Abstract
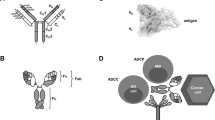
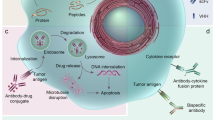
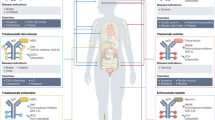
Monoclonal antibodies are a growing class of targeted cancer therapeutics, characterized by exquisite specificity, long serum half-life, high affinity and immune effector functions. In this review, we outline key advances in the field with a particular focus on recent and emerging classes of engineered antibody therapeutic candidates, discuss molecular structure and mechanisms of action and provide updates on clinical development and practice.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Goulet, D. R. & Atkins, W. M. Considerations for the design of antibody-based therapeutics. J. Pharm. Sci. 109, 74–103 (2020).
Sliwkowski, M. X. & Mellman, I. Antibody therapeutics in cancer. Science 341, 1192–1198 (2013).
Pierpont, T. M., Limper, C. B. & Richards, K. L. Past, present, and future of rituximab—the world’s first oncology monoclonal antibody therapy. Front. Oncol. 8, 163 (2018).
Forecast, M.D. Global Cancer Monoclonal Antibodies Market Size, Share, Trends, COVID-19 Impact & Growth Analysis Report – Segmented By Application, Type, Conjugate Cancer Therapies & Region – Industry Forecast (2021 to 2026), Vol. 2021 (Market Data Forecast, 2021).
Schroeder, H. W. Jr. & Cavacini, L. Structure and function of immunoglobulins. J. Allergy Clin. Immunol. 125, S41–S52 (2010).
Vidarsson, G., Dekkers, G. & Rispens, T. IgG subclasses and allotypes: from structure to effector functions. Front. Immunol. 5, 520–520 (2014).
Akilesh, S. et al. Podocytes use FcRn to clear IgG from the glomerular basement membrane. Proc. Natl Acad. Sci. USA 105, 967–972 (2008).
Skowronek, M. H., Hendershot, L. M. & Haas, I. G. The variable domain of nonassembled Ig light chains determines both their half-life and binding to the chaperone BiP. Proc. Natl Acad. Sci. USA 95, 1574–1578 (1998).
Mankarious, S. et al. The half-lives of IgG subclasses and specific antibodies in patients with primary immunodeficiency who are receiving intravenously administered immunoglobulin. J. Lab. Clin. Med. 112, 634–640 (1988).
Ghetie, V. et al. Increasing the serum persistence of an IgG fragment by random mutagenesis. Nat. Biotechnol. 15, 637–640 (1997).
Kim, J. K., Tsen, M. F., Ghetie, V. & Ward, E. S. Evidence that the hinge region plays a role in maintaining serum levels of the murine IgG1 molecule. Mol. Immunol. 32, 467–475 (1995).
Kontermann, R. E. Strategies for extended serum half-life of protein therapeutics. Curr. Opin. Biotechnol. 22, 868–876 (2011).
Liu, R., Oldham, R. J., Teal, E., Beers, S. A. & Cragg, M. S. Fc-engineering for modulated effector functions—improving antibodies for cancer treatment. Antibodies 9, 64 (2020).
Rouet, R. et al. Expression of high-affinity human antibody fragments in bacteria. Nat. Protoc. 7, 364 (2012).
Hwang, W. Y. & Foote, J. Immunogenicity of engineered antibodies. Methods 36, 3–10 (2005).
Jones, P. T., Dear, P. H., Foote, J., Neuberger, M. S. & Winter, G. Replacing the complementarity-determining regions in a human antibody with those from a mouse. Nature 321, 522–525 (1986).
Rouet, R., Lowe, D. & Christ, D. Stability engineering of the human antibody repertoire. FEBS Lett. 588, 269–277 (2014).
Dudgeon, K. et al. General strategy for the generation of human antibody variable domains with increased aggregation resistance. Proc. Natl Acad. Sci. USA 109, 10879–10884 (2012).
Weiner, G. J. Monoclonal antibody mechanisms of action in cancer. Immunol. Res. 39, 271–278 (2007).
Appert-Collin, A., Hubert, P., Crémel, G. & Bennasroune, A. Role of ErbB receptors in cancer cell migration and invasion. Front. Pharmacol. https://doi.org/10.3389/fphar.2015.00283 (2015).
Fan, X. et al. VEGF blockade inhibits angiogenesis and reepithelialization of endometrium. FASEB J. 22, 3571–3580 (2008).
Glade-Bender, J., Kandel, J. J. & Yamashiro, D. J. VEGF blocking therapy in the treatment of cancer. Expert Opin. Biol. Ther. 3, 263–276 (2003).
Baselga, J. & Albanell, J. Mechanism of action of anti-HER2 monoclonal antibodies. Ann. Oncol. 12, S35–S41 (2001).
Meyer, S. et al. New insights in Type I and II CD20 antibody mechanisms of action with a panel of novel CD20 antibodies. Br. J. Haematol. 180, 808–820 (2018).
