Abstract
Anthropogenic pollution from marine microplastic particles is a growing concern, both as a source of toxic compounds, and because they can transport pathogens and other pollutants. Airborne microplastic particles were previously observed over terrestrial and coastal locations, but not in the remote ocean. Here, we collected ambient aerosol samples in the North Atlantic Ocean, including the remote marine atmosphere, during the Tara Pacific expedition in May-June 2016, and chemically characterized them using micro-Raman spectroscopy. We detected a range of airborne microplastics, including polystyrene, polyethylene, polypropylene, and poly-silicone compounds. Polyethylene and polypropylene were also found in seawater, suggesting local production of airborne microplastic particles. Terminal velocity estimations and back trajectory analysis support this conclusion. For technical reasons, only particles larger than 5 µm, at the upper end of a typical marine atmospheric size distribution, were analyzed, suggesting that our analyses underestimate the presence of airborne microplastic particles in the remote marine atmosphere.
Similar content being viewed by others
Introduction
Marine microplastic particles (MP) are plastic particles of sizes up to 5 mm1,2. Primary microplastic is produced either for direct use or as a precursor for other products, while secondary microplastics are formed in the environment from breakdown of larger plastic material, especially marine debris1,2. Marine MP are a subject of elaborate research, and a matter of great concern in the fields of marine pollution, chemistry, and ecology3,4,5,6,7,8,9,10,11,12,13. MP may pose a risk to aquatic environments due to their documented ubiquity in the marine ecosystems, their long lifetime in the environment, their ability to transport potential pathogens and persistent organic pollutants14, their role as a new source of toxic compounds in the marine environment, and their propensity to be ingested by diverse organisms1,2,15,16,17,18,19,20. Marine microplastic persistence is a major source of concern, since plastic entering marine ecosystems doesn’t degrade, but rather undergoes mechanical erosion and photooxidation via interaction with ultraviolet (UV) radiation, resulting in fragmentation to micro- and nanoplastic sizes (<1 µm)1. The microplastic and nanoplastic fragments are easily incorporated in an increasing number of environmental matrices, making them impossible to remove. The effect of MP toxicity on marine biota increases with decreasing size, since smaller sizes facilitate ingestion, as well as chemical processes such as leaching1. Estimates of recent years suggest that ∼5.25 trillion plastic fragments are currently floating on the oceans’ surface21. Airborne microplastic particles (AMP) from terrestrial origins were found in both polluted and remote areas over land, as well as in coastal regions, ranging from micron to mm scales, and can travel tens of kilometers12,13,22,23,24,25,26,27,28,29,30,31,32,33. However, despite the overwhelming amounts of marine MP and their effect on the marine environment, atmospheric microplastic particles over the ocean are given little attention13. Marine AMP may have implications on marine ecology; facilitated by the winds, AMP can spread potential pathogens and persistent organic pollutants adsorbed onto their surface14, by an order of magnitude faster than the ocean currents34,35. Furthermore, the components adsorbed to the AMP are exposed to UV radiation and oxidation, which can increase their toxicity to marine biota1,2,36.
The ubiquity of AMP from terrestrial sources, and the ubiquity of MP in the world’s oceans raise the question whether AMP are present in the remote marine atmosphere. We searched for marine AMP during the Tara Pacific expedition, a 2.5 year research project that investigates coral reefs and plankton communities37,38,39. In this report, we focus on marine AMP collection and analysis conducted on May–June 2016, during the first North Atlantic crossing of the Tara Pacific Expedition.
Results and discussion
The main goals of this study were to determine whether AMP are present in the open ocean, to chemically identify them, and to investigate their possible sources. For these purposes we collected ambient atmospheric aerosol samples along the first North Atlantic transect of the Tara Pacific expedition (see Fig. 1) onto polycarbonate membranes. Overall, 43 samples were analyzed, and AMP were found in nine of them (~20%). Using micro-Raman spectroscopy, a highly novel and reliable method for polymer identification and characterization40,41,42,43 (see “Methods” section for details), we chemically identified AMP as small as 5 µm in the collected marine aerosol samples. Strict measures were taken to assure that the AMP we identified did not originate from the boat, nor from any stage of the sampling and analysis. The R/V Tara surfaces were thoroughly sampled, and we found that they may be a source of plastic colorants, polyester and cellulose in the marine environment (see “Methods SI” section for details). Materials found in the handling blanks or on Tara surfaces were excluded as AMP, since their source could be the R/V Tara itself. Only materials detected exclusively in the collected atmospheric samples were considered as AMP (full details in the “Methods” section).
a The presence of AMP and MP along the Atlantic transect. The different colors of the transect presented on the map represent the segments that the R/V Tara crossed during each airborne filter collection. Forty-six airborne samples (filters) were collected in total, with typical sampling durations of 12–24 h. Each airborne sample number where AMP was detected along the transect (2, 5, 10, 11, 17, 23, 30, 38, and 39) is presented on the map, along with the corresponding sampling location (circled in blue), and the detected AMP type (abbreviated; full names of the plastic compounds are provided in Table 1). The colored full circles along the transect represent seawater microplastic particle (MP) log abundance per m3. Note that the seawater concentrations vary by 4 orders of magnitude. b Raman spectra of the AMP types collected during the transect. The obtained Raman spectrum (arbitrary intensity vs. Raman shift in wavenumber) of each airborne AMP and its light microscope image are presented. The AMP sample number as appears on the map is written next to each Raman spectrum. The Raman spectra of the plastic polymer standards, corresponding to the sampled AMP types are presented in magenta above the samples’ spectra.
