Abstract
Biochar production via biomass pyrolysis with subsequent burial in soils provides a carbon dioxide removal technology that is ready for implementation, yet uptake requires acceleration; notably, through generation of cost reductions and co-benefits. Here we find that biomass enrichment (doping) with refined minerals, mineral by-products, or ground rocks reduces carbon loss during pyrolysis, lowering carbon dioxide removal costs by 17% to US$ 80–150 t−1 CO2, with 30% savings feasible at higher biomass costs. As a co-benefit, all three additives increase plant-available nutrient levels. Doping with potassium-bearing minerals can increase both potassium and phosphorus release. Mineral doping in biochar production therefore offers carbon dioxide removal at lower costs, while alleviating global phosphorus and potassium shortages. This makes it unique among carbon dioxide removal technologies.
Similar content being viewed by others
Introduction
It is now widely agreed that immediate action on climate change is needed and that this must include both dramatic emissions reduction and atmospheric carbon dioxide removal (CDR)1. If there are economic and environmental co-benefits to any CDR technology, then this could significantly accelerate rates of implementation2,3,4. Biochar production and burial into soils is a key example of a technology that combines CDR with co-benefits for agricultural yield and the soil environment4,5,6,7. Greenhouse gas emission life-cycle assessments confirm biochar’s CDR potential; its global annual carbon dioxide removal potential is estimated at 0.03–6.6 Gt CO2 eq. year−1 8.
Biochar is produced through thermochemical conversion at temperatures above 350 °C (most often 450 °C and higher) under oxygen-limited conditions. This process, called pyrolysis, transforms the labile feedstock into a carbon-rich product that is recalcitrant against decomposition9. The feedstock for biochar production can be any biomass, such as crop and forestry residue, purposefully grown plants, animal waste, or wastewater (sewage) sludge5. During pyrolysis, minerals in the feedstock play a pivotal role in catalysing biomass conversion into biochar and assist in carbon stabilisation10,11. The same inherent minerals in biochar also provide nutrients to soil and plants through leaching. Many of the observed influences in agricultural applications have been attributed to this nutrient provision and increased nutrient-use efficiency12. Plant-available phosphorus (P) is a limiting resource, and efficient resource recovery from residues is needed to overcome nutrient limitations13. Therefore, enhancement of P availability in biochar is of particular interest.
The content of inorganic nutrients within biochar can be enhanced by adding specific, refined minerals to feedstock prior to pyrolysis, which can create a slow-release fertiliser14,15 or increase biochar’s carbon sequestration potential10,16. However, most refined mineral additives are relatively expensive (e.g., potassium acetate at ~US$ 1000 t−1)16, reducing the potential benefits for biochar doping. To achieve large-scale CDR through mineral-enriched biochar, abundant, and low-cost minerals are required close to sites where biomass is available for biochar production to ensure a favourable carbon footprint. Therefore, we evaluate the suitability of minerals from residues and by-products of existing industrial operations, and ground rocks, as potential biomass dopants for enhanced biochar production.
There are evident benefits of biomass mineral-enrichment prior to pyrolysis to achieve both higher carbon sequestration potentials and better fertiliser values. So far, however, this synergistic effect has received little attention. Therefore, this paper explores how optimising the biochar-pyrolysis system for this dual purpose could give significant economic and agricultural benefits, which can accelerate biochar implementation. Here we (i) review the benefits of biomass mineral doping for the resultant biochar and (ii) assess the suitability of (a) soluble, refined minerals, (b) mineral by-products/residues, and (c) ground rocks for doping to increase carbon sequestration and nutrient provision potential of biochar. Finally, (iii) we evaluate the effect of mineral doping on atmospheric CO2 removal costs in a new analysis.
Benefits of biomass doping with minerals
Improving biochar’s carbon sequestration potential
During pyrolysis, biomass polymers (lignin, cellulose and hemicellulose) are converted into an aromatic carbon framework. This aromatic carbon is highly recalcitrant to decomposition, so that its persistence and longevity in soil is much higher (orders of magnitude) than that of the feedstock material, with an estimated residence time of centuries to millennia9. However, not all of the biomass carbon retained in biochar exhibits this level of recalcitrance. The more labile carbon fraction is relatively readily decomposed in soil, ultimately resulting in re-release of some carbon into the atmosphere17. The proportional yield of the stable fraction determines the carbon sequestration potential of biochar and is calculated as biochar yield per unit biomass input multiplied by the percentage of stable carbon content within biochar (Fig. 1a)17. Many different methods exist to determine this stable carbon content in biochar based on thermal, chemical or biological processes18,19,20. One of the most widely used techniques is proximate analysis, which determines biochar’s thermal stability in an oxygen free atmosphere, typically at 900 °C. The resulting proportion of stable (or fixed) carbon is considered to persist in soil for at least 100 years18,21,22,23, the timeframe most commonly used for assessing global warming potential of greenhouse gases and subsequent modelling24.
a Biochar stable carbon yield (%) based on biochar yield (% conversion of biomass into biochar) and stable carbon content within biochar (%) and b enhanced stable carbon formation from the same amount of biomass through mineral doping prior to pyrolysis. Following equations are used to calculate the biochar stable carbon yield: (1) biochar yield (%) = amount biochar/amount biomass; (2) biochar stable carbon content (%) = amount stable carbon/amount biochar; (3) stable carbon yield (%) = biochar yield (%) * biochar stable carbon content (%).
Biomass pyrolysis is affected by the presence of inherent and externally added minerals in the feedstock. This mineral load can either increase or decrease biochar yield, its stable carbon content, or both, depending on the nature of the feedstock biomass and the minerals involved. Appropriate selection of minerals can result in overall increase in the stable carbon yield per unit feedstock biomass10,16,25 (Fig. 1b). Two main mechanisms can be distinguished here: (1) physical protection of carbon through encapsulation and formation of stable C complexes, which reduces carbon losses during pyrolysis and increases carbon persistence in soil, and (2) promotion of biomass decomposition into low-molecular-weight fragments and subsequent carbon atom cross-linking into a polyaromatic network through catalytic activity, which is the reduction in activation energy that is required for biomass conversion processes26,27.
Si in the form of silica (quartz and amorphous silica) affects pyrolysis through encapsulation28,29, Fe also mainly through encapsulation30, P, Ca, and Mg through both encapsulation and catalytic reactions10,11,31 and Na and K mainly through catalytic reactions26,27. Of all potential additives, the effects of minerals containing alkali (K, Na) and alkaline earth metals (Ca, Mg) have been investigated most thoroughly; addition of minerals containing an alkali metal generally increased the biochar and stable carbon yield more than addition of minerals with alkaline earth metals31,32,33,34. This highlights the great potential of alkali metals to boost biochar’s carbon sequestration potential16.
Alkali metals attack the carbon bonds in biomass and bind themselves to carbon temporarily, catalyse decomposition via chelation to hydroxyl and ether groups, and promote cross-linking of carbon via dehydration reactions11. These reactions result in increased stable carbon formation. However, for this to happen, Na and K ions need to migrate, be reactive and available. Biomass-inherent K is released from its original binding sites and migrates within the biomass/biochar matrix already at 200–400 °C and re-condenses without gaseous release35, forming new chemical species36, which demonstrates its mobility. Added minerals need the same mobility and reactivity to catalyse stable carbon formation.