Chan, H. T. et al. CD20-induced lymphoma cell death is independent of both caspases and its redistribution into triton X-100 insoluble membrane rafts. Cancer Res. 63, 5480–5489 (2003).
Honeychurch, J. et al. Antibody-induced nonapoptotic cell death in human lymphoma and leukemia cells is mediated through a novel reactive oxygen species-dependent pathway. Blood 119, 3523–3533 (2012).
Ivanov, A. et al. Monoclonal antibodies directed to CD20 and HLA-DR can elicit homotypic adhesion followed by lysosome-mediated cell death in human lymphoma and leukemia cells. J. Clin. Invest. 119, 2143–2159 (2009).
Mone, A. P. et al. Alemtuzumab induces caspase-independent cell death in human chronic lymphocytic leukemia cells through a lipid raft-dependent mechanism. Leukemia 20, 272–279 (2006).
Tobinai, K., Klein, C., Oya, N. & Fingerle-Rowson, G. A review of obinutuzumab (GA101), a novel type II anti-CD20 monoclonal antibody, for the treatment of patients with B-cell malignancies. Adv. Ther. 34, 324–356 (2017).
Bologna, L. et al. Mechanism of action of type II, glycoengineered, anti-CD20 monoclonal antibody GA101 in B-chronic lymphocytic leukemia whole blood assays in comparison with rituximab and alemtuzumab. J. Immunol. 186, 3762–3769 (2011).
Snajdauf, M. et al. The TRAIL in the treatment of human cancer: an update on clinical trials. Front. Mol. Biosci. 8, 628332 (2021).
Cardoso Alves, L., Corazza, N., Micheau, O. & Krebs, P. The multifaceted role of TRAIL signaling in cancer and immunity. FEBS J. 288, 5530–5554 (2021).
Wang, B. T. et al. Multimeric anti-DR5 IgM agonist antibody IgM-8444 is a potent inducer of cancer cell apoptosis and synergizes with chemotherapy and BCL-2 inhibitor ABT-199. Mol. Cancer Ther. 20, 2483–2494 (2021).
van der Horst, H. J. et al. Potent preclinical activity of HexaBody-DR5/DR5 in relapsed and/or refractory multiple myeloma. Blood Adv. 5, 2165–2172 (2021).
Nimmerjahn, F., Gordan, S. & Lux, A. FcγR dependent mechanisms of cytotoxic, agonistic, and neutralizing antibody activities. Trends Immunol. 36, 325–336 (2015).
Clynes, R. A., Towers, T. L., Presta, L. G. & Ravetch, J. V. Inhibitory Fc receptors modulate in vivo cytoxicity against tumor targets. Nat. Med. 6, 443–446 (2000).
Nimmerjahn, F. & Ravetch, J. V. Divergent immunoglobulin G subclass activity through selective Fc receptor binding. Science 310, 1510–1512 (2005).
Stewart, R., Hammond, S.A., Oberst, M. & Wilkinson, R.W. The role of Fc γ receptors in the activity of immunomodulatory antibodies for cancer. J. ImmunoTher. Cancer https://doi.org/10.1186/s40425-014-0029-x (2014).
Brüggemann, M. et al. Comparison of the effector functions of human immunoglobulins using a matched set of chimeric antibodies. J. Exp. Med. 166, 1351–1361 (1987).
Kaneko, Y., Nimmerjahn, F., & Ravetch, J . V. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science 313, 670–673 (2006).
de Taeye, S. W. et al. FcγR binding and ADCC activity of human IgG allotypes. Front. Immunol. 11, 740 (2020).
Lu, L. L., Suscovich, T. J., Fortune, S. M. & Alter, G. Beyond binding: antibody effector functions in infectious diseases. Nat. Rev. Immunol. 18, 46–61 (2018).
Musolino, A. et al. Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu-positive metastatic breast cancer. J. Clin. Oncol. 26, 1789–1796 (2008).
Weng, W. K. & Levy, R. Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J. Clin. Oncol. 21, 3940–3947 (2003).
Kennedy, A. D. et al. Rituximab infusion promotes rapid complement depletion and acute CD20 loss in chronic lymphocytic leukemia. J. Immunol. 172, 3280–3288 (2004).
Vanderven, H. A., Jegaskanda, S., Wheatley, A. K. & Kent, S. J. Antibody-dependent cellular cytotoxicity and influenza virus. Curr. Opin. Virol. 22, 89–96 (2017).
Pereira, N. A., Chan, K. F., Lin, P. C. & Song, Z. The ‘less-is-more’ in therapeutic antibodies: afucosylated anti-cancer antibodies with enhanced antibody-dependent cellular cytotoxicity. mAbs 10, 693–711 (2018).
Wang, W., Erbe, A. K., Hank, J. A., Morris, Z. S. & Sondel, P. M. NK cell-mediated antibody-dependent cellular cytotoxicity in cancer immunotherapy. Front. Immunol. 6, 368 (2015).
