Abstract
Noble gases are important tracers of planetary accretion and acquisition of volatiles to planetary atmospheres and interiors. Earth’s mantle hosts solar-type helium and neon for which 20Ne/22Ne ratios advocate either incorporation of solar wind irradiated solids or solar nebula gas dissolution into an early magma ocean. However, the exact source location of primordial signatures remains unclear. Here we use high-resolution stepwise heating gas extraction experiments to analyse interior samples of the iron meteorite Washington County and find that they contain striking excesses of solar helium and neon. We infer that the Washington County protolith was irradiated by solar wind and that implanted noble gases were partitioned into segregating metal melts. The corollary that solar signatures are able to enter the cores of differentiated planetesimals and protoplanets validates hypotheses that Earth’s core may have incorporated solar noble gases and may be contributing to the solar signatures observed in Earth’s mantle.
Similar content being viewed by others
Introduction
Earth’s mantle contains solar-type helium and neon, which is in contrast to the situation for Earth’s atmosphere, where planetary-type noble gases dominate. The latter are distinguished by their strong depletion in He and Ne compared to solar abundances and the relative enrichment in heavy gases (Ar, Kr and Xe)1,2,3. In particular, plume-derived samples (a plume is a deep-rooted upwelling of abnormally hot material within Earth’s mantle) show high 3He/4He values2,3,4,5,6 and solar-like 20Ne/22Ne ratios2,3,4,7,8,9,10,11,12, in some cases indistinguishable from the solar wind (SW)-implanted Ne-B component in meteorites3,7,8,11 (20Ne/22NeNe-B ~12.5–12.7)13. The origin of the light solar noble gases within Earth7 has been explained either by SW implantation into Earth’s building blocks or by incorporation of captured nebular gases into an early magma ocean during accretion. Ratios for 20Ne/22Ne even higher than the Ne-B value have been observed, associated with samples from the deep mantle9,10,11, ranging up to 13.03 ± 0.04 (2σ) in a recent study of plume-influenced samples12. This, indeed, gives some credence to the capture idea (20Ne/22Nesolar nebula = 13.36 ± 0.09)14, but overall, this process is expected to be of limited efficiency only11,15. Whereas oceanic island basalts (OIBs), derived from the plume source, show high contributions of light solar noble gases as indicated by more primitive isotopic ratios3,11, systematically lower 3He/4He ratios and generally lower maximum 20Ne/22Ne ratios (≲12.5) are observed in samples derived from mid-ocean-ridge basalts (MORBs)3,8. This requires at least two separate reservoirs in Earth’s interior: A strongly degassed and well-homogenised reservoir in the upper mantle as a source for MORBs, and a more pristine, isolated and gas-rich source deep within Earth that is sampled by plumes. The reservoir of the primitive signatures, traditionally assumed to be isolated in the deep mantle, however, is not precisely located. To solve the requirement for a separate reservoir of solar noble gases in Earth’s mantle, some models also suggest Earth’s core as a possible source3,6,16,17,18, either for the total mantle flux or at least for the OIB flux, which contributes ca. 1–10% only of the former4,5,19.
A kind of precursor materials of planetary cores are iron meteorites, which represent our only available analogue to materials from the deep interior of Earth20. All studied iron meteorites in our collections are thought to have been derived from no more than ~75 asteroidal sources. Their chemical classification is mainly based on the composition of the highly volatile siderophile elements Ga and Ge relative to Ni and Ir (as well as other elements like Au and Co) and allows for assignment to either one of 14 geochemical groups or as being still “ungrouped”, with further subdivision into “magmatic” and “nonmagmatic”20,21 (Supplementary Fig. 1). Magmatic iron meteorites, including the largest known group IIIAB, show similar elemental fractionation trends within each group and are thus consistent with fractional crystallisation in the metallic cores of separate parent bodies20,21,22,23. Whereas the nonmagmatic, or “silicate-bearing”, groups (IAB, IIICD and IIE) exhibit substantially different chemical trends and are thought to have originated from processes involving partial differentiation, break-up and/or melting by impacts20,24,25, it is suggested that most ungrouped iron meteorites experienced fractional crystallisation like most magmatic irons22. The fractionally crystallised groups are considered as products of differentiation processes during the first few million years of our solar system26,27. While many of the differentiated protoplanets were incorporated into the growing terrestrial planets, yet some remained in the asteroid belt. During small body collisions, metal fragments can be liberated from the interior of larger bodies and injected into Earth-crossing orbits. For such iron meteoroids, transit times to Earth are generally long (typically hundreds of millions of years28), during which time interaction with galactic cosmic rays (GCRs) produces abundant cosmogenic noble gases29. This in situ produced component may easily obscure the signature of pristine noble gases, if also present within the metal. So far, only the iron meteorite Washington County (which is chemically different from nonmagmatic groups and more similar to the magmatic IIIAB or IIIE groups, Supplementary Figs. 1 and 2) was reported to contain a remarkable excess of non-cosmogenic light noble gases30,31,32,33,34,35,36 (i.e. 4He and 20Ne), which was taken as an indication for the presence of a primordial component32,33. An initial report34 of unfractionated solar He, Ne and Ar was, however, subsequently reinterpreted35 because the apparently detected SW34 was found in a sample containing material from the unablated rear surface of the meteorite (Supplementary Fig. 2), into which it might have been implanted recently during its transit to Earth. For the earlier observations31,32,33, no information about the location of the analysed samples is available, with the exception of an abstract36, in which, however, only preliminary data without in-depth evaluation were presented.