We detected AMP in the marine atmosphere from the airborne samples collected in the North Atlantic Ocean, and found that most of them correspond to the most ubiquitous MP found in the marine environment6,44,45,46,47 (see Fig. 1 and Table 1). AMP samples 2, 5, 17 and 30 contained polystyrene (PS), where the Raman spectrum of sample 17 is a combination of PS with the polycarbonate filter on which the samples were collected and analyzed. Polyethylene (PE) and polypropylene (PP) were observed in samples 38 and 39, respectively. The spectrum of sample 38 shows two peaks in the region of 500 cm−1 wavenumber (Figs. 1b), attributed to rutile (TiO2), which may serve as a whitening agent or part of a colorant for plastics. The PP found in sample 39 is low ethylene RCP (random co-polymer), i.e., PP containing low levels of an ethylene co-monomer (1–8%). We found mainly poly-silicone compounds in samples 10, 11, and 23, identified as Tegomer E-Si-2330, which is polydimethylsiloxane (PDMS).
a Average wind speed and direction (gray arrows)38 during AMP sample collection. The error bars represent the standard deviation of the wind speed. b AMP, total, and coarse mode aerosol concentrations per m3 for each sample. AMP are marked in magenta, total aerosol are dark blue, and coarse mode aerosol are marked in cyan and blue for >10 µm and >1 µm, respectively. Sample numbers are presented in the direction following the R/V Tara transect presented on the map. c Map of 48 h HYSPLIT back trajectory ensembles for each point along the R/V Tara transect in the Atlantic Ocean where AMP were detected38. These are average trajectories of the HYSPLIT “Ensemble option” that were calculated based on an endpoint at 250 m height, the minimum height for the optimal configuration of the ensemble. The vast majority of the air masses originate from the ocean for the full extent of the 48 h.
PS, PE, and PP were previously identified as common plastic pollutants in the waters of remote ocean locations and shores worldwide6,44,45,46,48. Here, they were detected in the marine atmosphere. Moreover, PS, PP, and PE were recently identified as the most abundant plastic compounds that were carried in the atmosphere to a remote terrestrial location22. PE and PP are the main components of airborne plastics in urban environments, according to several studies24,26,27. PDMS, which was detected in three samples, is essentially insoluble in water (<1 ng L−1 at 23 °C), and therefore is preserved in the seawater. Since PDMS is less dense than water it is expected to remain in the ocean’s surface microlayer, from where it can be subjected to aerosolization via wind driven processes. PDMS has been found in aerosol samples in several US cities49.
AMP concentrations are presented in Fig. 2, together with total and coarse mode aerosol concentrations38. Wind speed and direction prevailing during AMP sampling periods are presented for reference. AMP concentrations are in the order of 1–10% of the coarse mode aerosol concentrations for sizes >10 µm. The AMP concentrations obtained here are in good agreement with concentrations attributed to marine source AMP, which were obtained from measurements conducted at the French Atlantic coast30. Based on their coastal measurements, the authors suggested that AMP can be formed via bubble bursting, which may also occur in the open ocean. In order to investigate the open ocean as a source of the AMP obtained in our study, we conducted 48 h back trajectories at 250 m altitude (HYSPLIT; HYbrid Single-Particle Lagrangian Integrated Trajectory) for each point along the Tara North Atlantic transect where AMP were detected. The vast majority of the air masses passed over the ocean during the full 48 h trajectories (see Fig. 2c). Next, we assessed the atmospheric residence times of the AMP we found, by calculating their settling (terminal) velocities (Table 1; see “Methods” section for the detailed calculations). The residence time of atmospheric particles is likely to be affected by their atmospheric terminal velocities. Slower velocities (≤0.01 m s−1) imply higher likelihood to be lifted from near the water surface by eddies, and to be carried by the surrounding air50. We found AMP settling velocities in the range of 0.001–0.2 m s−1 for the particles (Table 1, see “Methods” section). By combining the HYSPLIT back trajectories with the obtained residence times, we can evaluate the source of the particles. Particles 2, 17, 30, and 38 were clearly a product of local emission, with residence times of minutes to several hours (Table 1). The rest, with residence times of 1–2 days, combined with the HYSPLIT back trajectories, also appear to have been formed in the ocean. This suggests that the AMP found in this study are emitted from the seawater, indicating wind-related processes via bubble bursting.
AMP particles with irregular and elongated shapes can have long atmospheric residence times. For the most elongated AMP, despite having long major axis, their atmospheric settling velocities are in the range of 0.001 m s−1, equivalent to typical submicron particles, which are suspended in the marine atmosphere in a timescale of days, and dominate the marine boundary layer51. We summarize that the AMP we found can remain in the atmosphere up to ~2 days, and for a typical marine wind speed of 8 ms−1 can be transported to 100’s–1000’s km. However, AMP with settling velocities in the order of ~0.1 m s−1 will remain in the atmosphere for ~5 min, and travel ~3 km51.
To further explore the role of the open ocean as a source of AMP, we compared the chemical composition of the AMP samples with microplastic particles collected from seawater sampled at the same approximate location to where the AMP were sampled. We chose AMP samples collected at 26°N 66°W–26°N 68°W and 26°N 68°W–26°N 72°W, (samples 38 and 39, respectively; Fig. 1a), where the HYSPLIT trajectories indicate the air masses are of purely oceanic source. Microplastic particles collected from the seawater on the 22.06.2016 at the area of 26°N 68°W were compared with airborne sample collected at 26°N 66°W–26°N 68°W (sample 38) containing polyethylene AMP, which was collected on the same day, and with airborne sample collected at 26°N 68°W–26°N 72°W (sample 39) containing polypropylene AMP, which was collected the next day (23.06.2016) (see “Methods” section and Fig. 1a).