Uchida, J. et al. The innate mononuclear phagocyte network depletes B lymphocytes through Fc receptor-dependent mechanisms during anti-CD20 antibody immunotherapy. J. Exp. Med. 199, 1659–1669 (2004).
Lehmann, B. et al. Tumor location determines tissue-specific recruitment of tumor-associated macrophages and antibody-dependent immunotherapy response. Sci. Immunol. 2, eaah6413 (2017).
Gül, N. & van Egmond, M. Antibody-dependent phagocytosis of tumor cells by macrophages: a potent effector mechanism of monoclonal antibody therapy of cancer. Cancer Res. 75, 5008–5013 (2015).
Herbst, C. et al. Combined modality treatment improves tumor control and overall survival in patients with early stage Hodgkin’s lymphoma: a systematic review. Haematologica 95, 494–500 (2010).
Beers, S. A. et al. Antigenic modulation limits the efficacy of anti-CD20 antibodies: implications for antibody selection. Blood 115, 5191–5201 (2010).
Albanesi, M. et al. Neutrophils mediate antibody-induced antitumor effects in mice. Blood 122, 3160–3164 (2013).
Siders, W. M. et al. Involvement of neutrophils and natural killer cells in the anti-tumor activity of alemtuzumab in xenograft tumor models. Leuk. Lymphoma 51, 1293–1304 (2010).
Zhu, E. F. et al. Synergistic innate and adaptive immune response to combination immunotherapy with anti-tumor antigen antibodies and extended serum half-life IL-2. Cancer Cell 27, 489–501 (2015).
Matlung, H. L. et al. Neutrophils kill antibody-opsonized cancer cells by trogoptosis. Cell Rep. 23, 3946–3959 (2018).
Ring, N. G. et al. Anti-SIRPα antibody immunotherapy enhances neutrophil and macrophage antitumor activity. Proc. Natl Acad. Sci. USA 114, E10578–E10585 (2017).
Vitale, M., Cantoni, C., Pietra, G., Mingari, M. C. & Moretta, L. Effect of tumor cells and tumor microenvironment on NK cell function. Eur. J. Immunol. 44, 1582–1592 (2014).
Melero, I., Rouzaut, A., Motz, G. T. & Coukos, G. T-cell and NK-cell infiltration into solid tumors: a key limiting factor for efficacious cancer immunotherapy. Cancer Discov. 4, 522–526 (2014).
Herter, S. et al. Glycoengineering of therapeutic antibodies enhances monocyte/macrophage-mediated phagocytosis and cytotoxicity. J. Immunol. 192, 2252–2260 (2014).
Gigon, L., Yousefi, S., Karaulov, A. & Simon, H. U. Mechanisms of toxicity mediated by neutrophil and eosinophil granule proteins. Allergol. Int. 70, 30–38 (2021).
Valgardsdottir, R. et al. Human neutrophils mediate trogocytosis rather than phagocytosis of CLL B cells opsonized with anti-CD20 antibodies. Blood 129, 2636–2644 (2017).
Velmurugan, R., Challa, D. K., Ram, S., Ober, R. J. & Ward, E. S. Macrophage-mediated trogocytosis leads to death of antibody-opsonized tumor cells. Mol. Cancer Ther. 15, 1879–1889 (2016).
Taylor, R. P. & Lindorfer, M. A. Fcγ-receptor-mediated trogocytosis impacts mAb-based therapies: historical precedence and recent developments. Blood 125, 762–766 (2015).
Vazquez-Lombardi, R., Nevoltris, D., Rouet, R. & Christ, D. Expression of IgG monoclonals with engineered immune effector functions. Methods Mol. Biol. 1827, 313–334 (2018).
Golay, J. & Taylor, R. P. The role of complement in the mechanism of action of therapeutic anti-cancer mAbs. Antibodies 9, 58 (2020).
Cragg, M. S. et al. Complement-mediated lysis by anti-CD20 mAb correlates with segregation into lipid rafts. Blood 101, 1045–1052 (2003).
Marshall, M. J. E., Stopforth, R. J. & Cragg, M. S. Therapeutic antibodies: what have we learnt from targeting CD20 and where are we going? Front. Immunol. 8, 1245 (2017).
Waldman, A. D., Fritz, J. M. & Lenardo, M. J. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat. Rev. Immunol. 20, 651–668 (2020).
Brunet, J.-F. et al. A new member of the immunoglobulin superfamily—CTLA-4. Nature 328, 267–270 (1987).
Krummel, M. F. & Allison, J. P. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J. Exp. Med. 182, 459–465 (1995).
Linsley, P. S. et al. CTLA-4 is a second receptor for the B cell activation antigen B7. J. Exp. Med. 174, 561–569 (1991).
Seidel, J. A., Otsuka, A. & Kabashima, K. Anti-PD-1 and anti-CTLA-4 therapies in cancer: mechanisms of action, efficacy, and limitations. Front. Oncol. 8, 86 (2018).