Results
Washington County noble gases
In view of the fundamental implications that the presence of SW gases in the bulk meteorite might have, and in order to overcome the problem of unknown sample location, we investigated noble gases in four aliquots (WC_2, WC_5, WC_11 and WC_14) of a 3-cm-long metal slab of the meteorite (Supplementary Fig. 3). Near-surface and interior samples were taken from between 0.2 and 2.8 cm distance from the fusion crust. In order to study the distribution of volume correlated noble gases and to identify distinct host phases (see Supplementary Fig. 3), we performed high-resolution He, Ne (Table 1) and Ar analyses (see “Methods” section for details) using, for individual samples, up to 25 gas extraction steps between 600 and 1800 °C (Supplementary Data 1). We also analysed a schreibersite etch residue and two randomly sampled bulk splits (WC_r, WC_g and WC_s) using extraction temperatures up to 2000 °C. The high-resolution stepwise heating extractions indicate two major degassing peaks for all samples (Supplementary Figs. 4–7), interpreted as gas release from schreibersite ((Fe,Ni)3P), at ~1100 °C, and kamacite–taenite (Fe,Ni), at ≳1400 °C.
In the current context, our Ar data (Supplementary Fig. 8) do not provide useful information and are not considered further here. This is because Ar has a much lower abundance in the SW compared to He and Ne14, while at the same time cosmogenic Ar is copiously produced by GCR interaction29. Instead, the crucial information comes from He and Ne (Table 1).
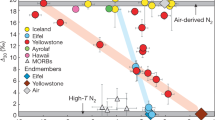
In a 4He/21Ne–4He/3He diagram (Fig. 1a), our new and previously reported bulk data31,32,33,34 indicate excesses of 4He with respect to the cosmogenic composition for a Washington County-sized meteoroid29, plotting instead along a mixing line between cosmogenic and SW. The same is observed if we plot selected extraction steps corresponding to the major release from schreibersite and kamacite–taenite, respectively (Fig. 1b and Supplementary Data 1), and also if we plot the numerous individual high-resolution extraction steps (Supplementary Fig. 9). While the latter data are susceptible to larger scatter (the He/Ne elemental ratios may become slightly fractionated during stepwise degassing), only the high-temperature gas extractions of WC_s (2000 °C) and of WC_14 kamacite–taenite (1480 °C) deviate discernibly from the mixing trend between GCR and SW, and point towards mixing with air.
a Literature31,32,33,34 and our new data (totals) for Washington County. b Selected noble gas extraction steps from our measurements that are assigned to major gas releases from schreibersite and kamacite–taenite, respectively. All data points, except for WC_14 (1480 °C kamacite–taenite gas extraction), plot (within 1σ uncertainties) directly on or close to the GCR-SW mixing line and indicate 4He excess of solar wind origin in all bulk samples and in both mineral constituents of Washington County. For reference, the GCR end-member composition (4He/21Ne = 150–200, 4He/3He = 2.4–2.9) for a Washington County-sized meteoroid29 (r ~ 10 cm)36 is shown as well as mixing lines towards the compositions of SW14, and air1 (both off-scale). The size of the GCR symbol equals the possible composition range of the spallation component.
In a classical Ne three-isotope plot (Fig. 2a), most bulk data suggest mixing between GCR and a solar component (SW or Ne-B). The presence of Ne-Q instead of solar Ne can be ruled out on the basis of elemental ratios (see Supplementary Note 1). Similarly, selected data for schreibersite and kamacite–taenite, respectively (Fig. 2b and Supplementary Data 1), as well as individual data of the high-resolution degassing steps (Supplementary Fig. 9) plot on the mixing lines between GCR and solar components. Only the data of WC_14, like in Fig. 1, deviate from this pronounced trend and plot within errors (1σ) on the GCR–air mixing line.
a Literature31,32,33,34 and our new data (totals) for Washington County. b Selected noble gas extraction steps from our measurements that are assigned to major gas releases from schreibersite and kamacite–taenite, respectively. All data points, except for WC_14, plot within errors (1σ) directly on or close to the GCR-Ne-B or GCR-SW mixing lines indicating a solar gas component within the bulk samples and in both mineral constituents of Washington County. Mixing lines are drawn from the most cosmogenic Ne values (20Ne/22Ne = 0.94 ± 0.05, 21Ne/22Ne = 0.936 ± 0.010) measured for Washington County (WC_14, 1050 °C, Supplementary Data 1) for which isotopic ratios plot closest to the GCR end-member of a Washington County-sized meteoroid. For reference, this GCR end-member composition (see Supplementary Fig. 9), SW14, Ne-B19 and air1 are shown.
