https://doi.org/10.1101/2020.05.22.111161 (2020).
Jin, S., Zhang, L. & Nie, Q. scAI: an unsupervised approach for the integrative analysis of parallel single-cell transcriptomic and epigenomic profiles. Genome Biol. 21, 25 (2020).
Chen, H. et al. Single-cell trajectories reconstruction, exploration and mapping of omics data with STREAM. Nat. Commun. 10, 1903 (2019).
Trapnell, C. & Cacchiarelli, D. Monocle. https://github.com/cole-trapnell-lab/monocle-release (2020).
Cao, J. et al. Joint profiling of chromatin accessibility and gene expression in thousands of single cells. Science 361, 1380–1385 (2018). This work establishes the first scalable genome-wide technique that allows simulations profiling transcription and chromatin accessibility on a single-cell level, illustrating the advantage of a multi-omics single-cell assay to link regulatory elements to regulated genes.
Kaplan, N. et al. The DNA-encoded nucleosome organization of a eukaryotic genome. Nature 458, 362–366 (2009).
Segal, E. et al. A genomic code for nucleosome positioning. Nature 442, 772–778 (2006).
Gaffney, D. J. et al. Controls of nucleosome positioning in the human genome. PLoS Genet. 8, e1003036 (2012).
Tillo, D. et al. High nucleosome occupancy is encoded at human regulatory sequences. PLoS ONE 5, e9129 (2010).
Gasperini, M., Tome, J. M. & Shendure, J. Towards a comprehensive catalogue of validated and target-linked human enhancers. Nat. Rev. Genet. 21, 292–310 (2020).
Harrison, M. M., Li, X.-Y., Kaplan, T., Botchan, M. R. & Eisen, M. B. Zelda binding in the early Drosophila melanogaster embryo marks regions subsequently activated at the maternal-to-zygotic transition. PLoS Genet. 7, e1002266 (2011).
Schulz, K. N. et al. Zelda is differentially required for chromatin accessibility, transcription factor binding, and gene expression in the early Drosophila embryo. Genome Res. 25, 1715–1726 (2015).
Sun, Y. et al. Zelda overcomes the high intrinsic nucleosome barrier at enhancers during Drosophila zygotic genome activation. Genome Res. 25, 1703–1714 (2015).
Gao, L. et al. Chromatin accessibility landscape in human early embryos and its association with evolution. Cell 173, 248–259 (2018).
Lee, M. T. et al. Nanog, Pou5f1 and SoxB1 activate zygotic gene expression during the maternal-to-zygotic transition. Nature 503, 360–364 (2013).
Leichsenring, M., Maes, J., Mössner, R., Driever, W. & Onichtchouk, D. Pou5f1 transcription factor controls zygotic gene activation in vertebrates. Science 341, 1005–1009 (2013).
Mayran, A. & Drouin, J. Pioneer transcription factors shape the epigenetic landscape. J. Biol. Chem. 293, 13795–13804 (2018).
Allshire, R. C. & Madhani, H. D. Ten principles of heterochromatin formation and function. Nat. Rev. Mol. Cell Biol. 19, 229–244 (2018).
Zhou, V. W., Goren, A. & Bernstein, B. E. Charting histone modifications and the functional organization of mammalian genomes. Nat. Rev. Genet. 12, 7–18 (2011).
Laurenti, E. & Göttgens, B. From haematopoietic stem cells to complex differentiation landscapes. Nature 553, 418–426 (2018).
Buenrostro, J. D. et al. Integrated single-cell analysis maps the continuous regulatory landscape of human hematopoietic differentiation. Cell 173, 1535–1548.e16 (2018).
Yoshida, H. et al. The cis-regulatory atlas of the mouse immune system. Cell 176, 897–912.e20 (2019).
Gosselin, D. et al. Environment drives selection and function of enhancers controlling tissue-specific macrophage identities. Cell 159, 1327–1340 (2014).
Lavin, Y. et al. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell 159, 1312–1326 (2014).