Twomey, J. D. & Zhang, B. Cancer immunotherapy update: FDA-approved checkpoint inhibitors and companion diagnostics. AAPS J. 23, 39 (2021).
Haymaker, C., Wu, R., Bernatchez, C. & Radvanyi, L. PD-1 and BTLA and CD8(+) T-cell ‘exhaustion’ in cancer: ‘exercising’ an alternative viewpoint. Oncoimmunology 1, 735–738 (2012).
Lee, J., Ahn, E., Kissick, H. T. & Ahmed, R. Reinvigorating exhausted T cells by blockade of the PD-1 pathway. For. Immunopathol. Dis. Therap. 6, 7–17 (2015).
Pardoll, D. M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 12, 252–264 (2012).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Akinleye, A. & Rasool, Z. Immune checkpoint inhibitors of PD-L1 as cancer therapeutics. J. Hematol. Oncol. https://doi.org/10.1186/s13045-019-0779-5 (2019).
Qin, S. et al. Novel immune checkpoint targets: moving beyond PD-1 and CTLA-4. Mol. Cancer 18, 155 (2019).
Huang, R. Y. et al. LAG3 and PD1 co-inhibitory molecules collaborate to limit CD8+ T cell signaling and dampen antitumor immunity in a murine ovarian cancer model. Oncotarget 6, 27359–27377 (2015).
Johnston, R. J. et al. The immunoreceptor TIGIT regulates antitumor and antiviral CD8(+) T cell effector function. Cancer Cell 26, 923–937 (2014).
Sakuishi, K. et al. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J. Exp. Med. 207, 2187–2194 (2010).
Long, G. V. et al. Relatlimab and nivolumab versus nivolumab in previously untreated metastatic or unresectable melanoma: overall survival and response rates from RELATIVITY-047 (CA224-047). J. Clin. Oncol. 40, 360385–360385 (2022).
Rudin, C. M. et al. Rudin, C.M. et al. SKYSCRAPER-02: primary results of a phase III, randomized, double-blind, placebo-controlled study of atezolizumab (atezo) + carboplatin + etoposide (CE) with or without tiragolumab (tira) in patients (pts) with untreated extensive-stage small cell lung cancer (ES-SCLC). J. Clin. Oncol. 40, LBA8507 (2022).
Garber, K. Immune agonist antibodies face critical test. Nat. Rev. Drug Discov. 19, 3–5 (2020).
Shi, S. Y. et al. A biparatopic agonistic antibody that mimics fibroblast growth factor 21 ligand activity. J. Biol. Chem. 293, 5909–5919 (2018).
Qi, X. et al. Optimization of 4-1BB antibody for cancer immunotherapy by balancing agonistic strength with FcγR affinity. Nat. Commun. 10, 2141 (2019).
Suntharalingam, G. et al. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N. Engl. J. Med. 355, 1018–1028 (2006).
Segal, N. H. et al. Results from an integrated safety analysis of urelumab, an agonist anti-CD137 monoclonal antibody. Clin. Cancer Res. 23, 1929–1936 (2017).
Segal, N. H. et al. Phase I study of single-agent utomilumab (PF-05082566), a 4-1BB/CD137 agonist, in patients with advanced cancer. Clin. Cancer Res. 24, 1816–1823 (2018).
Wilson, N. S. et al. An Fcγ receptor-dependent mechanism drives antibody-mediated target-receptor signaling in cancer cells. Cancer Cell 19, 101–113 (2011).
Bulliard, Y. et al. OX40 engagement depletes intratumoral Tregs via activating FcγRs, leading to antitumor efficacy. Immunol. Cell Biol. 92, 475–480 (2014).
Coe, D. et al. Depletion of regulatory T cells by anti-GITR mAb as a novel mechanism for cancer immunotherapy. Cancer Immunol. Immunother. 59, 1367–1377 (2010).
Simpson, T. R. et al. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J. Exp. Med. 210, 1695–1710 (2013).
Bulliard, Y. et al. Activating Fc γ receptors contribute to the antitumor activities of immunoregulatory receptor-targeting antibodies. J. Exp. Med. 210, 1685–1693 (2013).
Chau, I. et al. Nivolumab (NIVO) plus ipilimumab (IPI) or NIVO plus chemotherapy (chemo) versus chemo as first-line (1L) treatment for advanced esophageal squamous cell carcinoma (ESCC): first results of the CheckMate 648 study. J. Clin. Oncol. 39, LBA4001–LBA4001 (2021).
Vazquez-Lombardi, R. et al. Potent antitumour activity of interleukin-2-Fc fusion proteins requires Fc-mediated depletion of regulatory T-cells. Nat. Commun. 8, 1–12 (2017).
Ehrlich, P. Die Aufgaben der Chemotherapie. Frankfurter Zeitung und Handelsblatt (4 September 1906).
Chari, R. V. J., Miller, M. L. & Widdison, W. C. Antibody–drug conjugates: an emerging concept in cancer therapy. Angew. Chem. Int. Ed. 53, 3796–3827 (2014).