The seawater microplastic particles selected for analysis and comparison with the AMP underwent thorough morphological scanning, in order to assure their identification as plastic prior to micro-Raman spectroscopy measurements (see “Methods” section). Eight microplastic particles were analyzed from the seawater sample at 26°N 68°W, seven of which contained polyethylene or polypropylene (Fig. 3). Five were identified as polyethylene, out of which two were polyethylene compositions (one with Cobalt phthalocyanine and the second with acrylic acid), and two were identified as polypropylene (see Fig. 3).
a Raman spectra of airborne microplastic samples collected between June 22nd and 23rd 2016, between 26°N 66°W and 26°N 72°W (filters 38–39). b Raman spectra of seawater microplastic samples collected on June 22nd, 2016 at 26°N 68°W. Optical microscope images are presented for both airborne and seawater particles. Close up images of the locations where the Raman spectra were retrieved in the seawater microplastic particles are presented. The Raman spectra of the corresponding plastic polymer standards are shown in magenta.
The oceanic origin of the air masses as indicated by the HYSPLIT back trajectories, together with the chemical evidence from samples 38–39 and their corresponding seawater MP samples strongly suggest marine source of the AMP. Additionally, AMP sample 38 has a calculated terminal velocity of 0.2 m s−1, and 39 of 0.003 m s−1, yielding atmospheric residence times of ~5 min and ~48 h, respectively (Table 1). This strongly suggests that both were produced in the ocean. AMP sample 38 was most likely locally produced, and sample 39 with a potential residence time of 48 h, may have been produced far from the sample collection point (the residence time only indicates the maximal time a particle can spend in the atmosphere, and therefore could have also been emitted very shortly before sampling), but is also likely of oceanic source, as the full 48 h duration of the HYSPLIT back trajectory was spent over the ocean.
Furthermore, the specific densities of most AMP we found, and specifically polyethylene and polypropylene found in the seawater and in AMP samples 38 and 39, are lower than the specific density of seawater (Table 1), and are therefore likely to accumulate in the sea surface microlayer, and thus can be readily aerosolized.
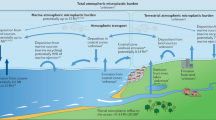
Our findings indicate a new possible source and formation mechanism for AMP, different to those found for the terrestrial and coastal AMP detected to date13,22,23,24,25,26,27,28,29,30,31,32,33. We have evidence from the open ocean, which suggest that AMP is emitted from the seawater into the atmosphere through wind related processes. MP is accumulated in the ocean surface microlayer, where it can be scavenged by rising air bubbles. The bursting of the bubbles injects the MP into the atmosphere in a process associated with sea spray aerosol formation51,52.
This implies that the ocean can be a constant global source of AMP, which may be transported to coastal regions and remote locations. However, we cannot dismiss the possibility that some AMP particles may have originated from other boats, continents or islands33, and transported into the remote marine atmosphere.
We found AMP in ~20% of our atmospheric samples. We postulate that these results underestimate the actual AMP concentration in the marine atmosphere due to the current technical challenges, which did not allow us to analyze airborne microplastic particles smaller than 5 µm (see “Methods” section for more details). Moreover, nanoplastic particles (<1 µm) were only very recently identified in the environment at the single particle level28, due to methodological limitations in nanoplastic collection and detection44,53. In a typical marine aerosol size distribution, the diameter range between 10 nm–1 µm constitutes >99% of the total marine airborne particle concentration52, as was also measured during the R/V Tara Pacific expedition38 (see Table 1 and Fig. 5 of their paper). It is therefore likely that a higher concentration of microplastic and nanoplastic particles, below our current size detection limit are suspended in the air. Despite the technical limitations in tracing them, it is widely accepted, and recently confirmed, that nanoplastic particles are abundant in the marine environment9,28,44,45,53,54. Therefore, we suggest that the marine atmosphere contains nanoplastic particles (10 nm–1 µm), which would be much more easily introduced and sustained in the marine atmosphere than the large particles presented in this study.
The presence of nanoplastic and microplastic in the marine atmosphere may have implications on the atmospheric composition, and may also increase microplastic toxicity due to exposure to UV radiation in the atmosphere1,2,36, with possible severe consequences for marine microbial ecology. The consequences may include increased stress response, and a decrease in metabolism, growth, and reproduction, which are some of the phenomena observed in aquatic organisms interacting with microplastics55. It is known that microplastics and nanoplastics, particularly polystyrene, polyethylene, and polypropylene (all found in our AMP samples), accumulate persistent organic pollutants56 as well as heavy metals44,57,58. The products of plastic degradation, particularly polystyrene, found in our AMP samples, are toxic58,59,60. Given that some AMP can persist in the air for days, according to our terminal velocity calculations, allowing long exposure times to UV radiation and oxidation, they can become highly toxic1,2,36 before being redeposited into the seawater, where they can cause further harm to the marine ecosystem than previously observed for non-airborne microplastic particles55,61. The ability of microplastic to carry pathogens1,2,14,15,16,55,61,62,63,64,65,66 may also increase the harm posed by AMP to the marine ecosystem, as airborne microplastic can carry pathogens further than typical ocean currents. According to our calculations, some AMP can travel 100’s to 1000’s of km, rendering distant marine ecosystems in danger of infection by these pathogens, provided they remain infective in the atmosphere, as shown possible in several studies67,68. Therefore, the effect of AMP can be devastating for marine microbial ecology, and must be thoroughly investigated17.
This study is the first to detect and identify AMP in the remote marine atmosphere. The implications of these findings on AMP emissions and their fate, the possible effects on the marine atmosphere and on marine microbial ecology, as suggested above, require further investigation17.