Optimising nutrient release from biochar
Biochar contains nutrients from the feedstock, which are mostly retained and enriched in the resulting biochar due to the loss of volatile organic matter. Pyrolysis modifies the availability of nutrients in biochar through altering the form in which nutrients are bound in the material. These effects differ for each nutrient considered.
Nitrogen is an important plant nutrient that is present in plant residues, but also animal manures and sewage sludge. Pyrolysis of biomass volatilises a significant proportion of N (30–70%) from the feedstock material37,38, resulting in mean N contents in biochar between ~1% (wood) and 3% (sewage sludge)39. The N remaining in the resultant biochar is largely unavailable for plant uptake; this limits N recycling through pyrolysis and biochar40. Doping with minerals, such as CaO, can form more stable N-containing compounds, which shows potential for increasing N retention during pyrolysis41. This could be an important research path towards producing biochar-based N fertilisers and should be explored further.
Potassium is a limited resource mined from salts; especially in the form of sylvine (KCl) and, as a newcomer to production at scale, polyhalite (K2Ca2Mg(SO4)4·2H2O). It is also present in forestry, farm, and urban residues such as woody materials, straw, animal manures, and sewage sludge, which enables K recycling through pyrolysis. Biochars produced from these feedstocks on average show K contents of 0.5–4.9%39. Below a pyrolysis temperature of 700 °C, the K minerals present in feedstock are mostly retained in biochar, although some fraction of the K may be lost through volatilisation35,42. The addition of phyllosilicate minerals (kaolin and mica) helps retain K during combustion43, and might also be an option for pyrolysis. The availability of biomass-inherent and added K in biochar is generally high (~50% of the total K content)16,39,44. This highlights a potential to produce K fertilisers from pyrolysis of K-rich biomass, or of K-poor biomass doped with K minerals.
The P contents in biochar from different feedstocks are on average 0.4% (wood), 0.8% (crop residues), 2.1% (animal manures), and 2.8% (sewage sludge)39. However, much higher P contents are reported in some sewage sludge biochars (e.g., 12%45). Phosphorus recycling from wastewater/sewage sludge is emerging as an essential strategy to overcome future P supply shortages, as well as frequently occurring water quality issues (eutrophication)46. Moreover, introduction of mandatory P recycling from wastewater in some countries highlights an urgent need to find effective P-recovery options for sewage sludge13,47,48. Sewage sludge pyrolysis is one of the most promising P recovery strategies, also because it ensures the safety of the material by sterilisation and removal of organic contaminants (e.g., pharmaceutical residues)49.
Loss of P is minor during pyrolysis, but the availability of biomass-inherent P and doped P in the resulting biochar is typically low at <1–10%, depending on extractant, feedstock, and pyrolysis temperature15,44,50. Mineral doping of biomass may alter the availability of biomass-inherent P. Doping with Ca, Fe, and Mg decreases biochar-P availability51,52 and could therefore be suitable if a particularly slow P-release fertiliser is desired; e.g., in areas of protected water sources52. Doping with low concentrations of K (as K acetate), on the other hand, increases P availability by forming K phosphates that are highly soluble44. Doping with sodium (Na) will likely have similar effects as doping with K, given that Na phosphates are also highly water-soluble. In sum, mineral doping demonstrates significant potential for optimising biochar for P provision and could increase the recycling efficiency of P from sewage sludge and animal manures.
Synthesis: mineral doping for dual-purpose biochar use
Mineral doping of biomass prior to pyrolysis can enhance the biochar carbon sequestration potential by increasing the stable carbon yield, and improve the biochar fertiliser value by adding nutrients and increasing the availability of P that was present in the biomass already.
Doping P-rich biomass, such as sewage sludge and manures, with minerals containing available K leads to natural synergies that increase the stable carbon yield, boost the K content (a nutrient in itself), and enhance the biochar P availability (Fig. 2)44. Low-P materials such as crop and forestry residues can also be doped with K-bearing minerals to increase the yield of stable biochar. In this case, minerals with easily available Ca, Mg, and Fe also seem suitable (Fig. 2). Doping P-poor biomass with minerals containing plant-available P or K is not only a valuable strategy for increasing the stable carbon yield, but simultaneously enhances P and K nutrient release14,16,53. Doping biomass with one mineral with low P-availability and a second with low K-availability could potentially trigger synergies that increase the availability of either or both added nutrients through formation of highly soluble potassium phosphates (Fig. 2) (further discussed in section “K-bearing silicates”).
Suitability of different minerals for dual-purpose biochar use
Soluble, refined minerals
Most studies on biomass doping used pure minerals or salts, typically testing Fe, P, Ca, Mg, K, and Na bound to different anions (inlcuding phosphate).
P addition boosted the biochar carbon sequestration potential when added in the form of ammonium phosphate31, phosphoric acid54,55, and calcium dihydrogen phosphate15,53. Fe-containing minerals increased biochar and stable carbon yield when added as sulphate, nitrate, and acetate31,54,56, but not as Fe2O3 and chloride57,58. Ca, Mg, Na, and K catalysed biochar formation when added as chlorides, hydroxides, oxides, carbonates, and acetates16,26,31,33,54,59. The available data demonstrate that most forms of soluble, pure minerals can successfully increase the carbon sequestration potential of biochar to some degree (Fig. 3), but the cations/anions (counter-ions) involved are also important. The key factor likely is the ability to interact with other compounds.
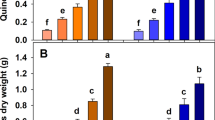
a Effect of K-rich minerals on the K-availability and P-availability in the resulting biochar and b effect of K-poor minerals on the biochar stable carbon yield. Only in a few cases experimental data exist and hence the effects are predicted effects based on the availability/mobility of a K and b phosphates/Na/Ca/Mg/Fe in the respective minerals (assessment details reported in Supplementary Tables 2 and 3). In a the uncertainty of the effect is highlighted by error bars. Predicting the effect of several components (phosphates/Na/Ca/Mg/Fe) as done in b has a much higher uncertainty, therefore, no error bars are reported. In b Na/Mg/Ca carbonates are set in brackets because carbonates increase the stable carbon yield but the overall effect on the carbon sequestration potential of biochar is uncertain due to CO2 release from carbonate decomposition.
Doping of biomass with carbonates containing Mg, Ca, K, and Na is very effective for increasing biochar formation, and generally shows greater impact than the use of other minerals; e.g., chlorides and acetates34,59. However, doping with carbonate minerals releases CO2 from carbonate decomposition during pyrolysis27,60. This could counterbalance the formation of extra stable carbon. Consequently, the carbon release from carbonate doping should be carefully weighed against the extra stable biochar formation.