Mullard, A. Maturing antibody–drug conjugate pipeline hits 30. Nat. Rev. Drug Discov. 12, 329–332 (2013).
Narayan, P. et al. FDA approval summary: Fam-trastuzumab deruxtecan-Nxki for the treatment of unresectable or metastatic HER2-positive breast cancer. Clin. Cancer Res. 27, 4478–4485 (2021).
Shitara, K. et al. Discovery and development of trastuzumab deruxtecan and safety management for patients with HER2-positive gastric cancer. Gastric Cancer 24, 780–789 (2021).
Chau, C. H., Steeg, P. S. & Figg, W. D. Antibody–drug conjugates for cancer. Lancet 394, 793–804 (2019).
Lassiter, G. et al. Belantamab mafodotin to treat multiple myeloma: a comprehensive review of disease, drug efficacy and side effects. Curr. Oncol. 28, 640–660 (2021).
Lamb, Y. N. Inotuzumab ozogamicin: first global approval. Drugs 77, 1603–1610 (2017).
Geman, D., d’Avignon, C., Naiman, D. Q. & Winslow, R. L. Classifying gene expression profiles from pairwise mRNA comparisons. Stat. Appl. Genet. Mol.Biol. 3, 19 (2004).
Razzaghdoust, A. et al. Data-driven discovery of molecular targets for antibody–drug conjugates in cancer treatment. BioMed. Res. Int. 2021, 2670573 (2021).
Dott, J., Abila, B. & Wuerthner, J. U. Current trends in the clinical development of antibody–drug conjugates in oncology. Pharmaceut. Med. 32, 259–273 (2018).
Hartley, J. A. et al. Pre-clinical pharmacology and mechanism of action of SG3199, the pyrrolobenzodiazepine (PBD) dimer warhead component of antibody-drug conjugate (ADC) payload tesirine. Sci. Rep. 8, 10479 (2018).
Damle, N. K. & Frost, P. Antibody-targeted chemotherapy with immunoconjugates of calicheamicin. Curr. Opin. Pharmacol. 3, 386–390 (2003).
Lee, A. Loncastuximab tesirine: first approval. Drugs 81, 1229–1233 (2021).
Jen, E. Y. et al. FDA approval: gemtuzumab ozogamicin for the treatment of adults with newly diagnosed CD33-positive acute myeloid leukemia. Clin. Cancer Res. 24, 3242–3246 (2018).
Rossi, C., Chrétien, M. L. & Casasnovas, R. O. Antibody–drug conjugates for the treatment of hematological malignancies: a comprehensive review. Target Oncol. 13, 287–308 (2018).
Chen, H. et al. Synthesis and antitumor activity of novel arylpiperazine derivatives containing the saccharin moiety. Molecules 22, 1857 (2017).
Ballantyne, A. & Dhillon, S. Trastuzumab emtansine: first global approval. Drugs 73, 755–765 (2013).
Chang, E. et al. FDA approval summary: enfortumab vedotin for locally advanced or metastatic urothelial carcinoma. Clin. Cancer Res. 27, 922–927 (2021).
Deeks, E. D. Polatuzumab vedotin: first global approval. Drugs 79, 1467–1475 (2019).
Perez, J. et al. Trastuzumab deruxtecan in HER2-positive metastatic breast cancer and beyond. Expert Opin. Biol. Ther. 21, 811–824 (2021).
Wahby, S. et al. FDA approval summary: accelerated approval of sacituzumab govitecan-hziy for third-line treatment of metastatic triple-negative breast cancer. Clin. Cancer Res. 27, 1850–1854 (2021).
Thomas, A. & Pommier, Y. Targeting topoisomerase I in the era of precision medicine. Clin. Cancer Res. 25, 6581–6589 (2019).
Tsuchikama, K. & An, Z. Antibody–drug conjugates: recent advances in conjugation and linker chemistries. Protein Cell 9, 33–46 (2018).
Beck, A., Goetsch, L., Dumontet, C. & Corvaïa, N. Strategies and challenges for the next generation of antibody–drug conjugates. Nat. Rev. Drug Discov. 16, 315–337 (2017).
Bargh, J. D., Isidro-Llobet, A., Parker, J. S. & Spring, D. R. Cleavable linkers in antibody–drug conjugates. Chem. Soc. Rev. 48, 4361–4374 (2019).
Kovtun, Y. V. et al. Antibody–drug conjugates designed to eradicate tumors with homogeneous and heterogeneous expression of the target antigen. Cancer Res. 66, 3214–3221 (2006).
Staudacher, A. H. & Brown, M. P. Antibody–drug conjugates and bystander killing: is antigen-dependent internalisation required? Br. J. Cancer 117, 1736–1742 (2017).
Walles, M., Connor, A. & Hainzl, D. ADME and safety aspects of non-cleavable linkers in drug discovery and development. Curr. Top. Med. Chem. 17, 3463–3475 (2017).