Satpathy, A. T. et al. Transcript-indexed ATAC-seq for precision immune profiling. Nat. Med. 24, 580–590 (2018).
Wei, G. et al. Global mapping of H3K4me3 and H3K27me3 reveals specificity and plasticity in lineage fate determination of differentiating CD4+ T cells. Immunity 30, 155–167 (2009).
Koues, O. I. et al. Distinct gene regulatory pathways for human innate versus adaptive lymphoid cells. Cell 165, 1134–1146 (2016).
Shih, H.-Y. et al. Developmental acquisition of regulomes underlies innate lymphoid cell functionality. Cell 165, 1120–1133 (2016).
Youngblood, B. et al. Effector CD8 T cells dedifferentiate into long-lived memory cells. Nature 552, 404–409 (2017).
van der Veeken, J. et al. Memory of inflammation in regulatory T cells. Cell 166, 977–990 (2016).
Netea, M. G. et al. Defining trained immunity and its role in health and disease. Nat. Rev. Immunol. 20, 375–388 (2020).
Novakovic, B. et al. β-Glucan reverses the epigenetic state of LPS-induced immunological tolerance. Cell 167, 1354–1368.e14 (2016).
Saeed, S. et al. Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science 345, 1251086 (2014).
Krausgruber, T. et al. Structural cells are key regulators of organ-specific immune responses. Nature 583, 296–302 (2020).
de la Torre-Ubieta, L. et al. The dynamic landscape of open chromatin during human cortical neurogenesis. Cell 172, 289–304.e18 (2018).
Prescott, S. L. et al. Enhancer divergence and cis-regulatory evolution in the human and chimp neural crest. Cell 163, 68–83 (2015).
Trevino, A. E. et al. Chromatin accessibility dynamics in a model of human forebrain development. Science 367, eaay1645 (2020).
Nott, A. et al. Brain cell type-specific enhancer-promoter interactome maps and disease-risk association. Science 366, 1134–1139 (2019).
Yin, S. et al. Transcriptomic and open chromatin atlas of high-resolution anatomical regions in the rhesus macaque brain. Nat. Commun. 11, 474 (2020).
Jia, G. et al. Single cell RNA-seq and ATAC-seq analysis of cardiac progenitor cell transition states and lineage settlement. Nat. Commun. 9, 4877 (2018).
Stone, N. R. et al. Context-specific transcription factor functions regulate epigenomic and transcriptional dynamics during cardiac reprogramming. Cell Stem Cell 25, 87–102.e9 (2019).
Fan, X. et al. Single cell and open chromatin analysis reveals molecular origin of epidermal cells of the skin. Dev. Cell 47, 21–37.e5 (2018).
Dravis, C. et al. Epigenetic and transcriptomic profiling of mammary gland development and tumor models disclose regulators of cell state plasticity. Cancer Cell 34, 466–482.e6 (2018).
Corces, M. R. et al. The chromatin accessibility landscape of primary human cancers. Science 362, eaav1898 (2018).
Beekman, R. et al. The reference epigenome and regulatory chromatin landscape of chronic lymphocytic leukemia. Nat. Med. 24, 868–880 (2018).
Ott, C. J. et al. Enhancer architecture and essential core regulatory circuitry of chronic lymphocytic leukemia. Cancer Cell 34, 982–995.e7 (2018).
Rendeiro, A. F. et al. Chromatin accessibility maps of chronic lymphocytic leukaemia identify subtype-specific epigenome signatures and transcription regulatory networks. Nat. Commun. 7, 11938 (2016).
Yi, G. et al. Chromatin-based classification of genetically heterogeneous AMLs into two distinct subtypes with diverse stemness phenotypes. Cell Rep. 26, 1059–1069.e6 (2019).
Rendeiro, A. F. et al. Chromatin mapping and single-cell immune profiling define the temporal dynamics of ibrutinib response in CLL. Nat. Commun. 11, 577 (2020).