Discussion
Solar noble gas origin
Planetary embryos with a mass >0.1% of that of Earth15—equivalent to a size larger than the dwarf planet Ceres—may have attracted a tenuous atmosphere while being immersed in the gas of the protosolar nebula. However, solar gas can only enter the protoplanet’s interior if both a dense atmosphere and a magma ocean form simultaneously. It was found15 that even embryos with 10% Earth’s mass could not have dissolved sufficient solar gas in a magma ocean to account for the Ne content of Earth’s mantle. Hence, we conclude that such a process is even more unrealistic in the case of asteroidal-sized parent bodies. This leaves SW irradiation of precursors as the more viable source15, which, moreover, is a frequently observed source of solar gases in meteorites3,7,8,13. Washington County, if considered as core analogue, thus, provides evidence that cores of planetary bodies may host noble gases of SW origin. The ubiquitous occurrence of solar-type helium and neon in the main phases of the meteorite (schreibersite and metal), in all interior samples up to several centimetres away from the fusion crust, documents that they cannot represent implanted SW from the recent passage to Earth. Surface-implanted components could only be present in a thin layer1,14, concentrated in the outermost few tens of nanometres2, which are usually ablated during atmospheric entry37,38. Although our measured variable concentrations of the solar component in Washington County argue for a heterogeneous distribution on a millimetre scale (variable concentrations of trapped noble gas in samples of adjacent areas were noted earlier32)—may be due to exsolution features of the kamacite–taenite structure during crystallisation—the occurrence of solar gases throughout the bulk meteorite indicates incorporation during metal formation within its parent body. This implies SW irradiation of the chondritic precursor protolith before metal segregation. A scenario involving irradiation of chondritic precursor material during the initial stages of our solar system appears reasonable, particularly after, but also before, dissipation of the solar nebula, a process that took place after a protoplanetary disk lifetime of ~6 Ma39 or possibly after more than ten million of years40. Although the accretion disk is considered as optically thick, due to its high gas and dust content, accreting solids in off-disk regions, which are less opaque, may have been repeatedly irradiated13,41. This process is valid for planetary building blocks with highly inclined orbits when appearing out of the settled disk at high distances from the midplane, for material on the surface of the disk, and actually also in parts of the midplane13. Consequently, it can be expected even during the earliest stages of solar system evolution that irradiated solids were continuously incorporated into accreting bodies.
Partitioning into planetary cores
Washington County samples contain ~4.8 × 10−9 cm3 g−1 solar 3He and 2.1 × 10−8 cm3 g−1 solar 20Ne, respectively (Supplementary Table 1), with a quasi-unfractionated average 3He/20Ne ratio of ~0.26 (3He/20NeSW ~0.3)14. If we assume an undifferentiated Washington County precursor protolith that also resembles a type of terrestrial precursor material, we might consider, for example, SW-irradiated, gas-rich CV chondrites42 (Vigarano-type carbonaceous chondrites) or enstatite chondrites43 (E chondrites) based on similarities in isotopic composition. With average 20Nesolar bulk concentrations of ~4.5 × 10−7 cm3 g−1 in CV and ~6.1 × 10−7 cm3 g−1 in E chondrites (Supplementary Data 2 and 3), the partition coefficient for Ne between Washington County-like metal and the Washington County parent body (DNe = 20Nesolar(WC)/20Nesolar(protolith), where 20Ne(protolith) corresponds to 20Nesolar in either CV or E chondrites) must have been ca. 4.6 × 10−2 and 3.4 × 10−2, respectively, in order to achieve the observed solar Ne abundance. For CI and CM chondrites (Ivuna- and Mighei-type carbonaceous chondrites, respectively) with bulk 20Nesolar concentrations of ~1.3 × 10−7 and ~2.2 × 10−7 cm3 g−1 (Supplementary Table 2 and Supplementary Data 4), the corresponding partition coefficients are ~1.6 × 10−1 and ~9.6 × 10−2, respectively. We emphasise, however, that CI–CM precursor material played only a limited role as building blocks during terrestrial accretion before core formation44 and rather contain low fractions of solar-like Ne isotopic ratios42.
A value for DNe in the range 10−2–10−1 was indeed recently determined between molten iron-rich metal and molten silicate under pressures up to 16 GPa17. At more relevant pressures of ≲1 GPa (corresponding to the maximum pressure at the centre of a planetesimal with 100 km radius), the partition coefficient of helium during percolative core formation and segregation between liquid metal and solid silicate is as high as 11.8 ± 1.818. With such a value, virtually all noble gases—having similar partitioning behaviour16,18,19,45—would enter the metal phase. In this case, even much lower protolith 20Nesolar concentrations of ~2 × 10−9 cm3 g−1 20Ne would yield the concentrations observed in Washington County.
Large planetary cores—like the terrestrial one—have largely been formed by merging cores from differentiated planetesimals26,46, of which a distinct fraction of Washington County-like metal can be expected. Despite some models argue for equilibration of a major part (70–100%) of the precursor metal with the terrestrial mantle before entering Earth’s core47, a certain fraction of the precursor cores might have undergone only limited (≥36%) re-equilibration and emulsification18,46,48. Hence, solar gases may have been delivered to Earth’s core either piggyback in planetesimal cores or may have entered core-forming metal phases by metal–silicate partitioning in the proto-mantle, which also contained solar gases2,3,4,5,7,8,19. An important question to be considered is if solar gases from the core can contribute significantly to mantle noble gases observed today.