Doronina, S. O. et al. Enhanced activity of monomethylauristatin F through monoclonal antibody delivery: effects of linker technology on efficacy and toxicity. Bioconjug. Chem. 17, 114–124 (2006).
Erickson, H. K. et al. Antibody–maytansinoid conjugates are activated in targeted cancer cells by lysosomal degradation and linker-dependent intracellular processing. Cancer Res. 66, 4426–4433 (2006).
Loganzo, X. T. Antibody–drug conjugate payloads induce markers of immunogenic cell death in cancer cells. Cancer Res. 78, 2757 (2018).
Müller, P. et al. Microtubule-depolymerizing agents used in antibody–drug conjugates induce antitumor immunity by stimulation of dendritic cells. Cancer Immunol. Res. 2, 741–755 (2014).
Müller, P. et al. Trastuzumab emtansine (T-DM1) renders HER2+ breast cancer highly susceptible to CTLA-4/PD-1 blockade. Sci. Transl. Med. 7, 315ra188 (2015).
Rios-Doria, J. et al. Antibody–drug conjugates bearing pyrrolobenzodiazepine or tubulysin payloads are immunomodulatory and synergize with multiple immunotherapies. Cancer Res. 77, 2686–2698 (2017).
Iwata, T. N. et al. A HER2-targeting antibody-drug conjugate, trastuzumab deruxtecan (DS-8201a), enhances antitumor immunity in a mouse model. Mol. Cancer Ther. 17, 1494–1503 (2018).
Galluzzi, L., Buqué, A., Kepp, O., Zitvogel, L. & Kroemer, G. Immunogenic cell death in cancer and infectious disease. Nat. Rev. Immunol. 17, 97–111 (2017).
Rosenberg, J. E. et al. Study EV-103: preliminary durability results of enfortumab vedotin plus pembrolizumab for locally advanced or metastatic urothelial carcinoma. J. Clin. Oncol. https://doi.org/10.1200/JCO.2020.38.6_suppl.441 (2020).
Schmid, P. et al. BEGONIA: phase 1b/2 study of durvalumab (D) combinations in locally advanced/metastatic triple-negative breast cancer (TNBC)—initial results from arm 1, d+paclitaxel (P), and arm 6, d+trastuzumab deruxtecan (T-DXd). J. Clin. Oncol. https://doi.org/10.1200/JCO.2021.39.15_suppl.1023 (2021).
Leppek, K., Das, R. & Barna, M. Functional 5′ UTR mRNA structures in eukaryotic translation regulation and how to find them. Nat. Rev. Mol. Cell Biol. 19, 158–174 (2018).
Indini, A., Rijavec, E. & Grossi, F. Trastuzumab deruxtecan: changing the destiny of HER2 expressing solid tumors. Int. J. Mol. Sci. 22, 4774 (2021).
Bardia, A. et al. Sacituzumab govitecan in metastatic triple-negative breast cancer. N. Engl. J. Med. 384, 1529–1541 (2021).
Li, Z. et al. Influence of molecular size on tissue distribution of antibody fragments. MAbs 8, 113–119 (2016).
Vazquez-Lombardi, R. et al. Challenges and opportunities for non-antibody scaffold drugs. Drug Discov. Today 20, 1271–1283 (2015).
Zhou, S., Liu, M., Ren, F., Meng, X. & Yu, J. The landscape of bispecific T cell engager in cancer treatment. Biomarker Res. 9, 38 (2021).
Nagorsen, D., Kufer, P., Baeuerle, P. A. & Bargou, R. Blinatumomab: a historical perspective. Pharmacol. Ther. 136, 334–342 (2012).
Ojemolon, P. E., Kalidindi, S., Ahlborn, T. A., Aihie, O. P. & Awoyomi, M. I. Cytokine release syndrome following blinatumomab therapy. Cureus 14, e21583 (2022).
Przepiorka, D. et al. FDA approval: blinatumomab. Clin. Cancer Res. 21, 4035–4039 (2015).
Tian, Z., Liu, M., Zhang, Y. & Wang, X. Bispecific T cell engagers: an emerging therapy for management of hematologic malignancies. J. Hematol. Oncol. 14, 75 (2021).
Wei, A. H. et al. Venetoclax plus LDAC for newly diagnosed AML ineligible for intensive chemotherapy: a phase 3 randomized placebo-controlled trial. Blood 135, 2137–2145 (2020).
Hutchings, M. et al. Glofitamab, a novel, bivalent CD20-targeting T-cell-engaging bispecific antibody, induces durable complete remissions in relapsed or refractory B-cell lymphoma: a phase I trial. J. Clin. Oncol. 39, 1959–1970 (2021).
Dickinson, M. et al. Glofitamab in patients with relapsed/refractory (R/R) diffuse large B-cell lymphoma (DLBCL) and ≥ 2 prior therapies: pivotal phase II expansion results. J. Clin. Oncol. 40, 7500 (2022).
Einsele, H. et al. The BiTE (bispecific T-cell engager) platform: development and future potential of a targeted immuno-oncology therapy across tumor types. Cancer 126, 3192–3201 (2020).