Methods
Atmospheric samples were collected during the Tara pacific expedition 2016–201837,39 using the collection system illustrated in Fig. 438. The collection system is composed of an inlet comprised of a funnel mounted on the rear backstay of the R/V Tara, and connected with conductive tubing (1.9 cm inner diameter) to filter holders (AHF analysentechnik AG). The air was pulled into the system by a vacuum pump at a flow rate of ~20 LPM (liter per minute). Aerosol samples were collected on polycarbonate membranes (Isopore membranes, Millipore Ltd., 0.8 µm pore size), with collection volumes of ~12–60 m3.
The system is composed of an inlet, positioned 15 m above sea level, which is connected to a flow splitter leading to the filter holders. Each filter holder contains a different filter, for biological analysis, for chemical characterization, and for morphology using SEM. Air is being pulled from the inlet through all filter holders by a vacuum pump. The air flow rate through each filter was approximately 20 LPM (liter per minute), as measured by a flow-meter38.
We used micro-Raman spectroscopy to analyze the samples collected in the North Atlantic Ocean (28th May–28th June 2016) in order to detect and chemically identify airborne microplastic particles (AMP). We note that during this period the inlet was installed halfway up the backstay, ~15 m above sea level.
We collected 46 filters (samples 1–46) from the Atlantic transect presented in Fig. 1. Three of the 46 samples had to be discarded for technical reasons, and therefore could not be analyzed.
We used the HORIBA micro-Raman system to detect and identify microplastic particles. The LabRAM HR Evolution (HORIBA, France) set-up was used for this analysis. The excitation was performed mostly with a 633 nm laser (but 532 and 785 nm were occasionally used as well). The set-up uses an 800 mm spectrograph, which allows for a high spectral resolution and low stray light. The pixel resolution is ≈1.8 cm−1 when working with a 600 g/mm grating and a 633 nm laser. The sample was illuminated using a ×50 LWD NA 0.5 objective (LMPlanFL N, Olympus). The Raman spectra were measured using a 1024 × 256 pixel open electrode front-illuminated CCD camera, cooled to −60 °C (Syncerity, HORIBA, USA). The system utilizes an open confocal microscope (Olympus BXFM) with a spatial resolution greater than 1 μm. The measurements were done with the laser beam focused on a single microplastic particle at a time. Raman spectroscopy is a documented method for polymer characterization, and micro-Raman spectroscopy can be used for marine microplastic detection due to its high spatial resolution. It is well suited for the study of (marine) microplastic due to the small spot size, addressing even minute samples40,41,42,43. Spectra were collected between 100–1800 cm−1 or 100–3500 cm−1, depending on the sample. For consistency, all spectra shown in this paper are in the 100–1800 cm−1 range.
We used a library of Raman spectra of polymers available in the BioRad know-it-all software, searching for matches for the spectra of our particles. Our method allows us to detect particles as small as ~5–10 µm, which is a limiting factor in this study, as plastic debris is known to produce also microplastic and nanoplastic particles smaller than 5 µm. The optical system can detect particles in this size range, or even smaller (up to 1 µm particles), but as the particles are deposited on polycarbonate filters, even the small depth of focus of the confocal microscope produces a strong background signal from the filter, overshadowing the signal from the smaller particles.
We used the particle finder module (Horiba, France) to detect particles on each filter, and automatically analyze each particle detected by the software using Raman spectroscopy. The spectra were treated for background subtraction as is common in Raman spectroscopy. An area of ~870 mm2 was scanned in search of particles in each filter (total of 43 filters). The area scanned was the same distance from the edge of the filter for each of the 43 filters. Upon finding a particle or group of particles, we obtained Raman spectra for an area of 0.018 mm2 in each scan of the particle finder software. Depending on the amount of particles found during the search executed for the entire filter (the ~870 mm2 scan), 30–100 scans were performed using the software, resulting in a total scanned area between 0.54 and 1.8 mm2. Since polycarbonate is the material from which the filters are composed, the Raman signal of polycarbonate often appeared in the obtained spectra, and was removed from the analyzed spectra of the particles when possible.
Sampling and handling blanks
In order to assure that the AMP we identified were not emitted from any surface onboard the R/V Tara or any part of the boat, and are not contaminations from any stage of sampling onboard the Tara, sample handling in the laboratory or the Raman spectra analysis, very strict measures were taken: two types of blanks were collected and analyzed using Raman spectroscopy to obtain their chemical identity in the exact same manner as the collected atmospheric samples. Firstly, all surfaces onboard the R/V Tara were sampled: the ropes (blue and red), the electricity cords, the flags, the masts and the sails. Raman spectra of the particles sampled from all the surfaces were obtained. The main component found was cellulose. Figure 5 shows Raman spectra of the R/V Tara surface blanks compared with the Raman spectra standards of the chemical compound corresponding to the blanks, which was cellulose. Figure 5a shows the Raman spectrum of cellulose, Fig. 5b shows typical Raman spectra of the sampled surfaces, corresponding to the spectra of cellulose, demonstrating the abundance of cellulose in various parts onboard the R/V Tara. Many of the surfaces onboard Tara emitted cellulose fibers and particles into our sampling inlet, and therefore cellulose particles from the Tara surfaces were detected in the atmospheric samples throughout the Atlantic transect (samples 1–46), as shown in Fig. 5c. The cellulose particles were disregarded as atmospheric particles (AMP) since they were found in the R/V Tara surface blanks, the same treatment was applied to any other materials found in any of the R/V Tara surfaces. The other materials identified on the surfaces were polyester, which was detected on one of the ropes (red rope), and Copper/Cobalt phthalocyanines (CoPhc), or Mortoperm blue, which was widely abundant in the handling blanks as elaborately explained below. Secondly, handling blanks from all aerosol sampling and analysis stages, from sampling into the filter holders (Fig. 4), and until analysis with micro-Raman Spectroscopy, were collected and analyzed with Raman spectroscopy. The handling blanks include the following treatments for filters: new filters taken out from the case, filters inserted and removed from the filter holders without aerosol collection, filters placed in the vicinity of the sample filter during microscopy and/or Raman analysis. One type of material in particular, which belongs to a family of very similar molecules called Copper/Cobalt phthalocyanines (CoPhc), or Mortoperm blue (a commercial name), was dominant in the handling blanks. It also appeared repeatedly in the atmospheric samples (in ~ 40 out of the 43 samples analyzed), as well as in a seawater sample from Tara from 22.6.2016 at 26°N 68°W (see main text; comparison between microplastic particles in airborne and seawater samples). It appeared in both handling blanks and the samples as a single material, or in composite with other materials, such as cellulose. CoPhc and Mortoperm blue have the same molecular structure (Fig. 6d), where the CoPhc contain either Copper (Cu) or Cobalt (Co) as the central ion of the molecule, and Mortoperm blue does not contain any additional metal in its center. An example of the Raman spectrum of Mortoperm blue (as a standard), an atmospheric sample containing Mortoperm blue, and a handling blank containing a composite of Mortoperm blue with cellulose is shown in Fig. 6a–c. CoPhc/Mortoperm blue are used as dyes and colorants in the plastic industry, and are therefore associated with plastics. However, although abundant in particles found in our atmospheric samples, and detected also in the Tara Pacific seawater samples, as well as in microplastic samples in aquatic environments from previous studies43,69, we ruled them out as valid samples originating from the marine atmosphere, and categorized them as contamination from handling blanks during sampling and analysis. The size range of the particles, which were found in the blanks and/or airborne samples was between ~1 and 100’s µm.