Schmidl, C. et al. Combined chemosensitivity and chromatin profiling prioritizes drug combinations in CLL. Nat. Chem. Biol. 15, 232–240 (2019).
Akhtar-Zaidi, B. et al. Epigenomic enhancer profiling defines a signature of colon cancer. Science 336, 736–739 (2012).
Cohen, A. J. et al. Hotspots of aberrant enhancer activity punctuate the colorectal cancer epigenome. Nat. Commun. 8, 14400 (2017).
Guilhamon, P. et al. Single-cell chromatin accessibility in glioblastoma delineates cancer stem cell heterogeneity predictive of survival. Preprint at bioRxiv https://doi.org/10.1101/370726 (2018).
Tome-Garcia, J. et al. Analysis of chromatin accessibility uncovers TEAD1 as a regulator of migration in human glioblastoma. Nat. Commun. 9, 4020 (2018).
Ooi, W. F. et al. Epigenomic profiling of primary gastric adenocarcinoma reveals super-enhancer heterogeneity. Nat. Commun. 7, 12983 (2016).
Denny, S. K. et al. Nfib promotes metastasis through a widespread increase in chromatin accessibility. Cell 166, 328–342 (2016).
Wang, Z. et al. The open chromatin landscape of non-small cell lung carcinoma. Cancer Res. 79, 4840–4854 (2019).
Riggi, N. et al. EWS-FLI1 utilizes divergent chromatin remodeling mechanisms to directly activate or repress enhancer elements in Ewing sarcoma. Cancer Cell 26, 668–681 (2014).
Tomazou, E. M. et al. Epigenome mapping reveals distinct modes of gene regulation and widespread enhancer reprogramming by the oncogenic fusion protein EWS-FLI1. Cell Rep. 10, 1082–1095 (2015).
Torchia, J. et al. Integrated (epi)-genomic analyses identify subgroup-specific therapeutic targets in CNS RHABDOID tumors. Cancer Cell 30, 891–908 (2016).
Halbritter, F. et al. Epigenomics and single-cell sequencing define a developmental hierarchy in langerhans cell histiocytosis. Cancer Discov. 9, 1406–1421 (2019).
Miller, B. C. et al. Subsets of exhausted CD8+ T cells differentially mediate tumor control and respond to checkpoint blockade. Nat. Immunol. 20, 326–336 (2019).
Sen, D. R. et al. The epigenetic landscape of T cell exhaustion. Science 354, 1165–1169 (2016).
Ghoneim, H. E. et al. De novo epigenetic programs inhibit PD-1 blockade-mediated T cell rejuvenation. Cell 170, 142–157.e19 (2017).
Pauken, K. E. et al. Epigenetic stability of exhausted T cells limits durability of reinvigoration by PD-1 blockade. Science 354, 1160–1165 (2016).
Waldman, A. D., Fritz, J. M. & Lenardo, M. J. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat. Rev. Immunol. 20, 651–668 (2020).
Boyd, M. et al. Characterization of the enhancer and promoter landscape of inflammatory bowel disease from human colon biopsies. Nat. Commun. 9, 1661 (2018).
Ai, R. et al. Comprehensive epigenetic landscape of rheumatoid arthritis fibroblast-like synoviocytes. Nat. Commun. 9, 1921 (2018).
Klein, H.-U. et al. Epigenome-wide study uncovers large-scale changes in histone acetylation driven by tau pathology in aging and Alzheimer’s human brains. Nat. Neurosci. 22, 37–46 (2019).
Bryois, J. et al. Evaluation of chromatin accessibility in prefrontal cortex of individuals with schizophrenia. Nat. Commun. 9, 3121 (2018).
Sun, W. et al. Histone acetylome-wide association study of autism spectrum disorder. Cell 167, 1385–1397.e11 (2016).
Schaub, M. A., Boyle, A. P., Kundaje, A., Batzoglou, S. & Snyder, M. Linking disease associations with regulatory information in the human genome. Genome Res. 22, 1748–1759 (2012).