While carbonates can release CO2, silicates, oxides, and hydroxides of K, Na, Ca, and Mg can react with and sequester CO2, forming carbonates61,62. The solid–gas reaction of Ca and Mg silicates and CO2 is very slow and needs high CO2 pressures; therefore, is not deemed economically viable61. The carbonation of pure Ca and Mg oxides and hydroxides at elevated temperature, however, can happen in minutes61. Doping of biomass with CaO significantly reduced CO2 release compared to pyrolysis of undoped (and zeolite-doped) biomass in the typical temperature range used for pyrolysis (300–700 °C)63. Consequently, the use of K, Na, Mg, and Ca oxides and hydroxides as biomass additives, e.g., as present in combustion ash, could have additional benefits for carbon sequestration62. Any CO2 release from the production of such oxides or hydroxides would need to be captured and stored, to optimise the overall carbon sequestration potential of the entire method.
The nutrients that are added as part of the soluble, refined minerals are typically plant-available14,16,44. So far, studies that investigate which K minerals enhance P availability in biochar are lacking. Only K acetate has been successfully tested44; additional research in this area is essential. It is likely that K-bearing minerals that increase the biochar stable carbon yield are also able to increase the availability of P, since K needs to be reactive and available in both cases (Fig. 3a).
Minerals from by-products and residues
A focus on by-products and residues from combustion, mining, or manufacturing processes would provide cost-effective sourcing of minerals, and facilitate the closing of resource cycles that promote the concept of a circular economy where waste is minimised.
Biomass doping with ash from wood combustion, a diverse material containing K, Mg, Ca, Fe, and Al in the form of oxides, hydroxides, carbonates, chlorides, phosphates, and sulphates62, can catalyse biochar formation and increase the stable carbon yield25. Besides benefits for carbon sequestration, the biochar matrix buffers the release of K contained in the wood-ash, which slows down K leaching in soil and avoids salinity issues, making biochar amended with combustion ash a potentially critical, slow-release K-fertiliser. The high content of available K in combustion ash could also make it suitable as amendment to P-rich feedstock, to increase the availability of P in the resulting biochar. Yet besides K, wood ash also contains Mg, Ca, Fe, and Al in large quantities, with potential to decrease P availability14,44,51,52. These opposing effects, and possibilities to change the balance between them, make research on wood ash and other biomass ashes as additives for P-rich biomass doping a priority.
P-rich residues, such as bone meal (calcium phosphate), might also be used directly as dopant to increase stable carbon yield and provide P in the resulting product15. However, the availability of P in bone meal is typically low and pyrolysis further reduces the P availability15,50. Adding K-minerals in addition to bone meal prior to pyrolysis may increase the P availability by forming soluble K-P minerals (Fig. 2).
Calcium-bearing residues, such as eggshells, oyster shells, and industrial lime mud (residue from paper mills) have demonstrated general catalytic capacity and could be suitable for doping P-poor materials to increase stable carbon yield and produce a Ca-providing biochar64,65.
For large-scale, global implementation of biochar production from mineral-enhanced biomass, mined rocks need to be used that are abundantly available in a cost-effective manner. Both primary minerals, formed during initial crystallisation of magma, and secondary minerals, i.e., chemically transformed primary minerals, are considered here (Table 1).
Non-silicate rock minerals
Phosphate rock is the primary raw material from which all commodity P chemicals are derived, with annual production of >200 million t66 at ~US$ 70 t−1 67. When incorporated into biochar, it behaves like bone meal: doping increased carbon retention68 and the availability of P was lower relative to that in the non-pyrolysed material69. Yet, phosphate rock addition decreased biochar’s aromaticity, the main indicator for biochar stability, which highlights the need to study the impacts on stable carbon yield (Fig. 1).
Iron ore (iron oxide) is produced in large quantities globally (3 billion t; Table 1) and has potential to catalyse biomass conversion70. However, its effectiveness in increasing biochar’s carbon sequestration potential remains untested.
K-bearing silicates
K silicates are highly abundant, distributed globally, and a potential source of K-fertiliser71. Their crystal structure determines the reactivity of the mineral; i.e., the mineral’s ease of dissolution by water and subsequent plant nutrient availability, and their ability to promote stable biochar formation and P availability during pyrolysis.
Structurally stable framework silicates (tectosilicates), such as K-feldspar (KAlSi3O8), have the highest level of crystallinity and hence are relatively unreactive since K is strongly bound within the structure. Therefore, they are unlikely to be suitable for biomass doping for the purpose of increasing P-availability, providing K and catalysing biochar formation (Fig. 3b). In fact, Na-feldspar (NaAlSi3O8; isostructural with K-feldspar) application did not significantly increase biochar yield in two feedstocks (olive bagasse, hazelnut bagasse)72.
While K in K-feldspars is tightly bound and mostly unavailable for plant uptake, mixing with chloride, sulphate, and carbonate salts of alkali and alkaline earth metals, and then heating to >600 °C, increased K availability and its suitability for application as fertiliser73,74,75. During this treatment, poorly soluble K-feldspar is partially converted into highly soluble K minerals (sulphate, chloride). Besides sulphates and chloride salts, minerals containing phosphates (in available or unavailable form) are potential candidates for this purpose (Fig. 2). This process could be used during biomass pyrolysis with K-feldspars to increase the K-availability, with potential further benefits for stable carbon yield formation. To catalyse stable biochar formation, K likely needs to be present in an available form at pyrolysis temperatures of 300–500 °C, while K-feldspar transformation reactions only start at 600–700 °C74, which highlights a need for process optimisation.
The feldspathoid nepheline ((Na,K)AlSiO4)—present, for example, in the rarer but commercially mined rock called nepheline syenite (refined for use as a raw material for the ceramics industry; US$ 157 t−1)—has shown potential as a fertiliser in studies dating back to pioneering work of Goldschmidt76. It is a tectosilicate with a relatively stable crystal structure71,77, but it has a much (~107 times) greater mineral dissolution rate than feldspars78. Leucite (KAlSi2O6) and kalsilite (KAlSiO4) are also feldspathoids with potential for K-provision, but are not as readily available as a global commodity71. Given that their mineral dissolution rates, and so their weathering rates, greatly exceed those of the feldspars, the catalytic potential of feldspathoids on stable carbon yield and P availability in biochar is worth investigating (Fig. 3a).
Micas, such as biotite (K(Mg,Fe)3(AlSi3O10)(F,OH)2), phlogopite (KMg3AlSi3O10(F,OH)2) and muscovite (KAl3Si3O10(OH)2), have K contents of 7–9%71 and are sheet silicate minerals (phyllosilicates) whose interlayer K is relatively easily exchanged in solution. These minerals demonstrate catalytic effects and undergo changes during heating, though mainly at temperatures higher than the typical range used for biochar production (>800 °C)79,80. The potential of muscovite to provide K to plants is low81. Biotite and phlogopite, on the other hand, demonstrated good K supply to plants and in several growing cycles performed as well as a one-time application of potash77,82,83. Their high available K content makes them very promising materials for biomass doping for dual-purpose biochar use (Fig. 3a).
There are ways to further increase the K availability in micas and, hence, their reactivity during pyrolysis. For example, the K-extractability from muscovite increased from 1–5% to 80% after application of NaOH and heating to 400 °C84. Increasing the K availability from K-feldspar and micas via application of other minerals and subsequent thermal treatment could ideally complement biomass pyrolysis, and bring further synergies for biochar production.