Subklewe, M. BiTEs better than CAR T cells. Blood Adv. 5, 607–612 (2021).
Jones, H. F., Molvi, Z., Klatt, M. G., Dao, T. & Scheinberg, D. A. Empirical and rational design of T cell receptor-based immunotherapies. Front. Immunol. 11, 585385 (2020).
Chang, A. Y. et al. Opportunities and challenges for TCR mimic antibodies in cancer therapy. Expert Opin. Biol. Ther. 16, 979–987 (2016).
Nathan, P. et al. Overall survival benefit with tebentafusp in metastatic uveal melanoma. N. Engl. J. Med. 385, 1196–1206 (2021).
Sgouros, G., Bodei, L., McDevitt, M. R. & Nedrow, J. R. Radiopharmaceutical therapy in cancer: clinical advances and challenges. Nat. Rev. Drug Discov. 19, 589–608 (2020).
Marcu, L., Bezak, E. & Allen, B. J. Global comparison of targeted α vs targeted β therapy for cancer: In vitro, in vivo and clinical trials. Crit. Rev. Oncol. Hematol. 123, 7–20 (2018).
Morris, M. J. et al. Phase III study of lutetium-177-PSMA-617 in patients with metastatic castration-resistant prostate cancer (VISION). J. Clin. Oncol. 39, LBA4 (2021).
Shields, R. L. et al. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcγ RIII and antibody-dependent cellular toxicity. J. Biol. Chem. 277, 26733–26740 (2002).
Narimatsu, Y. et al. Genetic glycoengineering in mammalian cells. J. Biol. Chem. 296, 100448 (2021).
Stapleton, N. M. et al. Competition for FcRn-mediated transport gives rise to short half-life of human IgG3 and offers therapeutic potential. Nat. Commun. 2, 599 (2011).
Lu, Q., Huang, H., Tang, S., Wang, Y. & Yang, D. H. Tafasitamab for refractory/relapsed diffuse large B-cell lymphoma. Drugs Today 57, 571–580 (2021).
Tarantino, P. et al. Margetuximab for the treatment of HER2-positive metastatic breast cancer. Expert Opin. Biol. Ther. 21, 127–133 (2021).
Roopenian, D. C. & Akilesh, S. FcRn: the neonatal Fc receptor comes of age. Nat. Rev. Immunol. 7, 715–725 (2007).
Beers, S. A., Glennie, M. J. & White, A. L. Influence of immunoglobulin isotype on therapeutic antibody function. Blood 127, 1097–1101 (2016).
White, A. L. et al. Interaction with FcγRIIB is critical for the agonistic activity of anti-CD40 monoclonal antibody. J. Immunol. 187, 1754–1763 (2011).
White, A. L., Beers, S. A. & Cragg, M. S. FcγRIIB as a key determinant of agonistic antibody efficacy. Curr. Top. Microbiol. Immunol. 382, 355–372 (2014).
Roschewski, M. & Hill, B. T. One size does not fit all: who benefits from maintenance after frontline therapy for follicular lymphoma? Am. Soc. Clin. Oncol. Educ. Book 39, 467–476 (2019).
Centanni, M., Moes, D. J. A. R., Trocóniz, I. F., Ciccolini, J. & van Hasselt, J. G. C. Clinical pharmacokinetics and pharmacodynamics of immune checkpoint inhibitors. Clin. Pharmacokinet. 58, 835–857 (2019).
Ugurlar, D. et al. Structures of C1-IgG1 provide insights into how danger pattern recognition activates complement. Science 359, 794–797 (2018).
Moore, G. L., Chen, H., Karki, S. & Lazar, G. A. Engineered Fc variant antibodies with enhanced ability to recruit complement and mediate effector functions. mAbs 2, 181–189 (2010).
Diebolder, C. A. et al. Complement is activated by IgG hexamers assembled at the cell surface. Science 343, 1260–1263 (2014).
Lazar, G. A. et al. Engineered antibody Fc variants with enhanced effector function. Proc. Natl Acad. Sci. USA 103, 4005–4010 (2006).
Ibrahim, R., Stewart, R. & Shalabi, A. PD-L1 blockade for cancer treatment: MEDI4736. Semin. Oncol. 42, 474–483 (2015).
Inman, B. A., Longo, T. A., Ramalingam, S. & Harrison, M. R. Atezolizumab: a PD-L1-blocking antibody for bladder cancer. Clin. Cancer Res. 23, 1886–1890 (2017).
Liu, S.-Y. & Wu, Y.-L. Tislelizumab: an investigational anti-PD-1 antibody for the treatment of advanced non-small cell lung cancer (NSCLC). Expert Opin. Investig. Drugs 29, 1355–1364 (2020).
Keyt, B. A., Baliga, R., Sinclair, A. M., Carroll, S. F. & Peterson, M. S. Structure, function, and therapeutic use of IgM antibodies. Antibodies 9, 53 (2020).