a The reference Raman spectrum of cellulose. b Raman spectra of blanks collected from various surfaces onboard the Tara, taken from the ropes, masts, and sails, all corresponding to cellulose. c The cellulose spectrum obtained from the Tara surface blanks, and the spectra obtained from particles found in atmospheric samples 3, 6, 11, 22, 24, 26, 27, 28, and 32. A strong agreement between the spectra of the blanks and the samples is observed.
a The Raman spectrum of Mortoperm blue. b The Raman spectrum of an atmospheric sample containing Mortoperm blue. c The Raman spectrum of a handling blank containing a composite of Mortoperm blue with cellulose. d The basic molecular structure of Mortoperm blue and Copper/Cobalt phthalocyanines. Copper/Cobalt phthalocyanines contain either a Copper (Cu) or a Cobalt (Co) ion at the center of the molecule, while Mortoperm blue doesn’t contain any metal ion in the center. The center of the molecule is marked with a red circle.
Other materials found in our blanks were not found in any of the atmospheric samples (1–46), or were ruled out and categorized as contamination, and are therefore not given any further attention here. We also note that cellulose and CoPhc/Mortoperm blue were detected in both R/V Tara surfaces, and in the handling blanks, as well as in the atmospheric samples. This suggests that these materials from the R/V Tara surfaces may reach the atmosphere, in which case the boat might be a source of cellulose and CoPhc/Mortoperm blue in the atmosphere, and maybe also in the seawater. Polyester, which was found on a surface onboard Tara was not found in any atmospheric sample, perhaps due to its high density (~1.35 g cm−3). It may settle from the Tara surfaces into the seawater, but we have no evidence that it is emitted into the atmosphere.
Most importantly, none of the plastics found in our AMP aerosol samples were found in any of the blanks we collected and measured. This rules out with a very high confidence level that our AMP samples are a result of sampling, measurement or handling contamination, and confirms that they indeed originate from the marine atmosphere.
AMP terminal velocity calculations
AMP terminal velocity was estimated using the well-established theory and measurements of settling velocity of water droplets and ice crystals with needle shapes, in the range of ~10–100 µm70. The particles shape, sizes, and densities that are close to water, allowed us to use this theory.
The terminal velocity of elongated particles in this size range, scales to the equivalent sphere of the same mass, but with a radius that scales to the largest dimension of the particle. This implies that the density of the equivalent sphere will be reduced by the ratio (α) of the real volume (Vr) of the particle and the volume (Vs) of the equivalent sphere:
Then, to a good approximation, the AMP terminal velocity (μ) could be scaled as:
where μs is the terminal velocity of a sphere (droplet) with diameter of the polymer’s major axis.
More specifically: The real volume of the MP particle can be approximated as a spheroid by \(V_{\mathrm{r}}\,=\,\frac{4}{3}\pi ab^2\), where a is the major and b is the minor axis, and the equivalent sphere volume is \(V_{\mathrm{s}} = \frac{4}{3}\pi a^3\). Assuming that the MP density is close to water’s density, ρmp ≈ ρw, yields:
The terminal velocity of spheres can be described as a power-low function of the radius r: Vt = κrη, where κ and η depend on the sizes. Droplets with radii r < 40 μm are in the laminar regime, for which κ ≈ 1.19 × 106 cm−1 s−1, and η = 2. Droplets in the range of 40 < r < 600 μm are in the intermediate regime, between laminar and turbulent, with κ ≈ 8 × 106 s−1 and η = 171,72.
Applying the above scaling to the measured AMP particles, yields settling velocities in the range of 0.001–0.2 m s−1 for the particles. For the most elongated cases, despite having long major axis, their settling velocities are in the range of 0.001 m s−1 (0.1 cm s−1).
Seawater microplastic collection
Surface microplastics were collected using the High Speed Net (HSN) tow, deployed in the Atlantic and Pacific Oceans, and split into two equal subsamples. The first for plastic and plankton analysis by the ZooScan imaging system73, and the second was used for genomic analysis39.
Seawater microplastic concentrations along the North Atlantic transect presented in Fig. 1a were obtained from the microplastics collected with the HSN, and scanned with the ZooScan Imaging system. The images were separated to “living organisms” and “detritus” categories, the organisms were identified to the deepest possible taxonomic level, and the microplastics were selected from the images determined as “detritus”.