Xiao, Y., Liu, H., Wu, L., Warburton, M. & Yan, J. Genome-wide association studies in maize: Praise and Stargaze. Mol. Plant 10, 359–374 (2017).
Degner, J. F. et al. DNase I sensitivity QTLs are a major determinant of human expression variation. Nature 482, 390–394 (2012).
Gate, R. E. et al. Genetic determinants of co-accessible chromatin regions in activated T cells across humans. Nat. Genet. 50, 1140–1150 (2018).
Jacobs, J. et al. The transcription factor Grainy head primes epithelial enhancers for spatiotemporal activation by displacing nucleosomes. Nat. Genet. 50, 1011–1020 (2018).
Atak, Z. K. et al. Prioritization of enhancer mutations by combining allele-specific chromatin accessibility with deep learning. Preprint at bioRxiv https://doi.org/10.1101/2019.12.21.885806 (2019).
Roscito, J. G. et al. Phenotype loss is associated with widespread divergence of the gene regulatory landscape in evolution. Nat. Commun. 9, 4737 (2018).
Van de Velde, J., Van Bel, M., Vaneechoutte, D. & Vandepoele, K. A collection of conserved noncoding sequences to study gene regulation in flowering plants. Plant Physiol. 171, 2586–2598 (2016).
Stone, J. R. & Wray, G. A. Rapid evolution of cis-regulatory sequences via local point mutations. Mol. Biol. Evol. 18, 1764–1770 (2001).
Lu, Z. et al. The prevalence, evolution and chromatin signatures of plant regulatory elements. Nat. Plants 5, 1250–1259 (2019).
Maher, K. A. et al. Profiling of accessible chromatin regions across multiple plant species and cell types reveals common gene regulatory principles and new control modules. Plant Cell 30, 15–36 (2018).
Sebé-Pedrós, A. et al. The dynamic regulatory genome of capsaspora and the origin of animal multicellularity. Cell 165, 1224–1237 (2016).
Barrett, T. et al. NCBI GEO: archive for functional genomics data sets—update. Nucleic Acids Res. 41, D991–D995 (2013).
Kolesnikov, N. et al. ArrayExpress update — simplifying data submissions. Nucleic Acids Res. 43, D1113–D1116 (2015).
Leinonen, R., Sugawara, H., Shumway, M. & International Nucleotide Sequence Database Collaboration. The Sequence Read Archive. Nucleic Acids Res. 39, D19–D21 (2011).
Leinonen, R. et al. The European Nucleotide Archive. Nucleic Acids Res. 39, D28–D31 (2011).
Kaminuma, E. et al. DDBJ launches a new archive database with analytical tools for next-generation sequence data. Nucleic Acids Res. 38, D33–D38 (2010).
Lappalainen, I. et al. The European Genome–phenome Archive of human data consented for biomedical research. Nat. Genet. 47, 692–695 (2015).
Mailman, M. D. et al. The NCBI dbGaP database of Genotypes and Phenotypes. Nat. Genet. 39, 1181–1186 (2007).
Bujold, D. et al. The International Human Epigenome Consortium data portal. Cell Syst. 3, 496–499.e2 (2016).
Oki, S. et al. ChIP-Atlas: a data-mining suite powered by full integration of public ChIP–seq data. EMBO Rep. 19, e46255 (2018).
Chèneby, J., Gheorghe, M., Artufel, M., Mathelier, A. & Ballester, B. ReMap 2018: an updated atlas of regulatory regions from an integrative analysis of DNA-binding ChIP–seq experiments. Nucleic Acids Res. 46, D267–D275 (2018).
Raney, B. J. et al. Track data hubs enable visualization of user-defined genome-wide annotations on the UCSC genome browser. Bioinformatics 30, 1003–1005 (2014).
Pijuan-Sala, B. et al. A single-cell molecular map of mouse gastrulation and early organogenesis. Nature 566, 490–495 (2019).