In a first step, we consider the present-day mantle degassing flux of 3He ranging from 267 to 1070 mol per year49, which includes the most recent estimate of 800 ± 170 (2σ) mol per year50. We further consider a two-stage degassing history of Earth3,51 with massive degassing in the first 100 Ma of accretion during a magma ocean stage followed by more “tranquil” fluxes3 soon after the moon-forming impact at ~4.45 Ga52, associated with degassing at mid-oceanic ridges, which continues until today. It is important to note here that the first stage of massive degassing is considered to be sourced by the proto-mantle19,51, in which most of the solar gases resided after metal–silicate partitioning—this degassing phase does not need to be sourced by the core. Only after the moon-forming impact, we assume mantle degassing to be supported by the core. Initially, e.g., during the Hadean, degassing of residual mantle noble gases may have contributed significantly, in addition to a core flux. Later, the core contribution could have become more and more important, particularly for the OIB mantle domains. In the extreme scenario that all degassed noble gases from the mantle after the moon-forming impact would be replenished by the underlying core, the upper limit present-day mantle flux of 1070 mol per year 3He (i.e. 4 atoms cm−2 s−1)53 throughout the past 4.4 Ga of Earth’s history yields an initial core concentration of ~5.5 × 10−11 cm3 g−1 3He. Hence, ~1% of Washington County-type metal (with 4.8 × 10−9 cm3 g−1 3He) is required to have contributed to proto-Earth. If we consider more active geodynamics and higher heat fluxes during the Hadean than today54, assuming an order of magnitude higher volatile fluxes from the mantle during 4.4–4.0 Ga, this would roughly double the required initial core concentrations to ~1 × 10−10 cm3 g−1 3He, which in turn would demand ~2% Washington County-type metal contribution to Earth’s core. It is of vital importance that these numbers are maximum values to cover the total flux of solar gases from the mantle. If only the much lower OIB noble gas flux4,5,19 is assumed to be sourced by the core, a factor of 10–100 lower inventory is required, i.e., only a 0.02–0.2% contribution of WC-type material to Earth’s core. The reasoning above is also valid for Ne, because both Washington County and the terrestrial mantle incorporated solar He and Ne2,3,4,7,8,19. Larger fractions of solar gas-bearing metal are possible and would provide correspondingly larger initial core inventories, while at the same time counterbalancing possible losses of noble gases from metal bodies during accretion. As mentioned above, such losses might, for instance, happen if a certain fraction of planetesimal core material equilibrated at high pressures when sinking through the magma ocean, before merging with Earth’s proto core18,46,47,48. For this fraction, the low partition coefficients measured for pressures up to 16 GPa between liquid metal and liquid silicate of ~10−3–10−2 for DHe and 10−2–10−1 for DNe would apply, which likely also apply for higher pressures at conditions of Earth’s core formation16,17. As exemplified earlier, even for such low partition coefficients typical solar gas concentrations in SW-irradiated precursor protoliths would be sufficient.
Earth’s core as a solar noble gas reservoir
Only minor SW-irradiation levels (when compared to typical solar gas-rich chondrites), associated with low-pressure metal segregation in small planetary bodies, is required to achieve Washington County-like concentrations of solar helium and neon. Furthermore, to explain the terrestrial noble gas inventory, only a minor fraction of such planetesimals, when containing a metallic core resembling Washington County-like material, is required to accrete to Earth’s core. This would still allow a substantial fraction to equilibrate with the mantle at higher pressures18 and even to lose some solar-type noble gases from the accreting metal before merging with the terrestrial core. Here, it has to be kept in mind that only ~1–2% of such material is needed, whereas Washington County represents ≥1% of all known iron meteorite parent bodies20, and a factor of 10–100 lower contribution is sufficient if only the OIB flux is sourced from Earth’s core. At the same time, the proportion of solar gas-bearing iron cores might have been higher in the zone of the terrestrial building blocks closer to the sun than the asteroid belt regions where most meteorite parent bodies reside. On the other hand, there is evidence for light solar noble gases in several other iron meteorites30 from both nonmagmatic and different magmatic groups (Supplementary Figs. 10 and 11), demonstrating that Washington County might not be a unique case. For these other iron meteorites, however, it remains so far unclear whether the solar gases are due to SW irradiation of the parent body or due to irradiation during transport in the solar system. Independent of the origin of the solar gases within the iron meteorites, this indicates that precursor metal from both incipient/partial and completed core formation processes on asteroids could have contributed to solar gas inventories of planetary cores, and deserves detailed investigations in upcoming studies. In this regard, our data represent the first solid proof of solar noble gases in metal from a small body, an important missing link en route to planetary core formation.