Seawater microplastic particles for micro-Raman analysis and comparison with AMP (see Fig. 3), were separated and identified after being scanned again with the ZooScan Imaging system, the second scanning was performed in order to assure that only particles identified as plastic with 100% confidence will be used for the micro-Raman analysis.
Data availability
Data are included in the article, and are publicly available through OSF at https://doi.org/10.17605/OSF.IO/R3Q5C. Additional data from Zooscan Tara Pacific 2016 2018 HSN 330 sn001, used in this paper (Fig. 1a), is available online: https://ecotaxa.obs-vlfr.fr/prj/1700.
Change history
19 February 2021
A Correction to this paper has been published: https://doi.org/10.1038/s43247-021-00119-5
References
Andrady, A. L. Microplastics in the marine environment. Mar. Pollut. Bull. 62, 1596–1605 (2011).
Arthur, C., Baker, J. E. & Bamford, H. A. Proceedings of the International Research Workshop on the Occurrence, Effects, and Fate of Microplastic Marine Debris (University of Washington Tacoma, Tacoma, WA, 2009).
Ostle, C. et al. The rise in ocean plastics evidenced from a 60-year time series. Nat. Commun. 10, 1622 (2019).
Bergmann, M., Gutow, L. & Klages, M. Marine Anthropogenic Litter (Springer, New York, 2015).
Bowmer, T. & Kershaw, P. Proceedings of the GESAMP International Workshop on Micro-plastic Particles as a Vector in Transporting Persistent, Bio-accumulating and Toxic Substances in the Oceans (UNESCO-IOC, Paris, 2010).
Browne, M. A. et al. Accumulation of microplastic on shorelines worldwide: sources and sinks. Environ. Sci. Technol. 45, 9175–9179 (2011).
Cole, M., Lindeque, P., Halsband, C. & Galloway, T. S. Microplastics as contaminants in the marine environment: a review. Mar. Pollut. Bull. 62, 2588–2597 (2011).
Cozar, A. et al. Plastic debris in the open ocean. Proc. Natl Acad. Sci. USA 111, 10239–10244 (2014).
Haward, M. Plastic pollution of the world’s seas and oceans as a contemporary challenge in ocean governance. Nat. Commun. https://doi.org/10.1038/s41467-018-03104-3 (2018).
Law, K. L. & Annual, R. Plastics in the Marine Environment. Ann Rev Mar Sci, 9, 205–229 (2017).
Song, Y. K. et al. Large accumulation of micro-sized synthetic polymer particles in the sea surface microlayer. Environ. Sci. Technol. 48, 9014–9021 (2014).
Rochman, C. M. & Hoellein, T. The global odyssey of plastic pollution. Science 368, 1184 (2020).
Evangeliou, N. et al. Atmospheric transport is a major pathway of microplastics to remote regions. Nat. Commun. 11, 3381 (2020).
Mato, Y. et al. Plastic resin pellets as a transport medium for toxic chemicals in the marine environment. Environ. Sci. Technol. 35, 318–324 (2001).
Wright, S. L., Thompson, R. C. & Galloway, T. S. The physical impacts of microplastics on marine organisms: a review. Environ. Pollut. 178, 483–492 (2013).
Mendoza, L. M. R. & Jones, P. R. Characterisation of microplastics and toxic chemicals extracted from microplastic samples from the North Pacific Gyre. Environ. Chem. 12, 611–617 (2015).
Burns, E. E. & Boxall, A. B. A. Microplastics in the aquatic environment: evidence for or against adverse impacts and major knowledge gaps. Environ. Toxicol. Chem. 37, 2776–2796 (2018).
Villarrubia-Gómez, P., Cornell, S. E. & Fabres, J. Marine plastic pollution as a planetary boundary threat—the drifting piece in the sustainability puzzle. Mar. Policy 96, 213–220 (2018).
Hammer, J., Kraak, M. & Parsons, J. Plastics in the marine environment: the dark side of a modern gift. Rev. Environ. Contam. Toxicol. 220, 1–44 (2012).
Worm, B., Lotze, H. K., Jubinville, I., Wilcox, C. & Jambeck, J. Plastic as a persistent marine pollutant. Annu. Rev. Environ. Res. 42, 1–26 (2017).
Eriksen, M. et al. Plastic pollution in the World’s oceans: more than 5 Trillion plastic pieces weighing over 250,000 Tons afloat at sea. PLoS ONE https://doi.org/10.1371/journal.pone.0111913 (2014).
Allen, S. et al. Atmospheric transport and deposition of microplastics in a remote mountain catchment. Nat. Geosci. 12, 339–344 (2019).
Cai, L. et al. Characteristic of microplastics in the atmospheric fallout from Dongguan city, China: preliminary research and first evidence. Environ. Sci. Pollut. Res. 24, 24928–24935 (2017).
Dris, R. et al. A first overview of textile fibers, including microplastics, in indoor and outdoor environments. Environ. Pollut. 221, 453–458 (2017).
Dris, R. et al. Microplastic contamination in an urban area: a case study in Greater Paris. Environ. Chem. 12, 592–599 (2015).
Dris, R., Gasperi, J., Saad, M., Mirande, C. & Tassin, B. Synthetic fibers in atmospheric fallout: a source of microplastics in the environment? Mar. Pollut. Bull. 104, 290–293 (2016).
Gasperi, J. et al. Microplastics in air: are we breathing it in? Curr. Opin. Environ. Sci. Health 1, 1–5 (2018).
Materić, D. et al. Micro- and nanoplastics in alpine snow: a new method for chemical identification and (Semi)quantification in the nanogram range. Environ. Sci. Technol. 54, 2353–2359 (2020).