Davie, K. et al. A single-cell transcriptome atlas of the aging Dosophila brain. Cell 174, 982–998.e20 (2018).
David, F. P. A., Litovchenko, M., Deplancke, B. & Gardeux, V. ASAP 2020 update: an open, scalable and interactive web-based portal for (single-cell) omics analyses. Nucleic Acids Res. 48, W403–W414 (2020).
Papatheodorou, I. et al. Expression Atlas update: from tissues to single cells. Nucleic Acids Res. 48, 77–83 (2019).
Avsec, Ž. et al. The Kipoi repository accelerates community exchange and reuse of predictive models for genomics. Nat. Biotechnol. 37, 592–600 (2019).
Creyghton, M. P. et al. Histone H3K27ac separates active from poised enhancers and predicts developmental state. Proc. Natl Acad. Sci. USA 107, 21931–21936 (2010).
Hah, N., Murakami, S., Nagari, A., Danko, C. G. & Kraus, W. L. Enhancer transcripts mark active estrogen receptor binding sites. Genome Res. 23, 1210–1223 (2013).
Wang, D. et al. Reprogramming transcription by distinct classes of enhancers functionally defined by eRNA. Nature 474, 390–394 (2011).
Berest, I. et al. Quantification of differential transcription factor activity and multiomics-based classification into activators and repressors: diffTF. Cell Rep. 29, 3147–3159.e12 (2019).
Colli, M. L. et al. An integrated multi-omics approach identifies the landscape of interferon-α-mediated responses of human pancreatic β cells. Nat. Commun. 11, 2584 (2020).
Chen, S., Lake, B. B. & Zhang, K. High-throughput sequencing of the transcriptome and chromatin accessibility in the same cell. Nat. Biotechnol. 37, 1452–1457 (2019).
Maheshwari, S. et al. Massively parallel simultaneous profiling of the transcriptomic and epigenomic landscape at single cell resolution. 10x Genomics https://pages.10xgenomics.com/rs/446-PBO-704/images/10x_AGBT_Poster_2020_Massively-parallel-simultaneous-profiling-of-the-transcriptomic-and-epigenomic-landscape-at-single-cell-resolution.pdf (2020).
Chen, X. et al. Joint single-cell DNA accessibility and protein epitope profiling reveals environmental regulation of epigenomic heterogeneity. Nat. Commun. 9, 4590 (2018).
Pott, S. Simultaneous measurement of chromatin accessibility, DNA methylation, and nucleosome phasing in single cells. eLife 6, e23203 (2017).
Guo, F. et al. Single-cell multi-omics sequencing of mouse early embryos and embryonic stem cells. Cell Res. 27, 967–988 (2017).
Lhoumaud, P. et al. EpiMethylTag: simultaneous detection of ATAC-seq or ChIP–seq signals with DNA methylation. Genome Biol. 20, 248 (2019).
Spektor, R., Tippens, N. D., Mimoso, C. A. & Soloway, P. D. methyl-ATAC-seq measures DNA methylation at accessible chromatin. Genome Res. 29, 969–977 (2019).
Barnett, K. R. et al. ATAC-Me captures prolonged DNA methylation of dynamic chromatin accessibility loci during cell fate transitions. Mol. Cell 77, 1350–1364.e6 (2020).
Clark, S. J. et al. scNMT-seq enables joint profiling of chromatin accessibility DNA methylation and transcription in single cells. Nat. Commun. 9, 781 (2018).
Argelaguet, R. et al. Multi-Omics Factor Analysis — a framework for unsupervised integration of multi-omics data sets. Mol. Syst. Biol. 14, e8124 (2018).
Rubin, A. J. et al. Coupled single-cell CRISPR screening and epigenomic profiling reveals causal gene regulatory networks. Cell 176, 361–376.e17 (2019).
Thornton, C. A. et al. Spatially-mapped single-cell chromatin accessibility. Preprint at bioRxiv https://doi.org/10.1101/815720 (2019).