We note that our model can not only explain why noble gases were entering the terrestrial core but also why they leave the core. While higher partition coefficients at low-pressure metal segregation can explain effective partitioning into metal45 (most efficiently from solid silicates5,18), lower partition coefficients prevailing at high pressures16,17 can easily explain noble gas back transfer into the mantle at the core-mantle boundary (CMB), especially after reducing concentrations of noble gases in the mantle by massive degassing in the Hadean3,19,51,54. Effective noble gas transport across the CMB is particularly valid if transient partial silicate melts occur above the core; as judged from the orders of magnitude higher partition coefficient (DHe) between liquid metal and solid silicate compared to partitioning between liquid metal and liquid silicate at 1 GPa16,18,45, the transfer into liquid silicate is much easier than diffusion into solid mantle material. It has to be noted, however, that, to our knowledge, no noble gas partition data for CMB conditions exist, whereas, in support of our model, a single value for DHe = 9 × 10−3 at 40 GPa obtained by molecular dynamics simulations55 is consistent with low partition coefficients between molten metal and liquid silicate approaching conditions at the CMB.
For our planet, this may offer a new solution for problems associated with keeping different mantle regimes with distinct noble gas signatures, by fluxing individual reservoirs from the underlying core4,5,6,16,17,18,19. At the same time, this would imply a considerable—previously neglected—active role of Earth’s core in mantle geochemistry and volatile geodynamics, which should be integrated into future studies.
Methods
Sample preparation
We obtained a 3-cm-long slab of Washington County (WC_3078A, 0.75 g; Supplementary Fig. 3) from Dr. Jutta Zipfel from the collection at the Senckenberg Forschungsinstitut und Naturmuseum Frankfurt, Germany. The fusion crust of the former surface of the meteorite is located at one end, while the other end exhibits the interior of Washington County. At the Institut für Geowissenschaften in Heidelberg, the slab surface was polished and cleaned to reexamine the previously reported56,57 primary mineral phases with a scanning electron microscope (SEM) (Supplementary Fig. 3). The distribution of potential (minor) noble gas carrier phases was of particular interest. Subsequent to SEM analyses, WC_3078A was cut in length to spare one half of the slab for additional investigations. The other half was cut into 15 aliquots (Supplementary Fig. 3; WC_1–WC_15 from surface to depth) of comparable size and weights of 7.3–21.1 mg to allow noble gas measurements of near-surface and interior samples. WC_g and WC_s were investigated at Max-Planck-Institut für Chemie in Mainz and come from different ends of a ~1-cm-long Washington County sample. WC_r is a residue obtained after dissolution in sulfuric acid of 1.03 g of original sample material. The acid treatment resulted in a 14.1 mg residue of almost pure schreibersite with particle sizes of 3–5 µm. The Mainz sample (0.5 cm × 0.5 cm × 1.5 cm) had been provided by O. Schaeffer and subsequently distributed via H. Voshage and F. Begemann. Noble gas data of another part of this sample were reported previously33.
Analytical methods
At the Institut für Geowissenschaften, Universität Heidelberg, Germany, four aliquots from near-surface and interior parts of the sample slab WC_3078A were selected (WC_2: 17.3 mg; WC_5: 18.3 mg; WC_11: 14.2 mg; WC_14: 20.9 mg; the “missing” pieces in Supplementary Fig. 3). After weighing, we wrapped all samples in aluminium foil. Noble gas extraction and measurements (Table 1 and Supplementary Data 1) were carried out in Heidelberg and followed procedures as described in Bartoschewitz et al.58, with some modifications. We applied a high-resolution stepwise heating procedure for the first measured sample piece (WC_5, 25 temperature steps between 600 and 1800 °C) in order to constrain the general noble gas release pattern (Supplementary Data 1). The observed degassing pattern was used to refine the measurement procedure for subsequently analysed samples with a lower resolution stepwise heating protocol (WC_2, WC_11 and WC_14). Analytical noble gas data for Washington County samples WC_g, WC_s and WC_r (WC_g: 22.40 mg bulk, WC_s: 21.87 mg bulk, WC_r: 8.54 mg residue; using extraction temperatures between 800 and 2000 °C; Table 1 and Supplementary Data 1) were obtained at the Max-Planck-Institut für Chemie in Mainz, Germany, using previously described experimental procedures59,60.
Noble gas extraction
Noble gas extractions and analyses of samples WC_2, WC_5, WC_11 and WC_14 were performed at the Institut für Geowissenschaften, Universität Heidelberg, Germany. For gas extraction, we used a resistance-heated furnace consisting of an outer Ta tube containing an inner crucible consisting of molybdenum. Purification from active gases was achieved by exposure to two cold Al-Zr-(SAES) getters (WC_5) and two cold Ti getters (WC_2, WC_5, WC_11 and WC_14) during and after heating until the separation of He, Ne from Ar. For samples WC_2, WC_11 and WC_14, we omitted the Al-Zr getters from the cleaning process because minor atmospheric contributions from a leak in their system volume were discovered during a consecutive blank series (see Supplementary Data 1). After each step of heating, Ar was separated from He and Ne by transfer to a charcoal trap cooled with liquid nitrogen. The remaining He and Ne were fixed at another cryostatically cooled charcoal trap at ~20 K for WC_5 and WC_14. Helium was then fully separated from neon at 48 K and subsequently measured. In case of WC_2 and WC_11, only Ne was transferred to the cryostatically cooled charcoal kept at ~48 K, whereas the remaining He was directly measured. Neon was released from the trap at 120 K and then introduced into the mass spectrometer. The argon fraction was additionally cleaned by two hot Al-Zr getters (ca. 400 °C, WC_5 only) and two hot Ti getters (ca. 300 and 600 °C, all samples) for 15 min, respectively. In case of analysis of WC_5, Ar was transferred to a cryostatically cooled stainless-steel sponge adsorber. Subsequent separation of argon from xenon was achieved at 90 K, resulting in ~93% of the Ar fraction that was present in the analysis. In case of WC_2, WC_11 and WC_14, the full amount of Ar was available for analysis by a direct transfer to another charcoal trap held at liquid nitrogen temperature close to the mass spectrometer.