Panko, J. M., Chu, J., Kreider, M. L. & Unice, K. M. Measurement of airborne concentrations of tire and road wear particles in urban and rural areas of France, Japan, and the United States. Atmos. Environ. 72, 192–199 (2013).
Allen, S. et al. Examination of the ocean as a source for atmospheric microplastics. PLoS ONE 15, e0232746 (2020).
Bergmann, M. et al. White and wonderful? Microplastics prevail in snow from the Alps to the Arctic. Sci. Adv. 5, eaax1157 (2019).
Chen, G., Feng, Q. & Wang, J. Mini-review of microplastics in the atmosphere and their risks to humans. Sci. Total Environ. 703, 135504 (2020).
Liu, K. et al. Consistent transport of terrestrial microplastics to the ocean through atmosphere. Environ. Sci. Technol. 53, 10612–10619 (2019).
McCallum, H., Harvell, D. & Dobson, A. Rates of spread of marine pathogens. Ecol. Lett. 6, 1062–1067 (2003).
Abraham, E. R. et al. Importance of stirring in the development of an iron-fertilized phytoplankton bloom. Nature 407, 727–730 (2000).
Galloway, T. S., Cole, M. & Lewis, C. Interactions of microplastic debris throughout the marine ecosystem. Nat. Ecol. Evol. https://doi.org/10.1038/s41559-017-0116 (2017).
Gorsky, G. et al. Expanding tara oceans protocols for underway, ecosystemic sampling of the ocean-atmosphere interface during tara Pacific expedition (2016–2018). Front. Mar. Sci. https://doi.org/10.3389/fmars.2019.00750 (2019).
Flores, J. M. et al. Tara Pacific expedition’s atmospheric measurements. Marine aerosols across the Atlantic and Pacific Oceans overview and preliminary results. Bull. Am. Meteorol. Soc. https://doi.org/10.1175/bams-d-18-0224.1 (2019).
Planes, S. et al. The Tara Pacific expedition—A pan-ecosystemic approach of the “-omics” complexity of coral reef holobionts across the Pacific Ocean. PLoS Biol. 17, e3000483 (2019).
Hidalgo-Ruz, V., Gutow, L., Thompson, R. C. & Thiel, M. Microplastics in the marine environment: a review of the methods used for identification and quantification. Environ. Sci. Technol. 46, 3060–3075 (2012).
Laura, F. et al. A semi-automated Raman micro-spectroscopy method for morphological and chemical characterizations of microplastic litter. Mar. Pollut. Bull. 113, 461–468 (2016).
Oßmann, B. et al. Development of an optimal filter substrate for the identification of small microplastic particles in food by micro-Raman spectroscopy. Anal. Bioanal. Chem. https://doi.org/10.1007/s00216-017-0358-y (2017).
Oßmann, B. E. et al. Small-sized microplastics and pigmented particles in bottled mineral water. Water Res. 141, 307–316 (2018).
Alimi, O. S., Budarz, J. F., Hernandez, L. M. & Tufenkji, N. Microplastics and nanoplastics in aquatic environments: aggregation, deposition, and enhanced contaminant transport. Environ. Sci. Technol. 52, 1704–1724 (2018).
GESAMP. Sources, Fate and Effects of Microplastics in the Marine Environment (Part 2). (Journal Series GESAMP Reports and Studies, 2016).
PlasticsEurope. Plastics—the facts 2017: an analysis of European plastics production, demand and waste data. (Brussels, 2017).
Thompson, R. C. et al. Lost at sea: Where is all the plastic? Science 304, 838–838 (2004).
Geyer, R., Jambeck, J. R. & Law, K. L. Production, use, and fate of all plastics ever made. Sci. Adv. https://doi.org/10.1126/sciadv.1700782 (2017).
Carmichael, N. JACC 055: Linear Polydimethylsiloxanes CAS No. 63148-62-9. (European Centre for Eotoxicology and Toxicology of Chemicals, Brussels, 2011).
Koren, I., Altaratz, O. & Dagan, G. Aerosol effect on the mobility of cloud droplets. Environ. Res. Lett. 10, 104011 (2015).
Lewis, E. R. & Schwartz, S. E. Fundamentals sea salt aerosol production: mechanisms, methods, measurements and models. https://doi.org/10.1002/9781118666050.ch2 (2004).
O’Dowd, C. D. & De Leeuw, G. Marine aerosol production: a review of the current knowledge. Philos. Trans. R. Soc. A 365, 1753–1774 (2007).
Koelmans, A. A., Besseling, E. & Shim, W. J. in Marine Anthropogenic Litter (eds Melanie, B. et al.) 325–340 (Springer, New York, 2015).
Lusher, A. in Marine Anthropogenic Litter (eds Melanie, B. et al.) 245–307 (Springer, New York, 2015).
Franzellitti, S., Canesi, L., Auguste, M., Wathsala, R. & Fabbri, E. Microplastic exposure and effects in aquatic organisms: a physiological perspective. Environ. Toxicol. Pharmacol. 68, 37–51 (2019).
Frias, J., Sobral, P. & Ferreira, A. M. Organic pollutants in microplastics from two beaches of the Portuguese coast. Mar. Pollut. Bull. 60, 1988–1992 (2010).
Rochman, C. M. in Marine Anthropogenic Litter (eds Melanie, B. et al.) 117–140 (Springer, New York, 2015).
Rochman, C. M., Hentschel, B. T. & Teh, S. J. Long-term sorption of metals is similar among plastic types: implications for plastic debris in aquatic environments. PLoS ONE https://doi.org/10.1371/journal.pone.0085433 (2014).
Rochman, C. M. et al. Classify plastic waste as hazardous. Nature 494, 169–171 (2013).