Packer, J. & Trapnell, C. Single-cell multi-omics: an engine for new quantitative models of gene regulation. Trends Genet. 34, 653–665 (2018).
Ponnaluri, V. K. C. et al. NicE-seq: high resolution open chromatin profiling. Genome Biol. 18, 122 (2017).
Giresi, P. G. & Lieb, J. D. Isolation of active regulatory elements from eukaryotic chromatin using FAIRE (formaldehyde assisted isolation of regulatory elements). Methods 48, 233–239 (2009).
Lai, B. et al. TrAC-looping measures genome structure and chromatin accessibility. Nat. Methods 15, 741–747 (2018).
Spracklin, G. & Pradhan, S. Protect-seq: genome-wide profiling of nuclease inaccessible domains reveals physical properties of chromatin. Nucleic Acids Res. 48, e16 (2020).
Tchasovnikarova, I. A. et al. Hyperactivation of HUSH complex function by Charcot–Marie–Tooth disease mutation in MORC2. Nat. Genet. 49, 1035–1044 (2017).
Timms, R. T., Tchasovnikarova, I. A. & Lehner, P. J. Differential viral accessibility (DIVA) identifies alterations in chromatin architecture through large-scale mapping of lentiviral integration sites. Nat. Protoc. 14, 153–170 (2019).
Aughey, G. N., Estacio Gomez, A., Thomson, J., Yin, H. & Southall, T. D. CATaDa reveals global remodelling of chromatin accessibility during stem cell differentiation in vivo. eLife 7, e32341 (2018).
Umeyama, T. & Ito, T. DMS-seq for in vivo genome-wide mapping of protein–DNA interactions and nucleosome centers. Cell Rep. 21, 289–300 (2017).
Ishii, H., Kadonaga, J. T. & Ren, B. MPE-seq, a new method for the genome-wide analysis of chromatin structure. Proc. Natl Acad. Sci. USA 112, E3457–E3465 (2015).
Gargiulo, G. et al. NA-seq: a discovery tool for the analysis of chromatin structure and dynamics during differentiation. Dev. Cell 16, 466–481 (2009).
Chen, P. B., Zhu, L. J., Hainer, S. J., McCannell, K. N. & Fazzio, T. G. Unbiased chromatin accessibility profiling by RED-seq uncovers unique features of nucleosome variants in vivo. BMC Genomics 15, 1104 (2014).
Chereji, R. V., Eriksson, P. R., Ocampo, J., Prajapati, H. K. & Clark, D. J. Accessibility of promoter DNA is not the primary determinant of chromatin-mediated gene regulation. Genome Res. 29, 1985–1995 (2019).
Oberbeckmann, E. et al. Absolute nucleosome occupancy map for the Saccharomyces cerevisiae genome. Genome Res. 29, 1996–2009 (2019).
Brogaard, K., Xi, L., Wang, J.-P. & Widom, J. A map of nucleosome positions in yeast at base-pair resolution. Nature 486, 496–501 (2012).
Voong, L. N. et al. Insights into nucleosome organization in mouse embryonic stem cells through chemical mapping. Cell 167, 1555–1570.e15 (2016).
Chereji, R. V., Ramachandran, S., Bryson, T. D. & Henikoff, S. Precise genome-wide mapping of single nucleosomes and linkers in vivo. Genome Biol. 19, 19 (2018).
Flaus, A., Luger, K., Tan, S. & Richmond, T. J. Mapping nucleosome position at single base-pair resolution by using site-directed hydroxyl radicals. Proc. Natl Acad. Sci. USA 93, 1370–1375 (1996).
Pott, S. & Lieb, J. D. Single-cell ATAC-seq: strength in numbers. Genome Biol. 16, 172–172 (2015).