Pellizzari, G. et al. Harnessing therapeutic IgE antibodies to re-educate macrophages against cancer. Trends Mol. Med. 26, 615–626 (2020).
Bruhns, P. & Jönsson, F. Mouse and human FcR effector functions. Immunol. Rev. 268, 25–51 (2015).
Brinkhaus, M. et al. Human IgE does not bind to human FcRn. Sci. Rep. 12, 62 (2022).
Blumberg, L. J. et al. Blocking FcRn in humans reduces circulating IgG levels and inhibits IgG immune complex-mediated immune responses. Sci. Adv. 5, eaax9586 (2019).
Vieira, P. & Rajewsky, K. The half-lives of serum immunoglobulins in adult mice. Eur. J. Immunol. 18, 313–316 (1988).
Neri, D. Antibody–cytokine fusions: versatile products for the modulation of anticancer immunity. Cancer Immunol. Res. 7, 348–354 (2019).
Bonati, L. & Tang, L. Cytokine engineering for targeted cancer immunotherapy. Curr. Opin. Chem. Biol. 62, 43–52 (2021).
Holder, P. G. et al. Engineering interferons and interleukins for cancer immunotherapy. Adv. Drug Deliv. Rev. 182, 114112 (2022).
Young, P. A., Morrison, S. L. & Timmerman, J. M. Antibody–cytokine fusion proteins for treatment of cancer: engineering cytokines for improved efficacy and safety. Semin. Oncol. 41, 623–636 (2014).
Overwijk, W. W., Tagliaferri, M. A. & Zalevsky, J. Engineering IL-2 to give new life to T cell immunotherapy. Annu. Rev. Med. 72, 281–311 (2021).
Waldhauer, I. et al. Simlukafusp alfa (FAP-IL2v) immunocytokine is a versatile combination partner for cancer immunotherapy. MAbs 13, 1913791 (2021).
Ratcliff, M., Zhou, R. X., Jermutus, L. & Hyvönen, M. The role of pro-domains in human growth factors and cytokines. Biochem. Soc. Trans. 49, 1963–1973 (2021).
Elter, A. et al. Protease-activation of Fc-masked therapeutic antibodies to alleviate off-tumor cytotoxicity. Front. Immunol. 12, 715719 (2021).
Beck, A., Wurch, T., Bailly, C. & Corvaia, N. Strategies and challenges for the next generation of therapeutic antibodies. Nat. Rev. Immunol. 10, 345–352 (2010).
Liu, H. et al. In vitro and in vivo modifications of recombinant and human IgG antibodies. MAbs 6, 1145–1154 (2014).
Lee, C. H. et al. IgG Fc domains that bind C1q but not effector Fcγ receptors delineate the importance of complement-mediated effector functions. Nat. Immunol. 18, 889–898 (2017).
Montoyo, H. P. et al. Conditional deletion of the MHC class I-related receptor FcRn reveals the sites of IgG homeostasis in mice. Proc. Natl Acad. Sci. USA 106, 2788–2793 (2009).
Pyzik, M., Rath, T., Lencer, W. I., Baker, K. & Blumberg, R. S. FcRn: the architect behind the immune and nonimmune functions of IgG and albumin. J. Immunol. 194, 4595–4603 (2015).
Ward, E. S. et al. From sorting endosomes to exocytosis: association of Rab4 and Rab11 GTPases with the Fc receptor, FcRn, during recycling. Mol. Biol. Cell 16, 2028–2038 (2005).
Kim, J. K. et al. Mapping the site on human IgG for binding of the MHC class I-related receptor, FcRn. Eur. J. Immunol. 29, 2819–2825 (1999).
Kontermann, R. E. Strategies to extend plasma half-lives of recombinant antibodies. BioDrugs 23, 93–109 (2009).
Acknowledgements
S.Z., R.V.L. and D.C. wrote the manuscript with input from all authors. R.V.L. generated the figures. D.C. acknowledges funding from the National Health and Medical Research Council (1157744).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
P.J. and L.J. are employees and shareholders of AstraZeneca. R.V.L. is an employee and shareholder of Engimmune Therapeutics.
Peer review
Peer review information
Nature Cancer thanks Falk Nimmerjahn and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zinn, S., Vazquez-Lombardi, R., Zimmermann, C. et al. Advances in antibody-based therapy in oncology. Nat Cancer 4, 165–180 (2023). https://doi.org/10.1038/s43018-023-00516-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s43018-023-00516-z
This article is cited by
-
Decoding of the surfaceome and endocytome in primary glioblastoma cells identifies potential target antigens in the hypoxic tumor niche
Acta Neuropathologica Communications (2024)
-
Fcγ receptors and immunomodulatory antibodies in cancer
Nature Reviews Cancer (2024)
-
Considerations for the design of antibody drug conjugates (ADCs) for clinical development: lessons learned
Journal of Hematology & Oncology (2023)
-
Efficient Construction of a Polyaniline-coated DNA Nanoparticle for Photothermal Therapy
Chemical Research in Chinese Universities (2023)