Illite (a clay mineral) is a weathered mica with the same general formula as muscovite (KAl3Si3O10(OH)2) and a K content of ~3–6%85. It is available cheaply (~US$ 8 t−1) in the form of bulk clays used for heavy ceramics and bricks/tiles, albeit mixed with other clay minerals, such as kaolinite (Al2Si2O5(OH)4). Generally, clay minerals have low crystallinity and high reactivity (see next section)85 and illite is one of the few clay minerals with significant amounts of available K81; hence it should be a key target material for future investigations (Fig. 3a).
K-poor primary and secondary silicates
Biomass doping with minerals poor in K does not bring the benefit of increasing the availability of P already present in the biomass, yet such minerals might be able to increase the biochar stable carbon yield (Fig. 2).
Olivine ((Mg,Fe)2SiO4), an orthosilicate that is common in ultramafic igneous rocks, can promote catalytic decomposition of biomass86. In ultramafic and mafic rocks, olivine is mainly accompanied by pyroxene and plagioclase, which both have a higher order of crystallinity than olivine and hence are less suitable for biomass doping. These rocks such as basalt and diabase/dolerite, are widely used in construction and form a large proportion of the crushed stone figure in Table 1. They can be cheap, at US$ 12 t−1 67.
Clay minerals are the weathering products of primary silicates and highly reactive85. Several clay minerals have been tested as additives to straw for their catalytic potential during pyrolysis. It was found that addition of kaolinite did not increase biochar yield, carbon retention, or carbon recalcitrance53. Moreover, kaolinite (kaolin) resources are typically available at high prices for refined products (US$ 160 t−1), which also makes kaolinite unsuitable as a biomass doping material. Co-application of montmorillonite—a smectite clay mineral—with a Fe-mineral (magnetite) increased the recalcitrance of straw-derived biochar30. Montmorillonite is present in the commodity bentonite (21 million t year−1), which also contains other smectite clays and is available at ~US$ 98 t−1. Addition of vermiculite to straw increased carbon retention and stability of the resulting biochar, demonstrating good potential of vermiculite for biomass doping87. Vermiculite, a three-layered clay mineral with a K content of 0–1.7%85, is used in horticulture as soil improver for aeration, water and nutrient retention but has also proven to provide K to plants77. It has a global market of over 500,000 t year−1 at a price of US$ 140 t−1 and is a material that should be investigated further (modelling scenario (c) in Fig. 4).
The calculations consider stable carbon content in biochar based on proximate analysis (stable for ~100 years) and greenhouse gas emissions associated with feedstock and mineral generation, biochar production and biochar application. Simulation of biomass enrichment with (a, d) a refined, soluble mineral (potassium acetate) to bioenergy crop biomass (miscanthus)16, b, e mineral residues (wood ash) to forestry residues (woody residues)25 and c, f ground rocks (vermiculite) to agricultural residues (rice straw)87. The baseline scenarios in a–f assume biomass pyrolysis without mineral addition. Scenarios (a–c) are based on fixed feedstock and mineral costs and scenarios (d–f) on variable feedstock and mineral costs. In e only the feedstock costs were varied as the wood ash costs were assumed to be US$ 0 t−1. In scenarios (d, f) the lower, middle, and upper lines reflect potassium acetate and vermiculite prices of US$ 750 t−1, US$ 1000 t−1 and US$ 1250 t−1 and US$ 105 t−1, US$ 140 t−1 and US$ 175 t−1, respectively. The vertical black line in scenarios (d–f) show the feedstock costs used in scenarios (a–c), respectively. The scenario parameters are reported in Table 2 and more details on the assumptions can be found in the Supplementary Methods and Supplementary Table 1.
Above (and in the Supplementary Discussions), we assessed the potential of a wide range of industrial minerals for use as additives in biochar production. Global annual production capacities for these materials vary from just over 250,000 t (mica) to >4 billion t (crushed rock), with prices ranging from US$ 8 (gypsum) to US$ 300 t−1 (wollastonite) (Table 1). Once the desired bio-geo-chemical specifications are decided, successful application requires selection of cheaper resource streams that do not meet the standards of high-value markets but are effective as biochar additives. Mineral addition can increase stable biochar yield, yet mineral generation releases greenhouse gases, e.g., mining and crushing. In the following, we therefore investigate the effect of mineral addition on biochar CO2 removal costs.
Costs—the path towards biochar implementation
Biochar’s atmospheric CO2 removal costs are here defined as the economic costs for feedstocks provision, production, and soil application to sequester 1 t CO2 in biochar for at least 100 years, while considering the life-cycle greenhouse gas emissions associated with the process.
When mineral doping catalyses biochar formation and increases the stable carbon yield per unit of biomass, this decreases feedstock costs per tonne of atmospheric CO2 removal (i.e., the costs for biomass feedstock collection, transport, and handling or commercial price). However, adding large amounts of minerals to biomass can also require (i) longer running times of the pyrolysis unit to produce 1 t of stable carbon because organic feedstock mass is replaced by mineral mass, and (ii) higher biochar application rates to spread amounts equivalent to 1 t of stable carbon because the stable carbon content per amount of biochar is reduced by dilution with the higher mineral content25. These factors can increase costs of biochar production and application when mineral doping is applied. There is, therefore, an optimal amount of mineral addition that balances mineral dilution and stable carbon yield increase25. In addition, the mineral costs themselves and the greenhouse gas emissions associated with mineral generation also influence the overall atmospheric CO2 removal costs. The carbon cost of producing a crushed rock product can be low, at 5.4 kg CO2 per tonne of rock for crushed dolerite (<5 mm; 32% of total carbon cost of 65 km transport and spreading 1 t ha−1)88.
To investigate the effect of feedstock costs, mineral costs, and stable carbon yield increase per amount of mineral added, we modelled and evaluated three contrasting mineral-enrichment scenarios based on data from the literature: the addition of (a) a refined, soluble mineral that is available at a relatively high cost (potassium acetate; costs US$ 1000 t−1) in low quantities (1–2%) to bioenergy crop biomass (miscanthus; costs US$ 87 t−1)6, (b) a complex mixture of minerals that is a by-product of existing operations (wood ash; costs US$ 0 t−1) in high quantities (5–50%) to forestry residues (woody residues)5, and (c) a ground rock (vermiculite; costs US$ 140 t−1) in high quantities (20%) to agricultural residues (rice straw; costs US$ 59 t−1)87. The baseline scenarios used as reference assumed biomass pyrolysis without mineral addition (model parameters for baseline and further scenarios are described in Table 2).
Water-soluble minerals, such as potassium acetate, can be added cost-effectively by dissolving them in water and spraying the solution onto the feedstock biomass for biochar production16. Non-soluble minerals such as wood ash can be added by mixing with feedstock biomass and subsequent pelleting, although this adds another cost factor to the process25. Coating of biomass with a slurry/suspension of minerals in water could be an option that needs studying89.