Acknowledgements
This work was supported by the Division of Intramural Research, National Heart, Lung and Blood Institute, National Institutes of Health (NIH) (K.Z.) and the National Science Foundation (NSF; IOS-1856627) (R.J.S). R.J.S. is a Pew Scholar in the Biomedical Sciences, supported by The Pew Charitable Trusts. L.M. was supported by a PhD fellowship from the FWO (no. 1S03317N), A.P.M. by an NSF Postdoctoral Fellowship in Biology (DBI-1905869), C.B. by a European Research Council (ERC) Starting Grant (European Union’s Horizon 2020 research and innovation programme, grant agreement no. 679146), E.E.M.F. by an ERC Advanced grant (DeCRypT) and S.A. by an ERC Consolidator Grant (cis_CONTROL). W.J.G. acknowledges support as a Chan-Zuckerberg Biohub Investigator, and from NIH grants UM1-HG009436, P50-HG007735, UM1-HG009442 and U19-AI057266. The authors thank J. Demeulemeester for insights into the long-read sequencing platforms.
Author information
Authors and Affiliations
Contributions
Introduction (S.A., L.M.); Experimentation (G.K.M., L.P., A.P.M., W.J.G., K.Z., R.J.S.); Results (L.M., S.A., G.K.M., W.J.G.); Applications (G.K.M., T.K., A.P.M., R.J.S., C.B., W.J.G.); Reproducibility and data deposition (A.P.M., R.J.S.); Limitations and optimizations (G.K.M., W.J.G.); Outlook (S.S., E.E.M.F.); Overview of the Primer (S.A., L.M.).
Corresponding author
Ethics declarations
Competing interests
C.B. is an inventor on a patent describing the ChIPmentation assay. R.J.S. is a co-founder of REquest Genomics, LLC, a company that provides epigenomics services. W.J.G is an inventor on a patent describing Assay for Transposase-Accessible Chromatin using sequencing (ATAC-seq), a consultant for 10x Genomics and Guardant Health, and a co-founder of Protillion biosciences. All other authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Methods Primers thanks T. Liu, B. Treutlein, L. Zhu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
ENCODE ATAC-seq Data Standards and Processing Pipeline: https://www.encodeproject.org/atac-seq/
FastQC: http://www.bioinformatics.babraham.ac.uk/projects/fastqc/
Supplementary information
Glossary
- Nucleosomes
-
The basic structural unit of DNA packaging, consisting of ~147 bp of DNA wrapped around an octamer of histones.
- Cis-regulatory elements
-
Non-coding DNA regions involved in the regulation of expression of neighbouring genes. The regions contain binding sites for transcription factors.
- Accessible chromatin
-
A permissive state of the chromatin in which nuclear macromolecules are able to physically access and interact with the DNA.
- Transcriptional condensates
-
Membraneless compartments of the genome formed by liquid–liquid phase separation, in which the transcription machinery is concentrated to efficiently activate transcription.
- Pioneer factors
-
Transcription factors that can recognize and bind their target sequence in closed chromatin and trigger opening of the chromatin, allowing binding of other transcription factors.
- Tagmentation
-
Transposases cut DNA into fragments while simultaneously adding adaptor sequences. Used in Assay for Transposase-Accessible Chromatin using sequencing (ATAC-seq), as well as for general sequencing library construction to randomly fragment double-stranded DNA.
- TF footprinting
-
Small stretches of nucleotides that are protected from cleavage or tagmentation and represent the location of transcription factor (TF) binding sites. TF footprints can be inferred from the analysis of high-resolution chromatin accessibility data.
- Mosaic end adapters
-
Hyperactive versions of the two inverted 19-bp end sequences of the wild-type Tn5 transposon that, during an Assay for Transposase-Accessible Chromatin using sequencing (ATAC-seq) experiment, are end-joined to accessible DNA by the transposase.
- Nucleosome ladder
-
A characteristic ‘ladder’ pattern that originates from the cleavage of the linker DNA between nucleosomes, due to the periodic arrangement of nucleosomes.
- Combinatorial indexing
-
A technique that uniquely labels a large number of single molecules or single cells by split-pool barcoding of nucleic acids.