Noble gas measurement
Noble gas measurements were performed with a VG 3600 noble gas mass spectrometer. We used ionisation settings of 120 µA trap current, 5 kV acceleration voltage and a nominal ionisation energy of 80 eV except for the Ar fraction of WC_5 (60 eV). All isotopes except 4He and 40Ar were detected by a channeltron in a peak-jumping single ion counting mode. Because of expected higher signals, the ion currents of 4He and 40Ar were measured with a Faraday cup. In order to reduce mass interferences (in particular from 40Ar) during measurement of He and Ne the mass spectrometer volume was connected with a charcoal trap cooled with liquid nitrogen. We checked potential interferences during Ne measurements by simultaneous measurement of masses 18 (H2O), 40 (Ar), 44 (dominantly CO2) and 42 (hydrocarbons) and applied corrections61. Frequent measurements of calibration gases bracketing the sample measurements enabled us to correct for instrumental mass fractionation. Calibration gas measurements further allowed calculating the absolute gas amounts. The isotopic composition of all calibration gases is equivalent to air ratios except for He. The He gas standard is an artificial gas enriched in 3He with an isotopic composition of 4He/3He: 40,183 ± 87.
System blank heights were determined from a sequence of blank measurements for all gases and samples between 800 and 1800 °C. Blank uncertainties were set to ±10% for 4He and 36Ar, and ±5% for 20Ne (1σ errors). In general, isotopic compositions of blanks were indistinguishable from the air. This is also assumed for He, although 3He was always below the detection limit (i.e. below ca. 5 c.p.s.). Hence, we assume an air composition (±20% for He, ±10% for Ar, ±5% for Ne, 1σ errors) of the blank. Blank contributions could be substantial for 4He, 20Ne, 22Ne, 36Ar and 40Ar, but were generally low (at most few %) for the typical cosmogenic nuclides 3He, 21Ne and 38Ar.
Data availability
All data shown in the figures and reported in the paper are provided as Supplementary information and Supplementary Data. Data are shown in Figs. 1 and 2 and Supplementary Figs. 4–9 are given in Supplementary Data 1. Supplementary Data 2–4 include compiled literature noble gas data for CV, E and CM chondrites, respectively. Primary data of noble gas measurements have been deposited in the Astromaterials Data System (AstroMat Repository) and are available for download at https://doi.org/10.26022/IEDA/111938.
References
Ozima, M. & Podosek, F. A. Noble Gas Geochemistry 286pp (Cambridge University Press, 2002).
Pepin, R. O. & Porcelli, D. Origin of noble gases in the terrestrial planets. Rev. Mineral. Geochem. 47, 191–246 (2002).
Moreira, M. Noble gas constraints on the origin and evolution of Earth’s volatiles. Geochem. Perspect. 2, 229–403 (2013).
Porcelli, D. & Ballentine, C. J. Models for the distribution of terrestrial noble gases and evolution of the atmosphere. Rev. Mineral. Geochem. 47, 411–480 (2002).
Porcelli, D. & Halliday, A. N. The core as a possible source of mantle helium. Earth Planet. Sci. Lett. 192, 45–56 (2001).
Macpherson, C. G., Hilton, D. R., Sinton, J. M., Poreda, R. J. & Craig, H. High 3He/4He ratios in the Manus backarc basin: Implications for mantle mixing and the origin of plumes in the western Pacific Ocean. Geology 26, 1007–1010 (1998).
Péron, S., Moreira, M. & Agranier, A. Origin of light noble gases (He, Ne, and Ar) on Earth: a review. Geochem. Geophys. Geosyst. 19, https://doi.org/10.1002/2017GC007388, https://doi.org/10.1002/2017GC007388 (2018).
Trieloff, M., Kunz, J., Clague, D. A., Harrison, D. & Allègre, C. J. The nature of pristine noble gases in mantle plumes. Science 288, 1036–1038 (2000).
Yokochi, R. & Marty, B. A determination of the neon isotopic composition of the deep mantle. Earth Planet. Sci. Lett. 225, 77–88 (2004).
Mukhopadhyay, S. Early differentiation and volatile accretion recorded in deep-mantle neon and xenon. Nature 486, 101–104 (2012).
Péron, S. et al. Neon isotopic composition of the mantle constrained by single vesicle analyses. Earth Planet. Sci. Lett. 449, 145–154 (2016).
Williams, C. D. & Mukhopadhyay, S. Capture of nebular gases during Earth’s accretion is preserved in deep-mantle neon. Nature 565, 78–81 (2019).