In the baseline scenarios the CO2 removal costs were estimated at US$ 140 for biochar from woody residues, US$ 149 for (rice) straw biochar and US$ 202 for miscanthus biochar. In all scenarios, mineral doping reduces the biochar feedstock costs and because feedstock costs comprise the largest proportion of the CO2 removal costs, mineral doping also effectively reduces the total costs relative to the baseline scenarios (Fig. 4). Only the addition of 50% wood ash increased the CO2 removal costs relative to the baseline because of high biochar production and application costs. Biochar production costs are the second largest contributor to biochar CO2 removal costs in all scenarios and as demonstrated in the 50% wood ash example, the production costs can increase with mineral doping. With greater uptake of biochar as a CO2 removal technology, economy of scale will help to bring down the production costs. This reduces the proportion of biochar production costs relative to the total costs and further boosts the importance of the feedstock costs.
Feedstock costs are already the main driver of biochar CO2 removal costs. With an increasing carbon price and competition for biomass as a carbon source for other carbon sequestration operations, such as soil organic carbon formation and bioenergy carbon capture and storage (BECCS), the value of biomass will likely increase. For future, large-scale implementation, this will drive a need for innovations to increase the carbon sequestration efficiency for each unit of biomass. Mineral-enrichment of biomass with subsequent pyrolysis promises exactly that. In an extension of scenarios (a), (b) and (c), we assessed the effect on the CO2 removal costs of varying feedstock costs (e) and varying feedstock and mineral costs (d, f).
With an increase in feedstock costs, mineral addition becomes even more attractive because it further reduces CO2 removal costs relative to the baseline scenarios. The higher the feedstock costs, the more these costs dominate the CO2 removal costs over the costs of biochar production and application, and minerals used for doping. Therefore, CO2 removal costs are only sensitive to changes in mineral costs at low feedstock costs (Fig. 4, scenarios (d) and (f)). The addition of 2% K acetate to miscanthus (d), 20% wood ash to woody residues (e), and 20% vermiculite to rice straw (f) at feedstock costs of US$ 200 t−1 brought cost savings of 20–30% relative to the baseline scenarios (Table 2). At high feedstock costs, CO2 removal costs are controlled by the conversion efficiency of biomass into stable carbon because it determines how much feedstock is needed to produce 1 t of stable carbon (i.e., removal and sequestration of 3.67 t of CO2 from the climate system). The conversion efficiency can be enhanced by matching mineral type and quantity to the available feedstock and studies are needed to do this optimisation.
Based on a voluntary carbon removal marketplace, the current biochar CO2 removal cost based on nine suppliers stands at US$ 100–180 t−1 CO2 (mean US$ 130)90,91, which matches with our modelled prices in the baseline scenarios of US$ 140–200 t−1 CO2 (Table 2) that take into account the life-cycle greenhouse gas emissions. Predicted pricing for other, land-based CO2 removal strategies is comparable: enhanced rock (basalt) weathering stands at US$ 50–200 t−1 CO2 (mean US$ 130)92 and BECCS is estimated at US$ 150–240 t−1 CO2 (mean US$ 200)93. In our simulations, the three mineral doping scenarios with experimental data reduced biochar CO2 removal cost by 12–17% at current feedstock costs to US$ 120–170 t−1 CO2 (Table 2) through more efficient conversion of feedstock into recalcitrant biochar with further reductions feasible. Savings of 17% would reduce CO2-removal costs of the biochars from the voluntary carbon removal marketplace to US$ 80–150 t−1 CO2. This brings the costs closer to current carbon prices, such as that in the EU (US$ 70)94, which may accelerate biochar implementation. At higher feedstock costs, the savings would be 20–30% (Table 2). Including agronomic benefits from biochar addition would further improve its economic attractiveness.
Amendment of biomass with 2% potassium acetate, 20% wood ash, and 20% vermiculite would enhance K contents of the resulting biochars by ~6, 4, and 0.7% (based on biochar yields in the modelled scenarios with wood ash and vermiculite K contents of 8.9%95 and 1.7%85, respectively). At a K2O fertiliser price of US$ 300–600 t−1, this brings an additional US$ 15–30, US$ 10–20, and US$ 2–4 per tonne of biochar in K fertiliser value. When the biochar application replaces some of the potassium fertiliser application in agriculture, it further reduces greenhouse gas emissions by ~10, 6, and 1 kg CO2 per kg of biochar application with 2% potassium acetate, 20% wood ash and 20% vermiculite, respectively (at 158 kg CO2 eq. emissions per t of produced K fertiliser96). The biochars in our modelling scenarios are relatively P-poor (0.15% P miscanthus, 0.002% P woody residues and 0.5% P rice straw) so that a potential effect of K-minerals on P-availability will unlikely affect their fertiliser value. For pyrolysis of P-rich biomass with K-minerals, we expect that the higher P-availability will result in a higher P-fertiliser value.
It is important to be cautious with the selection of mineral amendments because the addition of some rocks and residues can introduce potentially toxic elements (PTEs), such as copper, arsenic, cadmium, nickel or chromium. Potassium acetate is a refined mineral and, therefore, does not contain contaminants with potential risks for plant growth and soil health. However, a 20% wood ash-amended biochar investigated previously exceeded total cadmium and chromium content guidelines threshold values for safe biochar use14. Levels of PTEs in different types of wood ash and rocks can vary significantly depending on their origin, even within the same rock type category, such as basalt97,98. Hence, their use for mineral doping needs to be evaluated on a case-by-case basis.
Outlook
Mineral doping of biochar provides opportunities for integrating existing waste streams into active greenhouse gas removal strategies, while lowering biochar CO2 removal costs. Local P recycling is an essential sustainability approach on international political agendas, and biochar production from mineral doped, P-rich residues can contribute substantial gains toward P-recovery targets by making recycled P in sewage sludge plant-available. Biomass doping with K-rich rocks followed by pyrolysis could increase the K availability of otherwise plant-inaccessible K with further benefits for the carbon sequestration potential of biochar. Use of the mineral-enriched biochar in agriculture can replace (a proportion of) P and K fertilisers and the controlled-nutrient release in biochar further improves biochar’s economic and environmental benefits.
Mineral doping of biomass prior to pyrolysis clearly has prospects for large-scale use, in particular when ground rocks are used for biomass-enrichment. Refined, soluble minerals may demonstrate reliable effects on both stable carbon formation and nutrient provision potential of biochar but are relatively expensive. In contrast, minerals such as micas, vermiculite, nepheline, or illite that can be sourced from common rock types are available at much lower costs than refined minerals, but can have similar effects. While the impact of pure minerals on biochar formation is reasonably well investigated, there is still a lack of systematic studies with a large variety of feedstocks and minerals, including residue minerals and ground rocks, that assess the effect on the parameter key for biochar’s carbon dioxide removal potential, the stable carbon yield per amount of biomass input. Developing this understanding is essential to further build on our emissions LCA-cost assessment and identify the best minerals for large-scale mineral-biochar production. It is crucial that such further work includes investigation of nutrient provision potential and other co-benefits of mineral-biochars, such as the effects on water and nutrient retention or formation of extra organic carbon in soil (negative priming).