Royer, S.-J., Ferrón, S., Wilson, S. T. & Karl, D. M. Production of methane and ethylene from plastic in the environment. PLoS ONE 13, e0200574 (2018).
Lamb, J. B. et al. Plastic waste associated with disease on coral reefs. Science 359, 460–462 (2018).
Carson, H. S., Nerheim, M. S., Carroll, K. A. & Eriksen, M. The plastic-associated microorganisms of the North Pacific Gyre. Mar. Pollut. Bull. 75, 126–132 (2013).
Kirstein, I. V. et al. Dangerous hitchhikers? Evidence for potentially pathogenic Vibrio spp. on microplastic particles. Mar. Environ. Res. 120, 1–8 (2016).
Oberbeckmann, S., Kreikemeyer, B. & Labrenz, M. Environmental factors support the formation of specific bacterial assemblages on microplastics. Front. Microbiol. https://doi.org/10.3389/fmicb.2017.02709 (2018).
Oberbeckmann, S., Loder, M. G. J. & Labrenz, M. Marine microplastic- associated biofilms—a review. Environ. Chem. 12, 551–562 (2015).
Virsek, M. K., Lovsin, M. N., Koren, S., Krzan, A. & Peterlin, M. Microplastics as a vector for the transport of the bacterial fish pathogen species Aeromonas salmonicida. Mar. Pollut. Bull. 125, 301–309 (2017).
Despres, V. R. et al. Primary biological aerosol particles in the atmosphere: a review. Tellus Ser. B https://doi.org/10.3402/tellusb.v64i0.15598 (2012).
Sharoni, S. et al. Infection of phytoplankton by aerosolized marine viruses. Proc. Natl Acad. Sci. USA 112, 6643–6647 (2015).
Horton, A. A., Svendsen, C., Williams, R. J., Spurgeon, D. J. & Lahive, E. Large microplastic particles in sediments of tributaries of the River Thames, UK—abundance, sources and methods for effective quantification. Mar. Pollut. Bull. 114, 218–226 (2017).
Westbrook, C. D. The fall speeds of sub-100 mu m ice crystals. Q. J. R. Meteorol. Soc. 134, 1243–1251 (2008).
Khvorostyanov, V. I. & Curry, J. A. Terminal velocities of droplets and crystals: power laws with continuous parameters over the size spectrum. J. Atmos. Sci. 59, 1872–1884 (2002).
Rogers, R. R. & Yau, M. K. A Short Course in Cloud Physics, 3rd edn (Elsevier, Amsterdam, 1996).
Gorsky, G. et al. Digital zooplankton image analysis using the ZooScan integrated system. J. Plankton Res. 32, 285–303 (2010).
Acknowledgements
This research was supported by a research grant from Scott Jordan and Gina Valdez, the De Botton for Marine Science, the Yeda-Sela center for Basic research, and a research grant from the Yotam Project. Special thanks to the Tara Ocean Foundation, the R/V Tara crew and the Tara Pacific Expedition Participants (https://doi.org/10.5281/zenodo.3777760). We are keen to thank the commitment of the following institutions for their financial and scientific support that made this unique Tara Pacific Expedition possible: CNRS, PSL, CSM, EPHE, Genoscope, CEA, Inserm, Université Côte d’Azur, ANR, agnès b., UNESCO-IOC, the Veolia Foundation, the Prince Albert II de Monaco Foundation, Région Bretagne, Billerudkorsnas, AmerisourceBergen Company, Lorient Agglomération, Oceans by Disney, L’Oréal, Biotherm, France Collectivités, Fonds Français pour l’Environnement Mondial (FFEM), Etienne Bourgois, and the Tara Ocean Foundation teams. Tara Pacific would not exist without the continuous support of the participating institutes. The authors also particularly thank Serge Planes, Denis Allemand, and the Tara Pacific consortium. This study has been conducted using E.U. Copernicus Marine Service Information and Mercator Ocean products. This is publication number # 12 of the Tara Pacific Consortium. The authors gratefully acknowledge the NOAA Air Resources Laboratory (ARL) for the provision of the HYSPLIT transport and dispersion model and/or READY website (http://www.ready.noaa.gov) used in this publication.
Author information
Authors and Affiliations
Contributions
I.K. and A.V. conceived the basic idea and supervised the project. J.M.F and M.T. designed and installed the atmospheric measurements suite onboard the Tara. J.M.F. and G.B. collected the samples. M.T. and I.P. performed Raman analysis and interpreted the data. M.L.P. and F.L. performed seawater analyses. J.M.F, G.G., E.B. and Y.R. contributed to data interpretation and writing. M.T., I.K., and A.V. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest. Yinon Rudich is an Editorial Board Member for Communications Earth & Environment, but was not involved in the editorial review of, or the decision to publish this article.
Additional information
Peer review information Primary handling editor: Heike Langenberg.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Trainic, M., Flores, J.M., Pinkas, I. et al. Airborne microplastic particles detected in the remote marine atmosphere. Commun Earth Environ 1, 64 (2020). https://doi.org/10.1038/s43247-020-00061-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s43247-020-00061-y
This article is cited by
-
Water–air transfer rates of microplastic particles through bubble bursting as a function of particle size
Microplastics and Nanoplastics (2024)
-
Systematic review of microplastics and nanoplastics in indoor and outdoor air: identifying a framework and data needs for quantifying human inhalation exposures
Journal of Exposure Science & Environmental Epidemiology (2024)
-
Microplastic pollution as an environmental risk exacerbating the greenhouse effect and climate change: a review
Carbon Research (2024)
-
The effects of land use types on microplastics in river water: A case study on the mainstream of the Wei River, China
Environmental Monitoring and Assessment (2024)
-
Enhanced singular jet formation in oil-coated bubble bursting
Nature Physics (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.