Moreira, M. & Charnoz, S. The origin of the neon isotopes in chondrites and on Earth. Earth Planet. Sci. Lett. 433, 249–256 (2016).
Heber, V. S. et al. Isotopic mass fractionation of solar wind: evidence from fast and slow solar wind collected by the genesis mission. Astrophys. J. 759, 121 (2012).
Jaupart, E., Charnoz, S. & Moreira, M. Primordial atmosphere incorporation in planetary embryos and the origin of Neon in terrestrial planets. Icarus 293, 199–205 (2017).
Bouhifd, M. A., Jephcoat, A. P., Heber, V. S. & Kelley, S. P. Helium in Earth’s early core. Nat. Geosci. 6, 982–986 (2013).
Bouhifd, M. A., Jephcoat, A. P., Porcelli, D., Kelley, S. P. & Marty, B. Potential of Earth’s core as a reservoir for noble gases: case for helium and neon. Geochem. Perspect. Lett. 15, 15–18 (2020).
Roth, A. S. G. et al. The primordial He budget of the Earth set by percolative core formation in planetesimals. Geochem. Perspect. Lett. 9, 26–31 (2019).
Trieloff, M. & Kunz, J. Isotope systematics of noble gases in the Earth’s mantle: possible sources of primordial isotopes and implications for mantle structure. Phys. Earth Planet. Inter. 148, 13–38 (2005).
Benedix, G. K., Haack, H. & McCoy, T. J. in Treatise on Geochemistry 2nd edn (eds Holland, H. D. & Turekian, K. K.) 267–285 (Elsevier, 2014).
Krot, A. N., Keil, K., Scott, E. R. D., Goodrich, C. A. & Weisberg, M. K. in Treatise on Geochemistry 2nd edn (eds Holland, H. D. & Turekian, K. K.) 1–63 (Elsevier, 2014).
Goldstein, J. I., Scott, E. R. D. & Chabot, N. L. Iron meteorites: crystallization, thermal history, parent bodies, and origin. Chem. Erde 69, 293–325 (2009).
Chabot, N. L. & Haack, H. in Meteorites and the Early Solar System II (eds Lauretta, D. S. & McSween Jr., H. Y.) 747–771 (University of Arizona Press, 2006).
Benedix, G. K., McCoy, T. J., Keil, K. & Love, S. G. A petrologic study of the IAB iron meteorites: constraints on the formation of the IAB-Winonaite parent body. Meteorit. Planet. Sci. 35, 1127–1141 (2000).
Wasson, J. T. & Kallemeyn, G. W. The IAB iron-meteorite complex: a group, five subgroups, numerous grouplets, closely related, mainly formed by crystal segregation in rapidly cooling melts. Geochim. Cosmochim. Acta 66, 2445–2473 (2002).
Kleine, T. et al. Hf–W chronology of the accretion and early evolution of asteroids and terrestrial planets. Geochim. Cosmochim. Acta 73, 5150–5188 (2009).
Wadhwa, M., Srinivasan, G. & Carlson, R. in Meteorites and the Early Solar System II (eds Lauretta, D. S. & McSween Jr., H. Y.) 715–731 (University of Arizona Press, 2006).
Eugster, O., Herzog, G. F., Marti, K. & Caffee, M. W. in Meteorites and the Early Solar System II (eds Lauretta, D. S. & McSween Jr., H.Y.) 829–851 (University of Arizona Press, 2006).
Ammon, K., Masarik, J. & Leya, I. New model calculations for the production rates of cosmogenic nuclides in iron meteorites. Meteorit. Planet. Sci. 44, 485–503 (2009).
Schultz, L. & Franke, L. Helium, neon, and argon in meteorites: a data collection. Meteorit. Planet. Sci. 39, 1889–1890 (2004).
Schaeffer, O. A. & Fisher, D. E. Cosmogenic noble gases in the Washington County Meteorite. Nature 183, 660–661 (1959).
Signer, P. & Nier, A. O. C. in Research on Meteorites (ed. Moore, C. B.) 7–35 (Wiley, 1962).
Hintenberger, H., Schultz, L., Wänke, H. & Weber, H. Helium- und Neonisotope in Eisenmeteoriten und der Tritiumverlust in Hexaedriten. Z. Naturforsch. A 22, 780–787 (1967).
Becker, R. H. & Pepin, R. O. Solar composition noble gases in the Washington County iron meteorite. Earth Planet. Sci. Lett. 70, 1–10 (1984).
Becker, R. H. & Pepin, R. O. Solar composition noble gases in the Washington County iron meteorite: a correction. Earth Planet. Sci. Lett. 84, 356 (1987).
Murty, S. V. S. & Ranjith Kumar, P. M. Volume correlated solar noble gases in Washingtonm County Iron Meteorite. In Lunar Planetary Science Conference, Vol. 45, abstract #1110 (2014).
Bhandari, N. et al. Atmospheric ablation in meteorites: a study based on cosmic ray tracks and neon isotopes. Nuclear Tracks 4, 213–262 (1980).
Bland, P. A. & Artemieva, N. A. The rate of small impacts on Earth. Meteorit. Planet. Sci. 41, 607–631 (2006).