- Doublets
-
Artefactual libraries generated from two cells in single-cell omics experiments. For instance, in droplet-based methods, doublets arise if two cells are captured in a single droplet.
- BAM file
-
An alignment file format that is the compressed binary version of a SAM file, used to represent aligned sequences.
- Irreproducible discovery rate
-
(IDR). A measure of consistency between biological replicates of high-throughput sequencing experiments. Also used to determine highly stable peak calling thresholds based on reproducibility.
- Genomic intervals
-
Consecutive stretches on a genomic sequence, specified as a chromosomal location range or as a cytoband designation.
- Fraction of reads in called peaks
-
(FRiP score). The fraction of all mapped reads that fall into the called peak regions.
- Signal proportion of tags
-
(SPOT score). The fraction of reads that fall in tag-enriched regions identified using the Hotspot algorithm.
- Signature
-
A set of peaks that is differentially accessible between studied samples and can be used to define a studied cell type or state.
- Differential peak calling
-
A process in which peaks with significantly differentially accessibility between samples are identified.
- MA plots
-
Visual representations of genomic data used to compare two samples or two groups of samples. The x axis represents the base mean value of the samples and the y axis the difference between them.
- Hierarchical clustering and k-means clustering
-
Clustering algorithms that group similar objects in a data set into groups called clusters. In k-means clustering, the data are divided into a predefined number (‘k’) of clusters, whereas in hierarchical clustering, a hierarchy of clusters is built without requiring a predefined number of clusters.
- Pseudo-time trajectory
-
A computational reconstructed path of a dynamic biological process, such as differentiation, undergone by the cells in a single-cell omics experiment. Single cells are ordered along the trajectory based on their ‘pseudo-time’, or their inferred progression through the biological process.
- Zygotic genome activation
-
A process by which transcription is turned on after fertilization, making the switch from an unfertilized oocyte with nearly any gene expression to a state where up to thousands of genes are transcribed.
- Quantitative trait loci
-
(QTL). Small regions of the genome at which a genetic variant is associated with a quantitative trait of a cell or an organism, based on statistical association between genetic markers and the measurable trait.
- Yoruba HapMap
-
A resource set up by the Yoruba HapMap project that aims to catalogue the common patterns of human genetic variation and associate SNPs with genotypes across human populations.
- Hi-C
-
(High-throughput chromosome conformation capture). A genome-wide sequencing technique used to investigate 3D chromatin conformation.
- Chromatin accessibility QTL
-
Quantitative trait loci (QTL) associated with chromatin accessibility. Specifically, chromatin accessibility QTL represent an SNP that is correlated significantly with accessibility changes in their encompassing region.
- Morphogen gradients
-
Gradients of signalling molecules within developing tissues and embryos, which illicit different responses across the gradient, leading to diverse outcomes in terms of cell fate decisions, controlling pattern formation during embryogenesis.
Rights and permissions
About this article
Cite this article
Minnoye, L., Marinov, G.K., Krausgruber, T. et al. Chromatin accessibility profiling methods. Nat Rev Methods Primers 1, 10 (2021). https://doi.org/10.1038/s43586-020-00008-9
Accepted:
Published:
DOI: https://doi.org/10.1038/s43586-020-00008-9
This article is cited by
-
ATACAmp: a tool for detecting ecDNA/HSRs from bulk and single-cell ATAC-seq data
BMC Genomics (2023)
-
3D organization of regulatory elements for transcriptional regulation in Arabidopsis
Genome Biology (2023)
-
Epigenetic remodeling of the immune landscape in cancer: therapeutic hurdles and opportunities
Journal of Biomedical Science (2023)
-
Inferring chromatin accessibility during murine hematopoiesis through phylogenetic analysis
BMC Research Notes (2023)
-
Base-resolution UV footprinting by sequencing reveals distinctive damage signatures for DNA-binding proteins
Nature Communications (2023)