Mineral-enriched biochar has potential to boost incentives for biochar implementation as efficient, controlled-release fertiliser, P-recycling strategy, and as an important, readily available carbon dioxide removal technology, thus contributing to the ambitious 2030 and 2050 targets.
Data availability
The data sets analysed in this article are all fully cited in the respective sections.
Code availability
The excel tool used to conduct the modelling scenarios could be made available upon reasonable request.
References
IPCC. Climate Change 2021—The Physical Science Basis. Working Group I Contribution to the Sixth Asssessment Report of the Intergovernmental Panel on Climate Change (Cambridge University Press, 2021).
Buss, W., Yeates, K., Rohling, E. J. & Borevitz, J. Enhancing natural cycles in agro-ecosystems to boost plant carbon capture and soil storage. Oxford Open Clim. Change 1, kgab006 (2021).
Paustian, K. et al. Climate-smart soils. Nature 532, 49–57 (2016).
Smith, P. Soil carbon sequestration and biochar as negative emission technologies. Glob. Change Biol 22, 1315–1324 (2016).
Joseph, S. et al. How biochar works, and when it doesn’t: a review of mechanisms controlling soil and plant responses to biochar. GCB Bioenergy 13, 1731–1764 (2021).
Yang, Q. et al. Prospective contributions of biomass pyrolysis to China’s 2050 carbon reduction and renewable energy goals. Nat. Commun. 12, 1698 (2021).
Lehmann, J. et al. Biochar in climate change mitigation. Nat. Geosci. 14, 883–892 (2021).
Smith, P. et al. Which practices co-deliver food security, climate change mitigation and adaptation, and combat land degradation and desertification? Glob. Change Biol. 26, 1532–1575 (2020).
Lehmann, J. et al. Biochar for Environmental Management: Science and Technology and Implementation, 2nd edn 169–182 (Earthscan Ltd., 2015).
Nan, H. et al. Different alkaline minerals interacted with biomass carbon during pyrolysis: Which one improved biochar carbon sequestration? J. Clean. Prod. 255, 120162 (2020).
Giudicianni, P. et al. Inherent metal elements in biomass pyrolysis: a review. Energy Fuels 35, 5407–5478 (2021). Both alkali and earth alkaline metal demonstrate great potential for carbon retention during pyrolysis.
Jeffery, S. et al. Biochar boosts tropical but not temperate crop yields. Environ. Res. Lett. 12, 053001 (2017).
The European Parliament and the Council of the European Union. Regulation (EU) 2019/1009 of the European Parliament and of the Council of 5 June 2019 laying down rules on the making available on the market of EU fertilising products and amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and repealing Regula. Off. J. Eur. Union 62, 1–132 (2019).
Buss, W., Jansson, S. & Mašek, O. Unexplored potential of novel biochar-ash composites for use as organo-mineral fertilizers. J. Clean. Prod. 208, 960–967 (2019).
Zhao, L. et al. Copyrolysis of biomass with phosphate fertilizers to improve biochar carbon retention, slow nutrient release, and stabilize heavy metals in soil. ACS Sustain. Chem. Eng. 4, 1630–1636 (2016).
Mašek, O., Buss, W., Brownsort, P., Rovere, M. & Alberto, T. Potassium doping increases biochar carbon sequestration potential by 45%, facilitating decoupling of carbon sequestration from soil improvement. Sci. Rep. https://doi.org/10.1038/s41598-019-41953-0 (2019). Biomass doping with pure minerals can increase the carbon sequestration potential per biomass input.
Mašek, O., Brownsort, P., Cross, A. & Sohi, S. Influence of production conditions on the yield and environmental stability of biochar. Fuel 103, 151–155 (2013).
Crombie, K., Mašek, O., Sohi, S. P., Brownsort, P. & Cross, A. The effect of pyrolysis conditions on biochar stability as determined by three methods. GCB Bioenergy 5, 122–131 (2013).
Cross, A. & Sohi, S. P. A method for screening the relative long-term stability of biochar. GCB Bioenergy 5, 215–220 (2013).
Wang, J., Xiong, Z. & Kuzyakov, Y. Biochar stability in soil: meta-analysis of decomposition and priming effects. GCB Bioenergy 8, 512–523 (2016).
Budai, A. et al. International Biochar Initiative: biochar carbon stability test method: an assessment of methods to determine biochar carbon stability. (2013).
Spokas, K. A. Review of the stability of biochar in soils: predictability of O:C molar ratios. Carbon Manag. 1, 289–303 (2010).
Enders, A., Hanley, K., Whitman, T., Joseph, S. & Lehmann, J. Characterization of biochars to evaluate recalcitrance and agronomic performance. Bioresour. Technol. 114, 644–653 (2012).
IPCC. Chapter 8: Climate Change 2013: The physical science basis. Contribution of working group I to the fifth assessment report of the intergovernmental panel on climate change. https://doi.org/10.1017/CBO9781107415324 (2013).
Buss, W., Jansson, S., Wurzer, C. & Mašek, O. Synergies between BECCS and biochar—maximizing carbon sequestration potential by recycling wood ash. ACS Sustain. Chem. Eng. 7, 4204–4209 (2019).
Nowakowski, D. J. & Jones, J. M. Uncatalysed and potassium-catalysed pyrolysis of the cell-wall constituents of biomass and their model compounds. J. Anal. Appl. Pyrolysis 83, 12–25 (2008).
Nishimura, M., Iwasaki, S. & Horio, M. The role of potassium carbonate on cellulose pyrolysis. J. Taiwan Inst. Chem. Eng. 40, 630–637 (2009).
Ahmad, M. et al. Date palm waste-derived biochar composites with silica and zeolite: synthesis, characterization and implication for carbon stability and recalcitrant potential. Environ. Geochem. Health 41, 1687–1704 (2019).
Guo, J. & Chen, B. Insights on the molecular mechanism for the recalcitrance of biochars: interactive effects of carbon and silicon components. Environ. Sci. Technol. 48, 9103–9112 (2014).
Lu, J. et al. Iron-montmorillonite treated corn straw biochar: Interfacial chemical behavior and stability. Sci. Total Environ. 708, 134773 (2020).
Fuentes, M. E. et al. A survey of the influence of biomass mineral matter in the thermochemical conversion of short rotation willow coppice. J. Energy Inst. 81, 234–241 (2008).
Wang, Z., Wang, F., Cao, J. & Wang, J. Pyrolysis of pine wood in a slowly heating fixed-bed reactor: potassium carbonate versus calcium hydroxide as a catalyst. Fuel Process. Technol. 91, 942–950 (2010).
Patwardhan, P. R., Satrio, J. A., Brown, R. C. & Shanks, B. H. Influence of inorganic salts on the primary pyrolysis products of cellulose. Bioresour. Technol. 101, 4646–4655 (2010).
Feng, D., Zhang, Y., Zhao, Y. & Sun, S. Catalytic effects of ion-exchangeable K+ and Ca2+ on rice husk pyrolysis behavior and its gas–liquid–solid product properties. Energy 152, 166–177 (2018).