Haisch, K. E. J., Lada, E. A. & Lada, C. J. Disk frequencies and lifetimes in young clusters. Astrophys. J. Lett. 553, L153–L156 (2001).
Pfalzner, S., Steinhausen, M. & Menten, K. Short dissipation times of proto-planetary disks: an artifact of selection effects? Astrophys. J. Lett. 793, L34 (2014).
Sasaki, S. Off-disk penetration of ancient solar wind. Icarus 91, 29–38 (1991).
Trieloff, M., Kunz, J. & Allègre, C. J. Noble gas systematics of the Réunion mantle plume source and the origin of primordial noble gases in Earth’s mantle. Earth Planet. Sci. Lett. 200, 297–313 (2002).
Dauphas, N. The isotopic nature of the Earth’s accreting material through time. Nature 541, 521–524 (2017).
Braukmüller, N., Wombacher, F., Funk, C. & Münker, C. Earth’s volatile element depletion pattern inherited from a carbonaceous chondrite-like source. Nat. Geosci. 12, 564–568 (2019).
Matsuda, J. et al. Noble gas partitioning between metal and silicate under high pressures. Science 259, 788–790 (1993).
Nimmo, F. & Kleine, T. in The Early Earth - Accretion and Differentiation. Geophysical Monograph 212 (eds Badro, J. & Walter, M. J.) 83–102 (Wiley, 2015).
Rubie, D. C. et al. Accretion and differentiation of the terrestrial planets with implications for the compositions of early-formed Solar System bodies and accretion of water. Icarus 248, 89–108 (2015).
Rudge, J. F., Kleine, T. & Bourdon, B. Broad bounds on Earth/‘s accretion and core formation constrained by geochemical models. Nat. Geosci. 3, 439–443 (2010).
Holzer, M. et al. Objective estimates of mantle 3He in the ocean and implications for constraining the deep ocean circulation. Earth Planet. Sci. Lett. 458, 305–314 (2017).
Tucker, J. M., Mukhopadhyay, S. & Gonnermann, H. M. Reconstructing mantle carbon and noble gas contents from degassed mid-ocean ridge basalts. Earth Planet. Sci. Lett. 496, 108–119 (2018).
Allègre, C. J., Staudacher, T. & Sarda, P. Rare gas systematics: formation of the atmosphere, evolution and structure of the Earth’s mantle. Earth Planet. Sci. Lett. 81, 127–150 (1987).
Koeberl, C. The record of impact processes on the early Earth: a review of the first 2.5 billion years. In Processes on the Early Earth. Geological Society of America, Special Papers 405 (eds Reimold, U. W. & Gibson, R. L.) 1–22 (2006).
Craig, H., Clarke, W. B. & Beg, M. A. Excess 3He in deep water on the East Pacific Rise. Earth Planet. Sci. Lett. 26, 125–132 (1975).
Yokochi, R. & Marty, B. Geochemical constraints on mantle dynamics in the Hadean. Earth Planet. Sci. Lett. 238, 17–30 (2005).
Zhang, Y. & Yin, Q.-Z. Carbon and other light element contents in the Earth’s core based on first-principles molecular dynamics. Proc. Natl Acad. Sci. USA 109, 19579–19583 (2012).
Buchwald, V. F. Handbook of Iron Meteorites: Their History, Distribution, Composition and Structure (University of California Press, 1975).
Ray, D. & Ghosh, S. Washington County iron meteorite reclassified as IIIAB. In 77th Annual Meeting of the Meteoritical Society, Vol. 49, abstract #5159 (2014).
Bartoschewitz, R. et al. The Braunschweig meteorite − a recent L6 chondrite fall in Germany. Chem. Erde 77, 207–224 (2017).
Ott, U. Noble gases in SNC meteorites: Shergotty, Nakhla, Chassigny. Geochim. Cosmochim. Acta 52, 1937–1948 (1988).
Schelhaas, N., Ott, U. & Begemann, F. Trapped noble gases in unequilibrated ordinary chondrites. Geochim. Cosmochim. Acta 54, 2869–2882 (1990).
Hopp, J. & Viladkar, S. G. Noble gas composition of Indian carbonatites (Amba Dongar, Siriwasan): implications on mantle source compositions and late-stage hydrothermal processes. Earth Planet. Sci. Lett. 492, 186–196 (2018).
Acknowledgements
This work was supported by Klaus Tschira Stiftung gGmbH.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
M.V. performed the analyses at the Institut für Geowissenschaften in Heidelberg with the help from J.H. and W.H.S. U.O. contributed to the sample analyses at the Max-Planck-Institut für Chemie in Mainz. M.V., M.T. and U.O. wrote the manuscript. All authors contributed to the discussion and to the final draft of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Primary handling editor: Joe Aslin.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vogt, M., Trieloff, M., Ott, U. et al. Solar noble gases in an iron meteorite indicate terrestrial mantle signatures derive from Earth’s core. Commun Earth Environ 2, 92 (2021). https://doi.org/10.1038/s43247-021-00162-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s43247-021-00162-2
This article is cited by
-
Highest terrestrial 3He/4He credibly from the core
Nature (2023)
-
Primitive noble gases sampled from ocean island basalts cannot be from the Earth’s core
Nature Communications (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.