Jensen, P. A., Frandsen, F. J., Dam-Johansen, K. & Sander, B. Experimental investigation of the transformation and release to gas phase of potassium and chlorine during straw pyrolysis. Energy Fuels 14, 1280–1285 (2000).
Tian, S., Tan, Z., Kasiulienė, A. & Ai, P. Transformation mechanism of nutrient elements in the process of biochar preparation for returning biochar to soil. Chin. J. Chem. Eng. 25, 477–486 (2017).
Ye, Z., Liu, L., Tan, Z., Zhang, L. & Huang, Q. Effects of pyrolysis conditions on migration and distribution of biochar nitrogen in the soil-plant-atmosphere system. Sci. Total Environ. 723, 138006 (2020).
Yuan, S., Tan, Z. & Huang, Q. Migration and transformation mechanism of nitrogen in the biomass–biochar–plant transport process. Renew. Sustain. Energy Rev. 85, 1–13 (2018).
Xu, X. et al. Indispensable role of biochar-inherent mineral constituents in its environmental applications: a review. Bioresour. Technol. 241, 887–899 (2017).
Wang, Y., Villamil, M. B., Davidson, P. C. & Akdeniz, N. A quantitative understanding of the role of co-composted biochar in plant growth using meta-analysis. Sci. Total Environ. 685, 741–752 (2019).
Liu, H. et al. Catalytic role of conditioner CaO in nitrogen transformation during sewage sludge pyrolysis. Proc. Combust. Inst. 35, 2759–2766 (2015).
Okuno, T. et al. Primary release of alkali and alkaline earth metallic species during the pyrolysis of pulverized biomass. Energy Fuels 19, 2164–2171 (2005).
Liu, Y. et al. Experimental study of potassium release during biomass-pellet combustion and its interaction with inhibitive additives. Fuel 260, 116346 (2020).
Buss, W., Bogush, A., Ignatyev, K. & Mašek, O. Unlocking the fertilizer potential of waste-derived biochar. ACS Sustain. Chem. Eng. 8, 12295–12303 (2020). Pure potassium acetate minerals can increase the availability of phosphorus in biomass after pyrolysis.
Roberts, D. A., Cole, A. J., Whelan, A., de Nys, R. & Paul, N. A. Slow pyrolysis enhances the recovery and reuse of phosphorus and reduces metal leaching from biosolids. Waste Manag. 64, 133–139 (2017).
Cordell, D., Drangert, J. O. & White, S. The story of phosphorus: global food security and food for thought. Glob. Environ. Change 19, 292–305 (2009).
Schweizer Bundesrat. Verordnung ueber die Vermeidung und die Entsorgung von Abfaellen (VVEA); Ordinance on Avoidance and Diposal of Waste. (Swiss legislation, 2015).
AbfKlärV. Verordnung über die Verwertung von Klärschlamm, Klärschlammgemisch und Klärschlammkompost (Klärschlammverordnung - AbfKlärV). 1–47 (German legislation, 2017).
Buss, W. Pyrolysis solves the issue of organic contaminants in sewage sludge while retaining carbon—making the case for sewage sludge treatment via pyrolysis. ACS Sustain. Chem. Eng. 9, 1048–1053 (2021).
Zwetsloot, M. J., Lehmann, J. & Solomon, D. Recycling slaughterhouse waste into fertilizer: How do pyrolysis temperature and biomass additions affect phosphorus availability and chemistry? J. Sci. Food Agric. 95, 271–288 (2014).
Liu, Q. et al. Phosphorus speciation and bioavailability of sewage sludge derived biochar amended with CaO. Waste Manag. 87, 71–77 (2019).
Xiao, R. et al. Biochar produced from mineral salt-impregnated chicken manure: fertility properties and potential for carbon sequestration. Waste Manag. 78, 802–810 (2018).
Li, F., Cao, X., Zhao, L., Wang, J. & Ding, Z. Effects of mineral additives on biochar formation: carbon retention, stability, and properties. Environ. Sci. Technol. 48, 11211–11217 (2014).
Chen, M. et al. Catalytic effects of eight inorganic additives on pyrolysis of pine wood sawdust by microwave heating. J. Anal. Appl. Pyrolysis 82, 145–150 (2008).
Zhao, L. et al. Roles of phosphoric acid in biochar formation: synchronously improving carbon retention and sorption capacity. J. Environ. Qual. 46, 393–401 (2017).
Bru, K., Blin, J., Julbe, A. & Volle, G. Pyrolysis of metal impregnated biomass: an innovative catalytic way to produce gas fuel. J. Anal. Appl. Pyrolysis 78, 291–300 (2007).
Han, L. et al. Effect of Fe and Al ions on the production of biochar from agricultural biomass: properties, stability and adsorption efficiency of biochar. Renew. Sustain. Energy Rev. 145, 111133 (2021).
Yu, D. et al. Gas characteristics of pine sawdust catalyzed pyrolysis by additives. J. Therm. Sci. 30, 333–342 (2021).
Yang, H. et al. Influence of mineral matter on pyrolysis of palm oil wastes. Combust. Flame 146, 605–611 (2006).
Kwon, E. E. et al. Effects of calcium carbonate on pyrolysis of sewage sludge. Energy 153, 726–731 (2018).
Lackner, K. S., Butt, D. P. & Wendt, C. H. Progress on binding CO2 in mineral substrates. Energy Convers. Manag. 38, 259–264 (1997).
Vassilev, S. V. & Vassileva, C. G. Extra CO2 capture and storage by carbonation of biomass ashes. Energy Convers. Manag. 204, 112331 (2020).
Wang, D., Xiao, R., Zhang, H. & He, G. Comparison of catalytic pyrolysis of biomass with MCM-41 and CaO catalysts by using TGA-FTIR analysis. J. Anal. Appl. Pyrolysis 89, 171–177 (2010).
Yuan, R., Yu, S. & Shen, Y. Pyrolysis and combustion kinetics of lignocellulosic biomass pellets with calcium-rich wastes from agro-forestry residues. Waste Manag. 87, 86–96 (2019).
Choi, D. et al. Catalytic pyrolysis of brown algae using carbon dioxide and oyster shell. J. CO2 Util. 34, 668–675 (2019).
Brown, T. J. et al. World Mineral Production 2015–19. https://www2.bgs.ac.uk/mineralsuk/download/world_statistics/2010s/WMP_2015_2019.pdf (2021).
U.S. Geological Survey. Mineral commodity summaries 2020: U.S. Geological Survey. https://doi.org/10.3133/mcs2020 (2020).
Gao, R. et al. Co-pyrolysis biochar derived from rape straw and phosphate rock: carbon retention, aromaticity, and Pb removal capacity. Energy Fuels 33, 413–419 (2019).
Avornyo, V. K., Manu, A., Laird, D. A. & Thompson, M. L. Temperature effects on properties of rice husk biochar and calcinated Burkina phosphate rock. Agriculture 11, 432 (2021).
Song, Q. et al. Pyrolysis of municipal solid waste with iron-based additives: a study on the kinetic, product distribution and catalytic mechanisms. J. Clean. Prod. 258, 120682 (2020).
Manning, D. A. C. Mineral sources of potassium for plant nutrition. A review. Agron. Sustain. Dev. 30, 281–294 (2010).
Demiral, I. & Şensöz, S. The effects of different catalysts on the pyrolysis of industrial wastes (olive and hazelnut bagasse). Bioresour. Technol. 99, 8002–8007 (2008).
Haseli, P., Majewski, P., Christo, F. C., Hammond, B. & Bruno, F. Thermochemical and experimental kinetic analysis of potassium extraction from ultrapotassic syenite using Molten chloride salts. Ind. Eng. Chem. Res. 58, 7397–7407 (2019).
Yuan, B. et al. Extraction of potassium from K-feldspar via the CaCl2 calcination route. Chin. J. Chem. Eng. 23, 1557–1564 (2015).
Zhang, W. et al. Metal immobilization by sludge-derived biochar: roles of mineral oxides and carbonized organic compartment. Environ. Geochem. Health 39, 379–389 (2017).
Goldschmidt, V. Oversikgtskart over utbredelsen av de forskjellige kalimineraler I norsk fjeldgrund. Nor. Landmandshlad 41, 268–269 (1922).
Mohammed, S. M. O., Brandt, K., Gray, N. D., White, M. L. & Manning, D. A. C. Comparison of silicate minerals as sources of potassium for plant nutrition in sandy soil. Eur. J. Soil Sci. 65, 653–662 (2014).
Palandri, J. L. & Kharaka, Y. K. A Compilation of Rate Parameters of Water-Mineral Interaction Kinetics for Application to Geochemical Modeling (US Geological Survey, 2004).
Kozak, L. M. Exchangeability of Potassium in Heated and Oxidized Micas https://lib.dr.iastate.edu/rtd/6238 (Iowa State University, 1976).
Barlow, S. G. & Manning, D. A. C. Influence of time and temperature on reactions and transformations of muscovite mica. Br. Ceram. Trans. 98, 122–126 (1999).
Li, T., Wang, H., Wang, J., Zhou, Z. & Zhou, J. Exploring the potential of phyllosilicate minerals as potassium fertilizers using sodium tetraphenylboron and intensive cropping with perennial ryegrass. Sci. Rep. 5, 17–22 (2015).
Madaras, M., Mayerová, M., Kulhánek, M., Koubová, M. & Faltus, M. Waste silicate minerals as potassium sources: a greenhouse study on spring barley. Arch. Agron. Soil Sci. 59, 671–683 (2013).
Bakken, A. K., Gautneb, H., Sveistrup, T. & Myhr, K. Crushed rocks and mine tailings applied as K fertilizers on grassland. Nutr. Cycl. Agroecosyst. 56, 53–57 (2000).
Kumar, A., Tanvar, H., Pratap, Y. & Dhawan, N. Evaluation of mica as a source of potash. Min. Metall. Explor. 36, 547–555 (2019).
Blume, H.-P. et al. Scheffer/Schachtschabel: Soil Science 7–37 (Springer, 2016).
Choi, G. G., Oh, S. J. & Kim, J. S. Scrap tire pyrolysis using a new type two-stage pyrolyzer: effects of dolomite and olivine on producing a low-sulfur pyrolysis oil. Energy 114, 457–464 (2016).
Liu, Y. et al. Vermiculite modification increases carbon retention and stability of rice straw biochar at different carbonization temperatures. J. Clean. Prod. 254, 120111 (2020). Vermiculite addition to biomass prior to pyrolysis increases carbon retention and stability.
Lefebvre, D. et al. Assessing the potential of soil carbonation and enhanced weathering through Life Cycle Assessment: a case study for Sao Paulo State, Brazil. J. Clean. Prod. 233, 468–481 (2019).
Wurzer, C. & Mašek, O. Feedstock doping using iron rich waste increases the pyrolysis gas yield and adsorption performance of magnetic biochar for emerging contaminants. Bioresour. Technol. 321, 124473 (2021).
Fawzy, S., Osman, A. I., Yang, H., Doran, J. & Rooney, D. W. Industrial biochar systems for atmospheric carbon removal: a review. Environ. Chem. Lett. 19, 3023–3055 (2021).
Puro.Earth. Puro.Earth carbon removal suppliers. https://puro.earth/services/.
Beerling, D. J. et al. Potential for large-scale CO2 removal via enhanced rock weathering with croplands. Nature 583, 242–248 (2020).
Bhave, A. et al. Screening and techno-economic assessment of biomass-based power generation with CCS technologies to meet 2050 CO2 targets. Appl. Energy 190, 481–489 (2017).
EMBER. EUA (EU ETS) Future Prices. https://ember-climate.org/data/carbon-price-viewer/ (2021).
Vassilev, S. V., Baxter, D., Andersen, L. K. & Vassileva, C. G. An overview of the composition and application of biomass ash. Part 1. Phase—mineral and chemical composition and classification. Fuel 105, 40–76 (2013).
Chen, W., Geng, Y., Hong, J., Yang, D. & Ma, X. Life cycle assessment of potash fertilizer production in China. Resour. Conserv. Recycl. 138, 238–245 (2018).
Someshwar, A. V. Wood and combination wood-fired boiler ash characterization. J. Environ. Qual. 25, 962–972 (1996).
Marsh, J. S. Basalt geochemistry and tectonic discrimination within continental flood basalt provinces. J. Volcanol. Geotherm. Res. 32, 35–49 (1987).
Kremer, D. et al. Geological mapping and characterization of possible primary input materials for the mineral sequestration of carbon dioxide in Europe. Minerals 9, 485 (2019).
Veld, H., Roskam, G. D., & van Enk, R. Desk study on the feasibility of CO2 sequestration by mineral carbonation of olivine—TNO Report 2008-U-R0776/B. (2008).
Author information
Authors and Affiliations
Contributions
W.B. conceptualised the idea for the manuscript in collaboration with C.W., D.A.C.M., and O.M. W.B. performed the data analyses, prepared figures and wrote the manuscript. C.W., D.A.C.M., E.J.R., J.B., and O.M. supported further conceptualisation and data presentation and reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Earth & Environment thanks Dries Roobroeck and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Clare Davis. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Buss, W., Wurzer, C., Manning, D.A.C. et al. Mineral-enriched biochar delivers enhanced nutrient recovery and carbon dioxide removal. Commun Earth Environ 3, 67 (2022). https://doi.org/10.1038/s43247-022-00394-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s43247-022-00394-w
This article is cited by
-
Spatial and temporal patterns of fuelwood consumption and its associated CO2 emissions in Muzaffarabad division, a western Himalayan region
Energy, Sustainability and Society (2024)
-
Land-neutral negative emissions through biochar-based fertilization—assessing global potentials under varied management and pyrolysis conditions
Mitigation and Adaptation Strategies for Global Change (2024)
-
Circularity of Nutrients for Food Security: a Case Study of By-products from Meat Industry
Circular Economy and Sustainability (2024)
-
Salt-affected marginal lands: a solution for biochar production
Biochar (2023)
-
Sludge-based biochar adsorbent: pore tuning mechanisms, challenges, and role in carbon sequestration
Biochar (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.